The Density of Group I mGlu5 Receptors Is Reduced along the Neuronal Surface of Hippocampal Cells in a Mouse Model of Alzheimer’s Disease
Abstract
:1. Introduction
2. Results
2.1. mGlu5 and Downstream Molecules in APP/PS1 Mice
2.2. Similar Regional and Cellular Expression of mGlu5 in Control and APP/PS1 Mice
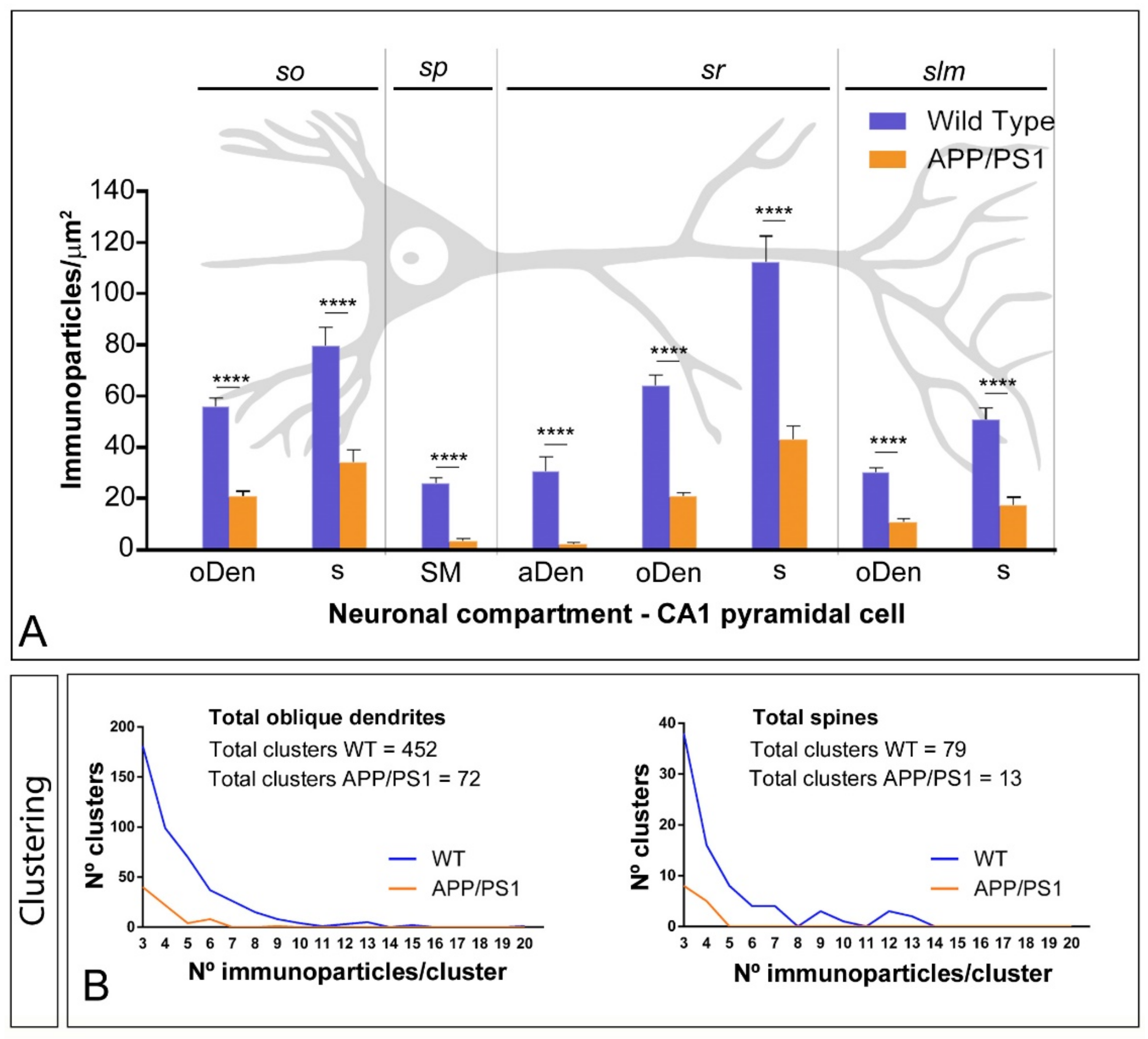
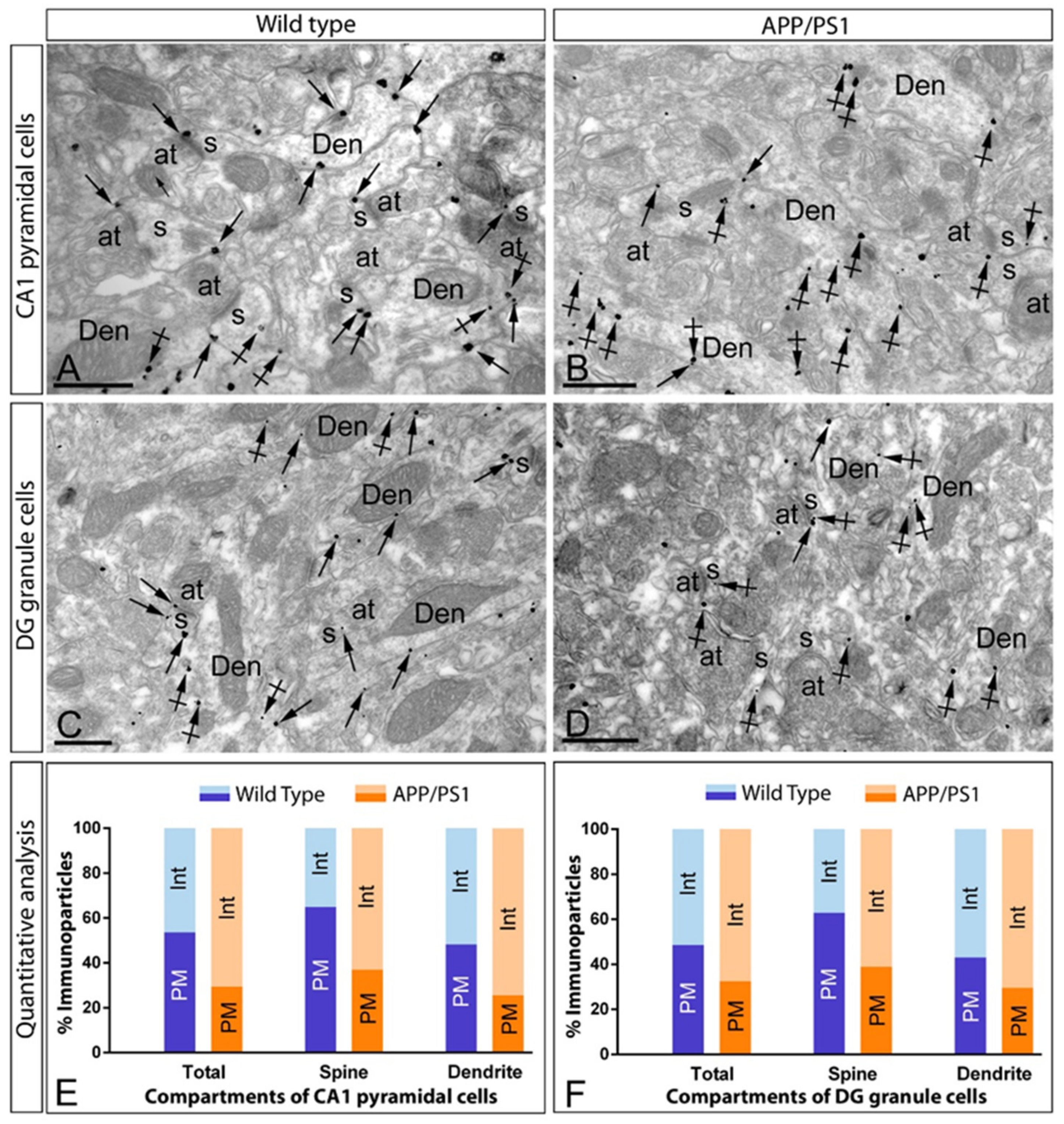
2.3. Reduction of mGlu5 in the Surface of CA1 Pyramidal Cells in APP/PS1 Mice
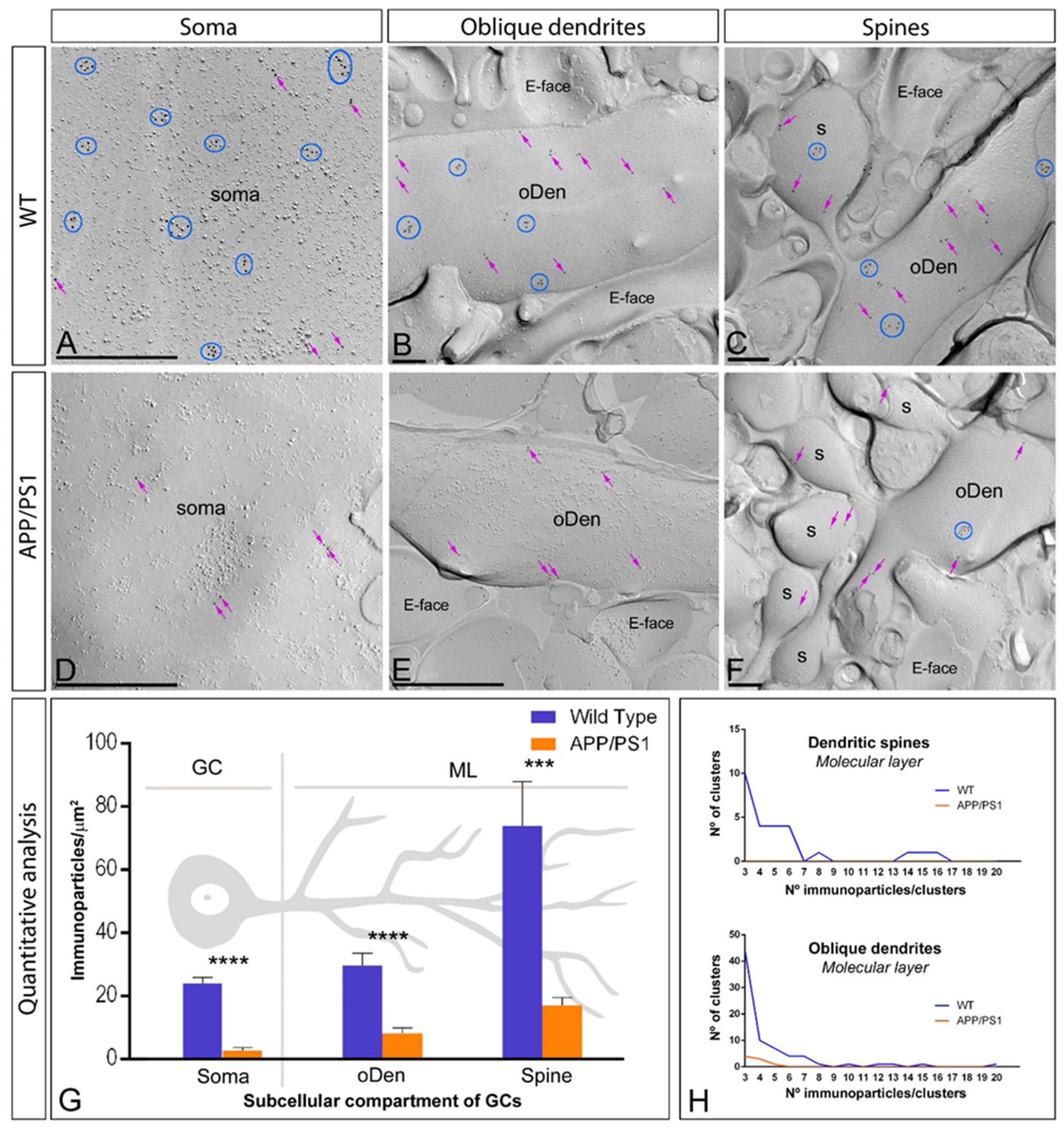
2.4. Reduction of mGlu5 in the Surface of DG Granule Cells in APP/PS1 Mice
2.5. Increase of mGlu5 in the Cytoplasm of Hippocampal Neurons in APP/PS1 Mice
3. Discussion
3.1. Stable Expression Levels of mGlu5 Protein in the Hippocampus of APP/PS1 Mice
3.2. Postsynaptic Arrangement of mGlu5 in Principal Cells
3.3. Evidence for the Reduction of mGlu5 on the Surface of Principal Cells
4. Material and Methods
4.1. Animals
4.2. Antibodies and Chemicals
4.3. Immunoblots
4.4. Histoblotting
4.5. Immunohistochemistry for Electron Microscopy
4.6. Quantification and Analysis of SDS-FRL Data
4.7. Controls
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyman, B.T.; Damasio, H.; Damasio, A.R.; Van Hoesen, G.W. Alzheimer’s disease. Annu. Rev. Public Health 1989, 10, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Spires-Jones, T.L.; Hyman, B.T. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaFerla, F.M.; Oddo, S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 2005, 11, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martín, M.; Blazquez-Llorca, L.; Benavides-Piccione, R.; Rabano, A.; Hernandez, F.; Avila, J.; DeFelipe, J. Selective alterations of neurons and circuits related to early memory loss in Alzheimer’s disease. Front. Neuroanat. 2014, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ondrejcak, T.; Klyubin, I.; Hu, N.-W.; Barry, A.E.; Cullen, W.K.; Rowan, M.J. Alzheimer’s Disease Amyloid β-Protein and Synaptic Function. NeuroMol. Med. 2010, 12, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Paula-Lima, A.C.; Brito-Moreira, J.; Ferreira, S.T. Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. J. Neurochem. 2013, 126, 191–202. [Google Scholar] [CrossRef]
- Revett, T.; Baker, G.; Jhamandas, J.; Kar, S. Glutamate system, amyloid β peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J. Psychiatry Neurosci. 2013, 38, 6–23. [Google Scholar] [CrossRef] [Green Version]
- Forner, S.; Baglietto-Vargas, D.; Martini, A.C.; Trujillo-Estrada, L.; LaFerla, F.M. Synaptic Impairment in Alzheimer’s Disease: A Dysregulated Symphony. Trends Neurosci. 2017, 40, 347–357. [Google Scholar] [CrossRef]
- Ottersen, O.P.; Storm-Mathisen, J.; Bramham, C.; Torp, R.; Laake, J.; Gundersen, V. A quantitative electron microscopic immunocytochemical study of the distribution and synaptic handling of glutamate in rat hippocampus. Prog. Brain Res. 1990, 83, 99–114. [Google Scholar] [CrossRef]
- Lehre, K.P.; Levy, L.M.; Ottersen, O.P.; Storm-Mathisen, J.; Danbolt, N.C. Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. J. Neurosci. 1995, 15, 1835–1853. [Google Scholar] [CrossRef]
- Hollmann, M.; Heinemann, S. Cloned Glutamate Receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef]
- Nakanishi, S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron 1994, 13, 1031–1037. [Google Scholar] [CrossRef]
- Pin, J.P.; Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [Green Version]
- Anwyl, R. Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res. Rev. 1999, 29, 83–120. [Google Scholar] [CrossRef]
- Lu, Y.M.; Jia, Z.; Janus, C.; Henderson, J.T.; Gerlai, R.; Wojtowicz, J.M.; Roder, J.C. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J. Neurosci. 1997, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Ayala, J.E.; Chen, Y.; Banko, J.L.; Sheffler, D.J.; Williams, R.; Telk, A.N.; Watson, N.L.; Xiang, Z.; Zhang, Y.; Jones, P.J.; et al. MGluR5 Positive Allosteric Modulators Facilitate both Hippocampal LTP and LTD and Enhance Spatial Learning. Neuropsychopharmacology 2009, 34, 2057–2071. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Sugihara, H.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992, 267, 13361–13368. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nomura, S.; Ohishi, H.; Sugihara, H.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993, 163, 53–57. [Google Scholar] [CrossRef]
- Luján, R.; Nusser, Z.; Roberts, J.D.B.; Shigemoto, R.; Somogyi, P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur. J. Neurosci. 1996, 8, 1488–1500. [Google Scholar] [CrossRef]
- Luján, R.; Roberts, J.D.B.; Shigemoto, R.; Ohishi, H.; Somogyi, P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanat. 1997, 13, 219–241. [Google Scholar] [CrossRef]
- Harney, S.C.; Rowan, M.; Anwyl, R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J. Neurosci. 2006, 26, 1128–1132. [Google Scholar] [CrossRef]
- Kumar, A.; Dhull, D.K.; Mishra, P.S. Therapeutic potential of mGluR5 targeting in Alzheimer’s disease. Front. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious Effects of Amyloid β Oligomers Acting as an Extracellular Scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef] [Green Version]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic Glutamate Receptor 5 Is a Coreceptor for Alzheimer Aβ Oligomer Bound to Cellular Prion Protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [Green Version]
- Luján, R.; Aguado, C.; Ciruela, F.; Cózar, J.; Kleindienst, D.; de la Ossa, L.; Bettler, B.; Wickman, K.; Watanabe, M.; Shigemoto, R.; et al. Differential association of GABA B receptors with their effector ion channels in Purkinje cells. Brain Struct. Funct. 2018, 223, 1565–1587. [Google Scholar] [CrossRef] [Green Version]
- Thathiah, A.; De Strooper, B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 73–87. [Google Scholar] [CrossRef]
- Cartmell, J.; Schoepp, D.D. Regulation of Neurotransmitter Release by Metabotropic Glutamate Receptors. J. Neurochem. 2002, 75, 889–907. [Google Scholar] [CrossRef]
- Neyman, S.; Manahan-Vaughan, D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur. J. Neurosci. 2008, 27, 1345–1352. [Google Scholar] [CrossRef]
- Dinamarca, M.C.; Ríos, J.A.; Inestrosa, N.C. Postsynaptic receptors for amyloid-β oligomers as mediators of neuronal damage in Alzheimer’s disease. Front. Physiol. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caraci, F.; Nicoletti, F.; Copani, A. Metabotropic glutamate receptors: The potential for therapeutic applications in Alzheimer’s disease. Curr. Opin. Pharmacol. 2018, 38, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Paquet, M.; Cregan, P.S.; Ferguson, S.G.S. Group I Metabotropic Glutamate Receptor Signalling and its Implication in Neurological Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 574–595. [Google Scholar] [CrossRef] [PubMed]
- Merino-Serrais, P.; Knafo, S.; Alonso-Nanclares, L.; Fernaud-Espinosa, I.; Defelipe, J. Layer-specific alterations to CA1 dendritic spines in a mouse model of Alzheimer’s disease. Hippocampus 2011, 21, 1037–1044. [Google Scholar] [CrossRef]
- Alonso-Nanclares, L.; Merino-Serrais, P.; Gonzalez, S.; Defelipe, J. Synaptic changes in the dentate gyrus of APP/PS1 transgenic mice revealed by electron microscopy. J. Neuropathol. Exp. Neurol. 2013, 72, 386–395. [Google Scholar] [CrossRef] [Green Version]
- Šišková, Z.; Justus, D.; Kaneko, H.; Friedrichs, D.; Henneberg, N.; Beutel, T.; Pitsch, J.; Schoch, S.; Becker, A.; vonderKammer, H.; et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of alzheimer’s disease. Neuron 2014, 84, 1023–1033. [Google Scholar] [CrossRef] [Green Version]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruíz, R.; Itakura, M.; Moreno-Martínez, A.E.; de la Ossa, L.; Molnár, E.; Fukazawa, Y.; Luján, R. Age-Dependent Shift of AMPA Receptors from Synapses to Intracellular Compartments in Alzheimer’s Disease: Immunocytochemical Analysis of the CA1 Hippocampal Region in APP/PS1 Transgenic Mouse Model. Front. Aging Neurosci. 2020, 12. [Google Scholar] [CrossRef]
- Haas, L.T.; Salazar, S.V.; Kostylev, M.A.; Um, J.W.; Kaufman, A.C.; Strittmatter, S.M. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer’s disease. Brain 2016, 139, 526–546. [Google Scholar] [CrossRef] [Green Version]
- Raka, F.; Di Sebastiano, A.R.; Kulhawy, S.C.; Ribeiro, F.M.; Godin, C.M.; Caetano, F.A.; Angers, S.; Ferguson, S.S.G. Ca2+/Calmodulin-dependent protein Kinase II interacts with group i Metabotropic Glutamate and facilitates Receptor Endocytosis and ERK1/2 signaling: Role of β-Amyloid. Mol. Brain 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Um, J.W.; Nygaard, H.B.; Heiss, J.K.; Kostylev, M.A.; Stagi, M.; Vortmeyer, A.; Wisniewski, T.; Gunther, E.C.; Strittmatter, S.M. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012, 15, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Findley, C.A.; Bartke, A.; Hascup, K.N.; Hascup, E.R. Amyloid Beta-Related Alterations to Glutamate Signaling Dynamics During Alzheimer’s Disease Progression. ASN Neuro 2019, 11. [Google Scholar] [CrossRef]
- Hamilton, A.; Esseltine, J.L.; Devries, R.A.; Cregan, S.P.; Ferguson, S.S.G. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elrahman, K.S.; Albaker, A.; de Souza, J.M.; Ribeiro, F.M.; Schlossmacher, M.G.; Tiberi, M.; Hamilton, A.; Ferguson, S.S.G. Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer’s disease model mice in a sex-selective manner. Sci. Signal. 2020, 13. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Dalfó, E.; Ferrer, I.; Martín, M. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol. Dis. 2005, 20, 685–693. [Google Scholar] [CrossRef]
- Simonyi, A.; Ngomba, R.T.; Storto, M.; Catania, M.V.; Miller, L.A.; Youngs, B.; DiGorgi-Gerevini, V.; Nicoletti, F.; Sun, G.Y. Expression of groups I and II metabotropic glutamate receptors in the rat brain during aging. Brain Res. 2005, 1043, 95–106. [Google Scholar] [CrossRef]
- Sánchez-Melgar, A.; Albasanz, J.L.; Pallàs, M.; Martín, M. Resveratrol Differently Modulates Group i Metabotropic Glutamate Receptors Depending on Age in SAMP8 Mice. ACS Chem. Neurosci. 2020, 11, 1770–1780. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.J.; Park, I.S.; Park, J.A.; Kwon, Y.J.; Ryu, Y.H.; Kim, C.H.; Kang, J.H.; Hyun, I.Y.; Lee, K.C.; et al. Aβ pathology downregulates brain mGluR5 density in a mouse model of Alzheimer. Neuropharmacology 2018, 133, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, H.J.; Jeong, Y.J.; Oh, S.J.; Kang, K.J.; Han, S.J.; Nam, K.R.; Lee, Y.J.; Lee, K.C.; Ryu, Y.H.; et al. Age dependency of mGluR5 availability in 5xFAD mice measured by PET. Neurobiol. Aging 2019, 84, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Masugi-Tokita, M.; Shigemoto, R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr. Opin. Neurobiol. 2007, 17, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.I.; Matsuzaki, M.; Tarusawa, E.; Momiyama, A.; Molnar, E.; Kasai, H.; Shigemoto, R. Number and density of AMPA receptors in single synapses in immature cerebellum. J. Neurosci. 2005, 25, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’malley, K.; van den Pol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nakanishi, S.; Mizuno, N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J. Comp. Neurol. 1992, 322, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Berthele, A.; Laurie, D.J.; Platzer, S.; Zieglgänsberger, W.; Tölle, T.R.; Sommer, B. Differential expression of rat and human type 1 metabotropic glutamate receptor splice variant messenger RNAs. Neuroscience 1998, 85, 733–749. [Google Scholar] [CrossRef]
- Poncer, J.C.; Shinozaki, H.; Miles, R. Dual modulation of synaptic inhibition by distinct metabotropic glutamate receptors in the rat hippocampus. J. Physiol. 1995, 485, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Popkirov, S.G.; Manahan-Vaughan, D. Involvement of the metabotropic glutamate receptor mGluR5 in NMDA receptor-dependent, learning-facilitated long-term depression in CA1 synapses. Cereb. Cortex 2011, 21, 501–509. [Google Scholar] [CrossRef] [Green Version]
- García-Negredo, G.; Soto, D.; Llorente, J.; Morató, X.; Galenkamp, K.M.O.; Gómez-Soler, M.; Fernández-Dueñas, V.; Watanabe, M.; Adelman, J.P.; Shigemoto, R.; et al. Coassembly and coupling of SK2 channels and mGlu5 receptors. J. Neurosci. 2014, 34, 14793–14802. [Google Scholar] [CrossRef] [Green Version]
- El-Hassar, L.; Hagenston, A.M.; D’Angelo, L.B.; Yeckel, M.F. Metabotropic glutamate receptors regulate hippocampal CA1 pyramidal neuron excitability via Ca 2+ wave-dependent activation of SK and TRPC channels. J. Physiol. 2011, 589, 3211–3229. [Google Scholar] [CrossRef]
- Kato, H.K.; Kassai, H.; Watabe, A.M.; Aiba, A.; Manabe, T. Functional coupling of the metabotropic glutamate receptor, InsP3 receptor and L-type Ca2+ channel in mouse CA1 pyramidal cells. J. Physiol. 2012, 590, 3019–3034. [Google Scholar] [CrossRef] [Green Version]
- Reiner, A.; Levitz, J. Glutamatergic Signaling in the Central Nervous System: Ionotropic and Metabotropic Receptors in Concert. Neuron 2018, 98, 1080–1098. [Google Scholar] [CrossRef] [Green Version]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruíz, R.; Moreno-Martínez, A.E.; de la Ossa, L.; Martínez-Hernández, J.; Buisson, A.; Shigemoto, R.; Fukazawa, Y.; Luján, R. Density of GABAB Receptors Is Reduced in Granule Cells of the Hippocampus in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2459. [Google Scholar] [CrossRef] [Green Version]
- Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruíz, R.; Moreno-Martínez, A.E.; de la Ossa, L.; Martínez-Hernández, J.; Buisson, A.; Früh, S.; Bettler, B.; Shigemoto, R.; et al. Reduction in the neuronal surface of post and presynaptic GABAB receptors in the hippocampus in a mouse model of Alzheimer’s disease. Brain Pathol. 2020, 30, 554–575. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.T.; Luján, R.; Watanabe, M.; Adelman, J.P.; Maylie, J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat. Neurosci. 2008, 11, 170–177. [Google Scholar] [CrossRef]
- Aniksztejn, L.; Otani, S.; Ben-Ari, Y. Quisqualate Metabotropic Receptors Modulate NMDA Currents and Facilitate Induction of Long-Term Potentiation Through Protein Kinase C. Eur. J. Neurosci. 1992, 4, 500–505. [Google Scholar] [CrossRef]
- Conquet, F.; Bashir, Z.I.; Davies, C.H.; Daniel, H.; Ferraguti, F.; Bordi, F.; Franz-Bacon, K.; Reggiani, A.; Matarese, V.; Condé, F.; et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 1994, 372, 237–243. [Google Scholar] [CrossRef]
- Jankowsky, J.L.; Slunt, H.H.; Ratovitski, T.; Jenkins, N.A.; Copeland, N.G.; Borchelt, D.R. Co-expression of multiple transgenes in mouse CNS: A comparison of strategies. Biomol. Eng. 2001, 17, 157–165. [Google Scholar] [CrossRef]
- Tanaka, J.; Nakagawa, S.; Kushiya, E.; Yamasaki, M.; Fukaya, M.; Iwanaga, T.; Simon, M.I.; Sakimura, K.; Kano, M.; Watanabe, M. Gq protein α subunits Gαq and Gα11 are localized at postsynaptic extra-junctional membrane of cerebellar Purkinje cells and hippocampal pyramidal cells. Eur. J. Neurosci. 2000, 12, 781–792. [Google Scholar] [CrossRef]
- Uchigashima, M.; Narushima, M.; Fukaya, M.; Katona, I.; Kano, M.; Watanabe, M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 2007, 27, 3663–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguado, C.; Luján, R. The Histoblot Technique: A Reliable Approach to Analyze Expression Profile of Proteins and to Predict Their Molecular Association BT—Co-Immunoprecipitation Methods for Brain Tissue. In Neuromethods; Odagaki, Y., Borroto-Escuela, D.O., Eds.; Springer: New York, NY, USA, 2019; pp. 65–88. ISBN 978-1-4939-8985-0. [Google Scholar]


| Wild Type | APP/PS1 | ||
|---|---|---|---|
| Mean ± SEM (Immunoparticles/µm2) | Mean ± SEM (Immunoparticles/µm2) | ||
| CA1 | Stratum oriens | ||
| Oblique Dendrites | 56.02 ± 3.16 | 20.83 ± 2.01 | |
| Spines | 79.54 ± 7.37 | 34.05 ± 4.93 | |
| Stratum pyramidale | |||
| Soma | 26.23 ± 1.75 | 3.57 ± 0.92 | |
| Stratum radiatum | |||
| Apical Dendrites | 30.58 ± 5.54 | 2.43 ± 0.47 | |
| Oblique Dendrites | 64.26 ± 3.91 | 20.79 ± 1.68 | |
| Spines | 112.18 ± 10.26 | 43.23 ± 5.00 | |
| Stratum lacunosum-moleculare | |||
| Oblique Dendrites | 30.32 ± 2.00 | 11.25 ± 1.24 | |
| Spines | 50.90 ± 4.51 | 17.27 ± 3.15 | |
| DG | Granule cell layer | ||
| Soma | 24.01 ± 1.84 | 2.70 ± 0.98 | |
| Molecular layer | |||
| Oblique Dendrites | 29.79 ± 3.75 | 8.25 ± 1.59 | |
| Spines | 73.86 ± 14.00 | 17.07 ± 2.43 | |
| Wild Type | APP/PS1 | ||||||
|---|---|---|---|---|---|---|---|
| No. Profiles | Area Profiles | No. Clusters | Range Gold Particles | No. Profiles | Area Profiles | No. Clusters | Range Gold Particles |
| 24 | 22 µm2 | 184 | 13–3 | 24 | 21 µm2 | 27 | 6–3 |
| 24 | 1.8 µm2 | 29 | 13–3 | 24 | 1.7 µm2 | 6 | 4–3 |
| 15 | 17 µm2 | 74 | 11–3 | 15 | 21 µm2 | 8 | 10–3 |
| 15 | 17 µm2 | 38 | 11–3 | 15 | 21 µm2 | 9 | 5–3 |
| 24 | 20 µm2 | 209 | 20–3 | 24 | 18 µm2 | 34 | 9–3 |
| 30 | 2 µm2 | 33 | 9–3 | 30 | 1.9 µm2 | 7 | 4–3 |
| 24 | 15 µm2 | 59 | 9–3 | 24 | 14 µm2 | 14 | 5–3 |
| 27 | 2 µm2 | 17 | 5–3 | 27 | 1.9 µm2 | 0 | |
| 183 | 97 µm2 | 643 | 183 | 100 µm2 | 103 | ||
| 10 | 14 µm2 | 49 | 13–3 | 10 | 13 µm2 | 2 | 3–3 |
| 18 | 22 µm2 | 75 | 20–3 | 18 | 21 µm2 | 8 | 5–3 |
| 21 | 4 µm2 | 26 | 16–3 | 21 | 4 µm2 | 0 | 0 |
| 49 | 40 µm2 | 150 | 49 | 38 µm2 | 11 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Belmonte, A.; Aguado, C.; Alfaro-Ruiz, R.; Albasanz, J.L.; Martín, M.; Moreno-Martínez, A.E.; Fukazawa, Y.; Luján, R. The Density of Group I mGlu5 Receptors Is Reduced along the Neuronal Surface of Hippocampal Cells in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 5867. https://doi.org/10.3390/ijms22115867
Martín-Belmonte A, Aguado C, Alfaro-Ruiz R, Albasanz JL, Martín M, Moreno-Martínez AE, Fukazawa Y, Luján R. The Density of Group I mGlu5 Receptors Is Reduced along the Neuronal Surface of Hippocampal Cells in a Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2021; 22(11):5867. https://doi.org/10.3390/ijms22115867
Chicago/Turabian StyleMartín-Belmonte, Alejandro, Carolina Aguado, Rocío Alfaro-Ruiz, José Luis Albasanz, Mairena Martín, Ana Esther Moreno-Martínez, Yugo Fukazawa, and Rafael Luján. 2021. "The Density of Group I mGlu5 Receptors Is Reduced along the Neuronal Surface of Hippocampal Cells in a Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 22, no. 11: 5867. https://doi.org/10.3390/ijms22115867






