Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature
Abstract
1. Introduction
2. Aging and Salivary Gland Degeneration
2.1. Structural Change
2.2. Saliva Composition
2.3. Salivary Flow Rate
3. Metabolic Changes in Salivary Gland
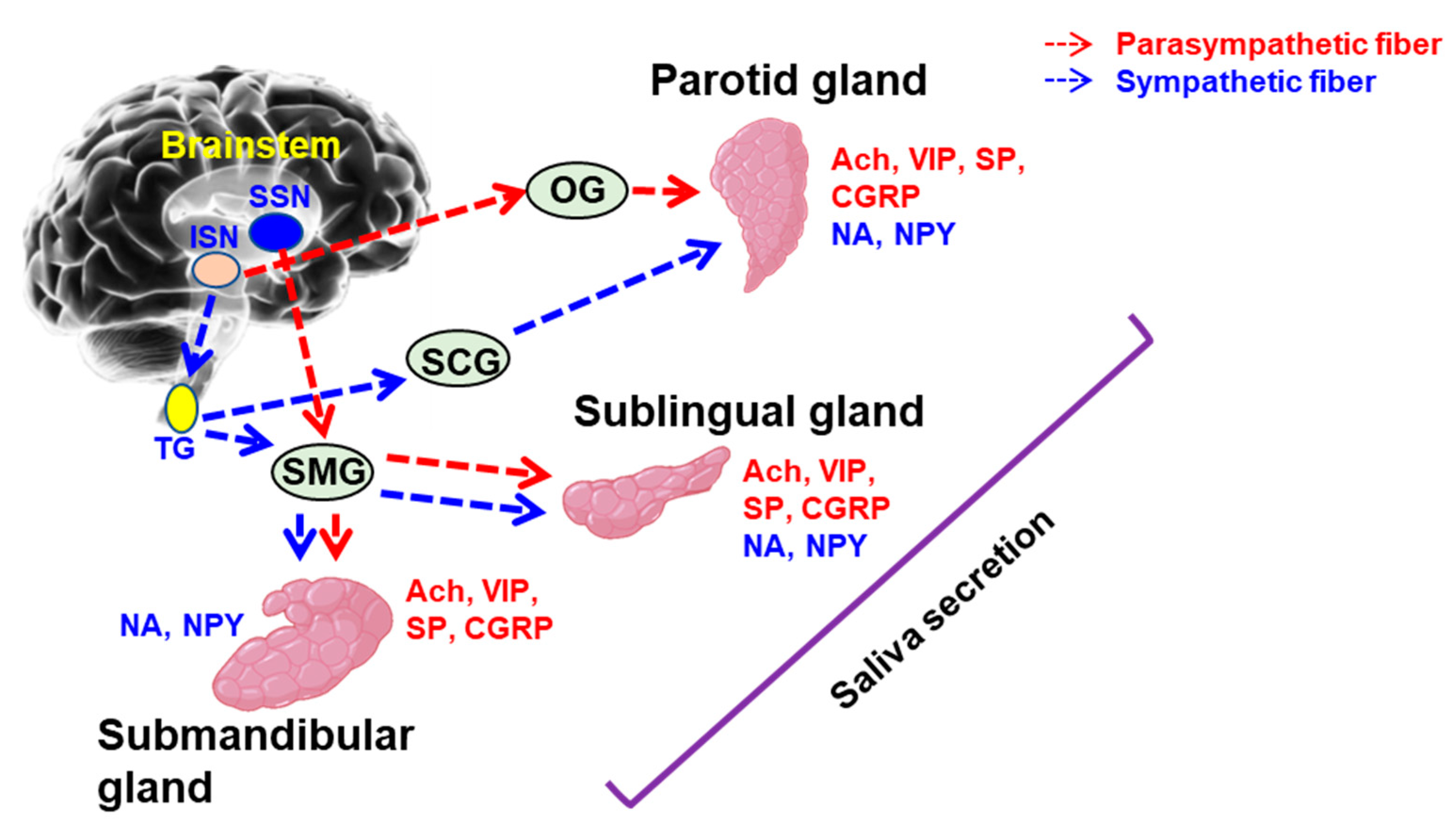
3.1. Innervation of Salivary Gland
3.2. Neurochemical Metabolites of Salivary Gland
4. Endocrine Metabolites of the Salivary Glands
4.1. Insulin
4.2. Melatonin
4.3. Estrogens and Androgens
5. Therapeutic Approach for Aging-Induced Salivary Gland Disorders
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scott, J. Quantitative age changes in the histological structure of human submandibular salivary glands. Arch. Oral Biol. 1977, 22, 221–227. [Google Scholar] [CrossRef]
- Ekström, J.; Khosravani, N.; Castagnola, M.; Messana, I. Saliva and the control of its secretion. In Dysphagia. Medical Radiology; Ekberg, O., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Age-Related changes in unstimulated salivary function and composition and its relations to medications and oral sensorial complaints. Aging Clin. Exp. Res. 2005, 17, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-Related changes in salivary biomarkers. J. Dent. Sci. 2014, 9, 85–90. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Zalewska, A.; Ładny, J.R. Salivary antioxidant barrier, redox status, and oxidative damage to proteins and lipids in healthy children, adults, and the elderly. Oxid. Med. Cell. Longev. 2019, 2019, 4393460. [Google Scholar] [CrossRef]
- Chang, W.I.; Chang, J.Y.; Kim, Y.Y.; Lee, G.; Kho, H.S. MUC1 expression in the oral mucosal epithelial cells of the elderly. Arch. Oral Biol. 2011, 56, 885–890. [Google Scholar] [CrossRef]
- Denny, P.C.; Denny, P.A.; Klauser, D.K.; Hong, S.H.; Navazesh, M.; Tabak, L.A. Age-Related changes in mucins from human whole saliva. J. Dent. Res. 1991, 70, 1320–1327. [Google Scholar] [CrossRef]
- Pushpass, R.A.G.; Daly, B.; Kelly, C.; Proctor, G.; Carpenter, G.H. Altered salivary flow, protein composition, and rheology following taste and TRP stimulation in older adults. Front. Physiol. 2019, 10, 652. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins in host defense and disease prevention. J. Oral Microbiol. 2015, 7, 29759. [Google Scholar] [CrossRef]
- Slomiany, B.L.; Murty, V.L.N.; Piotrowski, J.; Slomiany, A. Salivary mucins in oral mucosal defense. Gen. Pharmacol. 1996, 27, 761–771. [Google Scholar] [CrossRef]
- Mata, A.D.; Marques, D.; Rocha, S.; Francisco, H.; Santos, C.; Mesquita, M.F.; Singh, J. Effects of diabetes mellitus on salivary secretion and its composition in the human. Mol. Cell. Biochem. 2004, 261, 137–142. [Google Scholar] [CrossRef]
- Pijpe, J.; Kalk, W.W.I.; Bootsma, H.; Spijkervet, F.K.L.; Kallenberg, C.G.M.; Vissink, A. Progression of salivary gland dysfunction in patients with Sjögren’s syndrome. Ann. Rheum. Dis. 2007, 66, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Proulx, M.; de Courval, F.P.; Wiseman, M.A.; Panisset, M. Salivary production in Parkinson’s disease. Mov. Disord. 2005, 20, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Sreebny, L.M.; Schwartz, S.S. A reference guide to drugs and dry mouth—2nd edition. Gerodontology 1997, 14, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Nolan, N.E.; Puckett, S.A. Longitudinal analysis of parotid and submandibular salivary flow rates in healthy, different-aged adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, M285–M289. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Boland, B.; Daureeawoo, Y.; Donaldson, E.; Small, K.; Tuomainen, J. Effect of aging on stimulated salivary flow in adults. J. Am. Geriatr. Soc. 2013, 61, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of salivary flow rates in young and older adults. J. Am. Geriatr. Soc. 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Scott, J.; Flower, E.A.; Burns, J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J. Oral Pathol. Med. 1987, 16, 505–510. [Google Scholar] [CrossRef]
- Scott, J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J. Biol. Buccale 1980, 8, 187–200. [Google Scholar]
- Srivastava, S. Emerging insights into the metabolic alterations in aging using metabolomics. Metabolites 2019, 9, 301. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-Omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Herrala, M.; Mikkonen, J.J.W.; Pesonen, P.; Lappalainen, R.; Tjäderhane, L.; Niemelä, R.K.; Seitsalo, H.; Salo, T.; Myllymaa, S.; Kullaa, A.M. Variability of salivary metabolite levels in patients with sjögren’s syndrome. J. Oral Sci. 2021, 63, 22–26. [Google Scholar] [CrossRef]
- Kageyama, G.; Saegusa, J.; Irino, Y.; Tanaka, S.; Tsuda, K.; Takahashi, S.; Sendo, S.; Morinobu, A. Metabolomics analysis of saliva from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2015, 182, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, C.; Pitchika, V.; Pink, C.; Samietz, S.; Kastenmüller, G.; Artati, A.; Suhre, K.; Adamski, J.; Nauck, M.; Völzke, H.; et al. The saliva metabolome in association to oral health status. J. Dent. Res. 2019, 98, 642–651. [Google Scholar] [CrossRef]
- Mikkonen, J.J.W.; Singh, S.P.; Herrala, M.; Lappalainen, R.; Myllymaa, S.; Kullaa, A.M. Salivary metabolomics in the diagnosis of oral cancer and periodontal diseases. J. Periodontal Res. 2016, 51, 431–437. [Google Scholar] [CrossRef]
- Baima, G.; Iaderosa, G.; Citterio, F.; Grossi, S.; Romano, F.; Berta, G.N.; Buduneli, N.; Aimetti, M. Salivary metabolomics for the diagnosis of periodontal diseases: A systematic review with methodological quality assessment. Metabolomics 2021, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Goyal, V.; Kumaran, S.S.; Dwivedi, S.N.; Srivastava, A.; Jagannathan, N.R. Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls. Neurol. Sci. 2020, 1, 1201–1210. [Google Scholar] [CrossRef]
- Tsuruoka, M.; Hara, J.; Hirayama, A.; Sugimoto, M.; Soga, T.; Shankle, W.R.; Tomita, M. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis 2013, 34, 2865–2872. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef]
- Wang, X.; Kaczor-Urbanowicz, K.E.; Wong, D.T.W. Salivary biomarkers in cancer detection. Med. Oncol. 2017, 34, 7. [Google Scholar] [CrossRef]
- Mikkonen, J.J.; Herrala, M.; Soininen, P.; Lappalainen, R.; Tjäderhane, L.; Seitsalo, H.; Niemelä, R.; Salo, T.; Kullaa, A.M.; Myllymaa, S. Metabolic profiling of saliva in patients with primary Sjögren’s syndrome. Metabolomics 2012, 3, 1. [Google Scholar]
- Lohavanichbutr, P.; Zhang, Y.; Wang, P.; Gu, H.; Nagana Gowda, G.A.; Djukovic, D.; Buas, M.F.; Raftery, D.; Chen, C. Salivary metabolite profiling distinguishes patients with oral cavity squamous cell carcinoma from normal controls. PLoS ONE 2018, 13, e0204249. [Google Scholar] [CrossRef]
- Assad, D.X.; Mascarenhas, E.C.P.; de Lima, C.L.; de Toledo, I.P.; Chardin, H.; Combes, A.; Acevedo, A.C.; Guerra, E.N.S. Salivary metabolites to detect patients with cancer: A systematic review. Int. J. Clin. Oncol. 2020, 25, 1016–1036. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, H.; Li, X.; Zhang, A.H. High-Throughput metabolomics analysis discovers salivary biomarkers for predicting mild cognitive impairment and Alzheimer’s disease. RSC Adv. 2016, 6, 75499–75504. [Google Scholar] [CrossRef]
- Kim, S.A.; Lam, T.G.; Yook, J.I.; Ahn, S.G. Antioxidant modifications induced by the new metformin derivative HL156A regulate metabolic reprogramming in SAMP1/kl (-/-) mice. Aging 2018, 10, 2338–2355. [Google Scholar] [CrossRef] [PubMed]
- Toan, N.K.; Tai, N.C.; Kim, S.A.; Ahn, S.G. Choline acetyltransferase induces the functional regeneration of the salivary gland in aging SAMP1/KL -/- mice. Int. J. Mol. Sci. 2021, 22, 404. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H. Regulation of salivary gland function by autonomic nerves. Auton. Neurosci. Basic Clin. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- Kochhar, A.; Larian, B.; Azizzadeh, B. Facial nerve and parotid gland anatomy. Otolaryngol. Clin. North Am. 2016, 49, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.N.; Hoffman, M.P. Interactions between developing nerves and salivary glands. Organogenesis 2013, 9, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Kahle, W.; Frotscher, M. Color atlas of human anatomy. In Nervous System and Sensory Organs; Thieme: New York, NY, USA, 2021; Volume 3. [Google Scholar]
- Proctor, G.B.; Carpenter, G.H. Salivary secretion: Mechanism and neural regulation. Monogr. Oral Sci. 2014, 24, 14–29. [Google Scholar]
- Straub, S.V.; Giovannucci, D.R.; Bruce, J.I.E.; Yule, D.I. A role for phosphorylation of inositol 1,4,5-trisphosphate receptors in defining calcium signals induced by peptide agonists in pancreatic acinar cells. J. Biol. Chem. 2002, 277, 31949–31956. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Nonaka, N.; Takagi, Y.; Imamura, E.; Narukawa, M.; Nakamachi, T.; Shioda, S.; Banks, W.A.; Nakamura, M. Pituitary adenylate cyclase-activating polypeptide enhances saliva secretion via direct binding to PACAP receptors of major salivary glands in mice. Anat. Rec. 2016, 299, 1293–1299. [Google Scholar] [CrossRef]
- Qi, B.; Narita, T.; Satoh, K.; Guo, M.Y.; Katsumata-Kato, O.; Murakami, M.; Fujita-Yoshigaki, J.; Sugiya, H. Characteristics of neurokinin A-induced salivary fluid secretion in perfused rat submandibular gland. Arch. Oral Biol. 2010, 55, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Krause, J.E. Neuropeptide K potently stimulates salivary gland secretion and potentiates substance P-induced salivation. Proc. Natl. Acad. Sci. USA 1989, 86, 392–396. [Google Scholar] [CrossRef]
- Yu, J.H.; Burns, S.M.; Schneyer, C.A. Salivary secretion induced by substance P. Proc. Soc. Exp. Biol. Med. 1983, 173, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.N.; Carpenter, G.H.; Paterson, K.L.; Proctor, G.B. Inducible nitric oxide synthase increases secretion from inflamed salivary glands. Rheumatology 2010, 49, 48–56. [Google Scholar] [CrossRef]
- Endoh, T.; Shibukawa, Y.; Tsumura, M.; Ichikawa, H.; Tazaki, M.; Inoue, T. Calcitonin gene-related peptide- and adrenomedullin-induced facilitation of calcium current in submandibular ganglion. Arch. Oral Biol. 2011, 56, 187–193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, W.; Cong, X.; Zhang, X.M.; Wang, Z.Y.; Yang, N.Y.; Ding, C.; Shan, X.F.; Wu, L.L.; Yu, G.Y. Parasympathectomy increases resting salivary secretion in normal and irradiated submandibular glands of rats. Eur. J. Oral Sci. 2017, 125, 110–118. [Google Scholar] [CrossRef]
- Zhang, X.M.; Huang, Y.; Cong, X.; Qu, L.H.; Zhang, K.; Wu, L.L.; Zhang, Y.; Yu, G.Y. Parasympathectomy increases resting secretion of the submandibular gland in minipigs in the long term. J. Cell. Physiol. 2019, 234, 9515–9524. [Google Scholar] [CrossRef]
- Bottaro, B.; Cutler, L.S. An electrophysiological study of the postnatal development of the autonomic innervation of the rat submandibular salivary gland. Arch. Oral Biol. 1984, 29, 237–242. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef]
- Coppes, R.P.; Zeilstra, L.J.W.; Kampinga, H.H.; Konings, A.W.T. Early to late sparing of radiation damage to the parotid gland by adrenergic and muscarinic receptor agonists. Br. J. Cancer 2001, 85, 1055–1063. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.A.; Haddox, C.L.; Abrams, S.R.; Cotrim, A.; Wilson, A.J.; Hoffman, M.P. Parasympathetic stimulation improves epithelial organ regeneration. Nat. Commun. 2013, 4, 1494. [Google Scholar] [CrossRef]
- Proctor, G.B. The physiology of salivary secretion. Periodontol. 2000 2016, 70, 11–25. [Google Scholar] [CrossRef]
- Ekström, J.; Ekman, R.; Luts, A.; Sundler, F.; Tobin, G. Neuropeptide Y in salivary glands of the rat: Origin, release and secretory effects. Regul. Pept. 1996, 61, 125–134. [Google Scholar] [CrossRef]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, N.; Ohuchi, K.; Watanabe, M.; Tsurufuji, S. Role of endogenous histamine in postanaphylactic phase of allergic inflammation in rats. J. Pharmacol. Exp. Ther. 1987, 241, 967–973. [Google Scholar]
- Boison, D. Adenosinergic signaling in epilepsy. Neuropharmacology 2016, 104, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Boison, D.; Shen, H.Y. Adenosine kinase is a new therapeutic target to prevent ischemic neuronal death. Open Drug Discov. J. 2010, 2, 108–118. [Google Scholar]
- Zylka, M.J. Pain-Relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol. Med. 2011, 17, 188–196. [Google Scholar] [CrossRef]
- Pedata, F.; Pugliese, A.M.; Coppi, E.; Dettori, I.; Maraula, G.; Cellai, L.; Melani, A. Adenosine A 2A receptors modulate acute injury and neuroinflammation in brain ischemia. Mediat. Inflamm. 2014, 2014, 805198. [Google Scholar] [CrossRef]
- Rauck, R.L.; North, J.; Eisenach, J.C. Intrathecal clonidine and adenosine: Effects on pain and sensory processing in patients with chronic regional pain syndrome. Pain 2015, 156, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Pacher, P. Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef]
- Linden, J.; Cekic, C. Regulation of lymphocyte function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Barletta, K.E.; Ley, K.; Mehrad, B. Regulation of neutrophil function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Gao, Z.W.; Zhang, H.Z. The role of adenosinergic pathway in human autoimmune diseases. Immunol. Res. 2016, 64, 1133–1141. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pathological overproduction: The bad side of adenosine. Br. J. Pharmacol. 2017, 174, 1945–1960. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Cytidine 5′-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem. Res. 2005, 30, 15–23. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef]
- Diederich, K.; Frauenknecht, K.; Minnerup, J.; Schneider, B.K.; Schmidt, A.; Altach, E.; Eggert, V.; Sommer, C.J.; Schäbitz, W.R. Citicoline enhances neuroregenerative processes after experimental stroke in rats. Stroke 2012, 43, 1931–1940. [Google Scholar] [CrossRef]
- Cotroneo, A.M.; Castagna, A.; Putignano, S.; Lacava, R.; Fantò, F.; Monteleone, F.; Rocca, F.; Malara, A.; Gareri, P. Effectiveness and safety of citicoline in mild vascular cognitive impairment: The IDEALE study. Clin. Interv. Aging 2013, 8, 131–137. [Google Scholar] [CrossRef]
- Van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; van der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Égéa, J.C.; Hirtz, C.; Gross, R.; Lajoix, A.D.; Traskawka, E.; Ribes, G.; Deville De Périère, D. Preproinsulin I and II mRNA expression in adult rat submandibular glands. Eur. J. Oral Sci. 2000, 108, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Gvazava, I.G.; Vasil’ev, A.V.; Balan, O.V.; Terskikh, V.V. Cells of mouse submandibular salivary gland in culture in vitro. Cell Tissue Biol. 2011, 5, 165–170. [Google Scholar] [CrossRef]
- Baquero, A.F.; Gilbertson, T.A. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am. J. Physiol. Cell Physiol. 2011, 300, C860–C871. [Google Scholar] [CrossRef]
- López-Pintor, R.M.; Casañas, E.; González-Serrano, J.; Serrano, J.; Ramírez, L.; De Arriba, L.; Hernández, G. Xerostomia, hyposalivation, and salivary flow in diabetes patients. J. Diabetes Res. 2016, 2016, 4372852. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.K.; Åman, P.; Stenman, G. IGF2/IGF1R Signaling as a therapeutic target in MYB-positive adenoid cystic carcinomas and other fusion gene-driven tumors. Cells 2019, 8, 913. [Google Scholar] [CrossRef]
- Sumida, T.; Kamata, Y.U.; Kobayashi, Y.; Ishikawa, A.; Mori, Y. ID1 Controls aggressiveness of salivary gland cancer cells via crosstalk of IGF and AKT pathways. Anticancer Res. 2016, 36, 3865–3870. [Google Scholar]
- Liu, F.T.Y.; Lin, H.S. Role of insulin in body growth and the growth of salivary and endocrine glands in rats. J. Dent. Res. 1969, 48, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.L.; Huang, Y.; Zhang, Y.; Cong, X.; Zhang, Z.J.; Wu, L.L.; Yu, G.Y. Type 2 diabetes-induced hyposalivation of the submandibular gland through PINK1/Parkin-mediated mitophagy. J. Cell. Physiol. 2020, 235, 232–244. [Google Scholar] [CrossRef]
- Kolodziej, U.; Maciejczyk, M.; Miasko, A.; Matczuk, J.; Knas, M.; Zukowski, P.; Zendzian-Piotrowska, M.; Borys, J.; Zalewska, A. Oxidative modification in the salivary glands of high fat-diet induced insulin resistant rats. Front. Physiol. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Acuña-Castroviejo, D.; Reiter, R.J. Melatonin: Potential functions in the oral cavity. J. Periodontol. 2007, 78, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Czesnikiewicz-Guzik, M.; Konturek, S.J.; Loster, B.; Wisniewska, G.; Majewski, S. Melatonin and its role in oxidative stress related diseases of oral cavity. J. Physiol. Pharmacol. 2007, 58 (Suppl. 3), 5–19. [Google Scholar]
- Gómez-Moreno, G.; Guardia, J.; Ferrera, M.J.; Cutando, A.; Reiter, R.J. Melatonin in diseases of the oral cavity. Oral Dis. 2010, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.I.; Cengiz, S.; Wang, H.L. Melatonin and oral cavity. Int. J. Dent. 2012, 2012, 491872. [Google Scholar] [CrossRef]
- Shimozuma, M.; Tokuyama, R.; Tatehara, S.; Umeki, H.; Ide, S.; Mishima, K.; Saito, I.; Satomura, K. Expression and cellular localizaion of melatonin-synthesizing enzymes in rat and human salivary glands. Histochem. Cell Biol. 2011, 135, 389–396. [Google Scholar] [CrossRef]
- Isola, M.; Ekström, J.; Diana, M.; Solinas, P.; Cossu, M.; Lilliu, M.A.; Loy, F.; Isola, R. Subcellular distribution of melatonin receptors in human parotid glands. J. Anat. 2013, 223, 519–524. [Google Scholar] [CrossRef]
- Isola, M.; Lilliu, M.A. Melatonin localization in human salivary glands. J. Oral Pathol. Med. 2016, 45, 510–515. [Google Scholar] [CrossRef]
- Zhou, J.N.; Liu, R.Y.; Van Heerikhuize, J.; Hofman, M.A.; Swaab, D.F. Alterations in the circadian rhythm of salivary melatonin begin during middle-age. J. Pineal Res. 2003, 34, 11–16. [Google Scholar] [CrossRef]
- Pévet, P. Melatonin. Dialogues Clin. Neurosci. 2002, 4, 57–72. [Google Scholar] [PubMed]
- Ashour, M.A. Long-Term effect of melatonin on submandibular salivary glands in old rats. East. Mediterr. Heal. J. 1998, 4, 324–331. [Google Scholar]
- Elsherbini, A.M.; Ezzat, S.K. Effect of melatonin versus injectable platelet rich fibrin on critical wound healing in submandibular salivary glands of diabetic rats. J. Oral Biol. Craniofacial Res. 2020, 10, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Aras, H.Ç.; Ekström, J. Melatonin-Evoked in vivo secretion of protein and amylase from the parotid gland of the anaesthetised rat. J. Pineal Res. 2008, 45, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Obana-Koshino, A.; Ono, H.; Miura, J.; Sakai, M.; Uchida, H.; Nakamura, W.; Nohara, K.; Maruyama, Y.; Hattori, A.; Sakai, T. Melatonin inhibits embryonic salivary gland branching morphogenesis by regulating both epithelial cell adhesion and morphology. PLoS ONE 2015, 10, e0119960. [Google Scholar] [CrossRef]
- Kozloski, M.J.; Schumm, L.P.; McClintock, M.K. The utility and dynamics of salivary sex hormone measurements in the national social life, health, and aging project, wave 2. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2014, 69 (Suppl. 2), S215–S228. [Google Scholar] [CrossRef] [PubMed]
- Colella, G.; Izzo, G.; Carinci, F.; Campisi, G.; Lo Muzio, L.; D’Amato, S.; Mazzotta, M.; Cannavale, R.; Ferrara, D.; Minucci, S. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int. J. Immunopathol. Pharmacol. 2011, 24, 129–132. [Google Scholar] [CrossRef]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.A.; Laine, M. Estrogen receptor-β is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef]
- Meurman, J.H.; Tarkkila, L.; Tiitinen, A. The menopause and oral health. Maturitas 2009, 63, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Aufdemorte, T.B.; Chen, J.R.; Montoya, A.I.; Olive, D.; Talal, N. Estrogen induces the development of autoantibodies and promotes salivary gland lymphoid infiltrates in normal mice. J. Autoimmun. 1989, 2, 543–552. [Google Scholar] [CrossRef]
- Da, Y.; Niu, K.; Wang, K.; Cui, G.; Wang, W.; Jin, B.; Sun, Y.; Jia, J.; Qin, L.; Bai, W. A comparison of the effects of estrogen and Cimicifuga racemosa on the lacrimal gland and submandibular gland in ovariectomized rats. PLoS ONE 2015, 10, e0121470. [Google Scholar] [CrossRef]
- Sumida, T.; Ishikawa, A.; Kamata, Y.; Nakano, H.; Yamada, T.; Mori, Y. Estrogen enhances malignant phenotypes in human salivary adenoid cystic carcinoma cells. Anticancer Res. 2016, 36, 2793–2798. [Google Scholar]
- Laine, M.; Porola, P.; Udby, L.; Kjeldsen, L.; Cowland, J.B.; Borregaard, N.; Hietanen, J.; Ståhle, M.; Pihakari, A.; Konttinen, Y.T. Low salivary dehydroepiandrosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjögren’s syndrome. Arthritis Rheum. 2007, 56, 2575–2584. [Google Scholar] [CrossRef]
- Forsblad-d’Elia, H.; Carlsten, H.; Labrie, F.; Konttinen, Y.T.; Ohlsson, C. Low serum levels of sex steroids are associated with disease characteristics in primary Sjogren’s syndrome; supplementation with dehydroepiandrosterone restores the concentrations. J. Clin. Endocrinol. Metab. 2009, 94, 2044–2051. [Google Scholar] [CrossRef]
- Sato, S.; Maruyama, S.; Azuma, T. Androgenic action of glucocorticoids on the granular ducts of mouse submandibular glands. J. Endocrinol. 1981, 89, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kurabuchi, S. Repeated androgen and thyroid hormone injection modulates the morphology of hormone-responsive duct cells in the mouse parotid gland. Odontology 2006, 94, 29–37. [Google Scholar] [CrossRef]
- Kurabuchi, S.; Hosoi, K. Immunocytochemical study of granular duct cells with a hormonally enhanced granular cell phenotype in the mouse parotid gland. Odontology 2009, 97, 57–61. [Google Scholar] [CrossRef]
- Rocchi, C.; Emmerson, E. Mouth-Watering results: Clinical need, current approaches, and future directions for salivary gland regeneration. Trends Mol. Med. 2020, 26, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.I.; Konttinen, Y.; Fisher, A. Use of muscarinic agonists in the treatment of Sjögren’s syndrome. Clin. Immunol. 2001, 101, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Noaiseh, G.; Baker, J.F.; Vivino, F.B. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in Primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2014, 32, 575–577. [Google Scholar] [PubMed]
- Suzuki, Y.; Itoh, H.; Amada, K.; Yamamura, R.; Sato, Y.; Takeyama, M. Significant increase in salivary substance P level after a single oral dose of cevimeline in humans. Int. J. Pept. 2013, 2013, 284765. [Google Scholar] [CrossRef][Green Version]
- Brimhall, J.; Jhaveri, M.A.; Yepes, J.F. Efficacy of cevimeline vs. pilocarpine in the secretion of saliva: A pilot study. Spec. Care Dent. 2013, 33, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Q.; Xu, H.; Liu, L.; Wang, R.N.; Liu, Y.T.; Li, J.; Zhou, X.K. Efficacy and safety of pilocarpine for radiation-induced xerostomia in patients with head and neck cancer: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2016, 147, 236–243. [Google Scholar] [CrossRef]
- Cifuentes, M.; Del Barrio-Díaz, P.; Vera-Kellet, C. Pilocarpine and artificial saliva for the treatment of xerostomia and xerophthalmia in Sjögren syndrome: A double-blind randomized controlled trial. Br. J. Dermatol. 2018, 179, 1056–1061. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef]
- Güneri, P.; Alpöz, E.; Epstein, J.B.; Çankaya, H.; Ates, M. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec. Care Dent. 2011, 31, 123–128. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the oral microbiome: Beyond bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, G.; Guardia, J.; Aguilar-Salvatierra, A.; Cabrera-Ayala, M.; de-Val, J.E.M.S.; Calvo-Guirado, J.L. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med. Oral Patol. Oral Cir. Bucal 2013, 8, e49–e55. [Google Scholar] [CrossRef]
- Bardellini, E.; Amadori, F.; Conti, G.; Veneri, F.; Majorana, A. Effectiveness of a spray containing 1% malic acid in patients with xerostomia induced by graft-versus-host disease. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e190–e194. [Google Scholar] [CrossRef]
- Abashev, T.M.; Metzler, M.A.; Wright, D.M.; Sandell, L.L. Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium. Dev. Dyn. 2017, 246, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Metzler, M.A.; Raja, S.; Elliott, K.H.; Friedl, R.M.; Tran, N.Q.H.; Brugmann, S.A.; Larsen, M.; Sandell, L.L. RDH10-mediated retinol metabolism and RARα-mediated retinoic acid signaling are required for submandibular salivary gland initiation. Development 2018, 145, dev164822. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, D.; Waib, P.H.; Jordão Júnior, A.A. Mechanisms of action and effects of the administration of Coenzyme Q10 on metabolic syndrome. J. Nutr. Intermed. Metab. 2018, 13, 26–32. [Google Scholar] [CrossRef]
- Molyneux, S.L.; Young, J.M.; Florkowski, C.M.; Lever, M.; George, P.M. Coenzyme Q10: Is there a clinical role and a case for measurement? Clin. Biochem. Rev. 2008, 29, 71. [Google Scholar] [PubMed]
- Ushikoshi-Nakayama, R.; Ryo, K.; Yamazaki, T.; Kaneko, M.; Sugano, T.; Ito, Y.; Matsumoto, N.; Saito, I. Effect of gummy candy containing ubiquinol on secretion of saliva: A randomized, double-blind, placebo-controlled parallel-group comparative study and an in vitro study. PLoS ONE 2019, 14, e0214495. [Google Scholar] [CrossRef]
- Kaluarachchi, M.; Lewis, M.R.; Lindon, J.C. Standardized protocols for MS-based metabolic phenotyping. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppenaal, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 224–231. [Google Scholar]
| Reference | Study Design | N of Candidates | Results |
|---|---|---|---|
| Scott et al., 1987 [19] | Histological analysis of parotid salivary glands from dead people | N = 63 | Adipose content, fibrotic tissue, and ductal irregularities increase with age. Proportion of acinar structure declines by 30%. |
| J. Scott, 1977 [1] | Histological analysis of submandibular salivary glands from dead people | N = 96 | Reduction in parenchymal tissue and acinar structure. Percentage of adipose tissue increases. Duct volume also increases due to duct dilatation. |
| J Scott, 1980 [20] | Histological analysis of labial salivary glands from dead people | N = 70 | Acinar atrophy, ductal dilatation and hyperplasia increase with age. Acinar volume decreases while the fibrotic tissue proportion increases. |
| Nagler and Hershkovich, 2005 [4] | Sialometrical and sialochemical analysis of unstimulated saliva | N = 80 | Concentrations of K+, Ca2+, P, amylase and IgA increase. Total amounts of Na+, Ca2+, Mg2+, IgG, and IgA decrease. |
| Nassar et al., 2014 [5] | Analysis of unstimulated saliva | N = 40 | Salivary flow rate and concentrations of Ca2+, collagenase type 1 and MMP-8 decrease. |
| Maciejczyk et al., 2019 [6] | Redox and antioxidant analysis of both resting and stimulated saliva | N = 90 | Salivary peroxidase and catalase decrease while peroxidase increases with age. |
| Chang et al., 2011 [7] | Mucin and cytokine analysis of stimulated saliva | N = 60 | MUC1 levels and salivary flow rate decrease in the old age group. |
| Pushpass et al., 2019 [9] | Analysis of unstimulated and taste stimulated saliva | N = 56 | Salivary flow rate and MUC7 levels are decreased in old age group. |
| Affoo et al., 2015 [18] | Meta-analysis of previous studies involves salivary flow rate and age | N = 47 | Salivary flow rate decreased significantly with aging in every gland. |
| Name | Function | References |
|---|---|---|
| Acetylcholine (Ach) | Invokes water secretion through M1/M3 AchR; maintains the stemness of the epithelial salivary gland stem cells during organogenesis | Proctor, 2016 Knox et al., 2010 [54,57] |
| Norepinephrine | Invokes protein secretion through β1 adrenergic receptors | Straub et al., 2002 [44] |
| Vasoactive intestinal peptide (VIP) | Invokes protein secretion through β1 adrenergic receptors | Straub et al., 2002 [44] |
| Neuropeptide Y (NPY) | Induces protein and ion secretion | Ekstrom et al., 1996 [58] |
| Neurokinin A (NKA) | Stimulates saliva secretion by manipulating intracellular Ca2+ signaling | Qi et al., 2010 [46] |
| Substance P (SP) | Stimulates saliva secretion through tachykinis receptors NK1 | Yu et al., 1983 [48] |
| Nitric oxide synthase (NOS) | Induces saliva secretion through the free radical nitric oxide | Correia et al., 2010 [49] |
| Pituitary adenylate cyclase activating peptide (PACAP) | Invokes saliva secretion by binding to its receptor PAC1R; increases the EGF level in saliva. | Matoba et al., 2016 [45] |
| Calcitonin gene-related peptide (CGRP) | Modulates the voltage-dependent calcium channels; enhances NPY-induced saliva secretion | Endoh et al., 2011. [50] |
| Name | Function | References |
|---|---|---|
| Insulin | Dysfunction of insulin metabolism can induce acinar enlargement, ductal atrophy, mitochondrial dysfunction, mitophagy, oxidative stress, and oxidative lipid accumulation. | Liu and Lin, 1969 Xiang et al., 2020 [82,83] |
| Melatonin | Induces protein secretion through melatonin receptors and nitric oxide synthase. Induces cellular activity and regulates the organogenesis of embryonic salivary glands | Aras & Ekstrom, 2008 Ashour, 1998 Obana-Koshino et al., 2015 [94,96,97] |
| Estrogens | Lack of estrogen is highly associated with the development of salivary gland-related diseases. Ovariectomized rats developed cell apoptosis, gland atrophy, and mitochondrial defects, which are all reversible with estrogen administration. Can induce the production of antibodies and increase the lymphocyte infiltration in salivary glands. | Meurman et al., 2009 Ahmed et al., 1989 Da et al., 2015 [101,102,103] |
| Androgens | Castrated mice have significantly smaller salivary gland size, granular duct cells and duct diameter. Can induce the development of granular cells in salivary glands. DHEA treatment improves the salivary flow rate and acinar cells in Sjögren’s syndrome patients. | Sato et al., 1981 Kurabuchi, 2006 Kurabuchi and Hosoi, 2009 [107,108,109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toan, N.K.; Ahn, S.-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 5835. https://doi.org/10.3390/ijms22115835
Toan NK, Ahn S-G. Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. International Journal of Molecular Sciences. 2021; 22(11):5835. https://doi.org/10.3390/ijms22115835
Chicago/Turabian StyleToan, Nguyen Khanh, and Sang-Gun Ahn. 2021. "Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature" International Journal of Molecular Sciences 22, no. 11: 5835. https://doi.org/10.3390/ijms22115835
APA StyleToan, N. K., & Ahn, S.-G. (2021). Aging-Related Metabolic Dysfunction in the Salivary Gland: A Review of the Literature. International Journal of Molecular Sciences, 22(11), 5835. https://doi.org/10.3390/ijms22115835






