Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation
Abstract
1. Introduction
2. Results
2.1. Study Participants
2.2. Microbiome Signatures
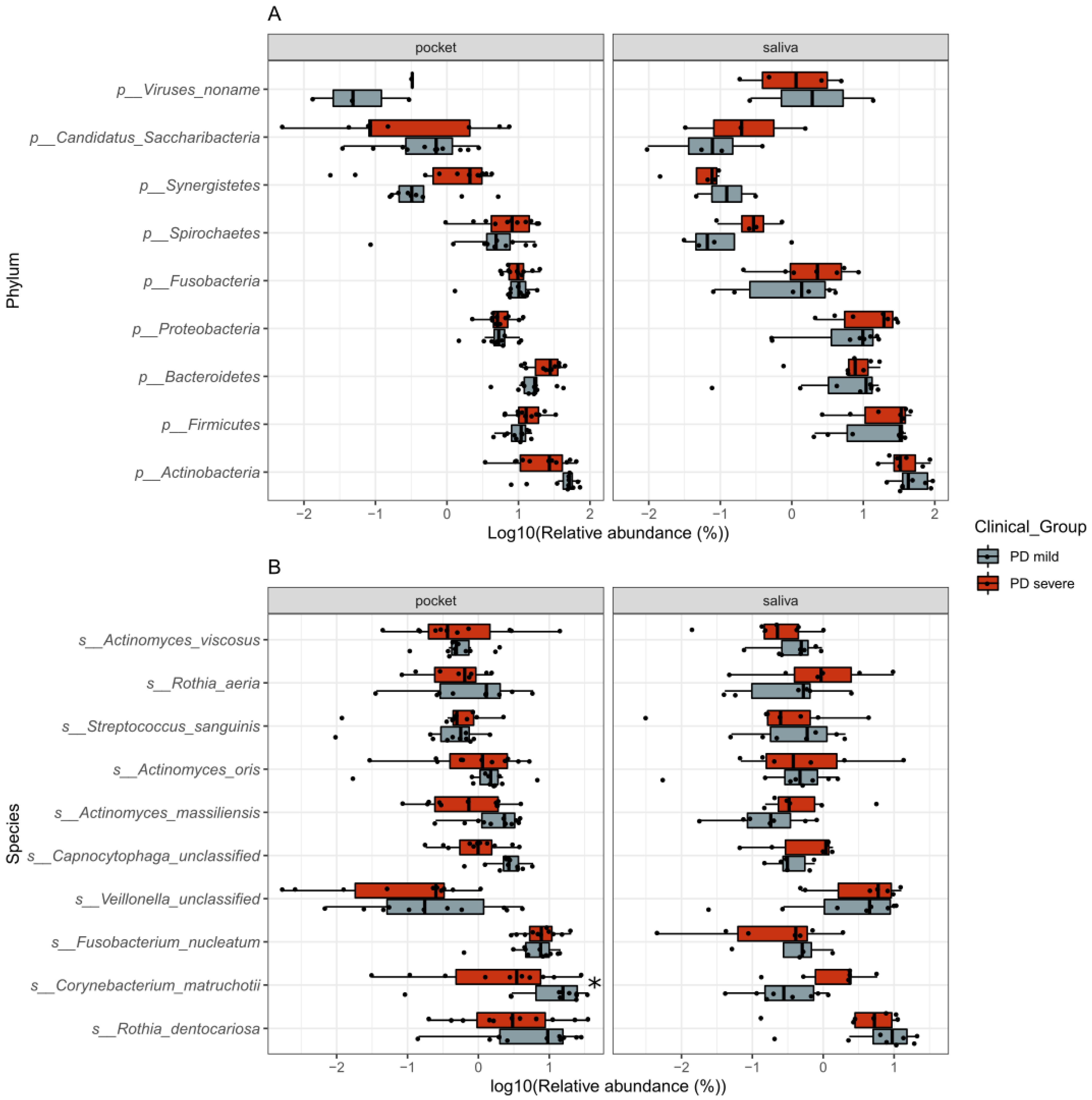
2.2.1. Composition of the Oral Microbiome and Its Association with PD Disease Severity
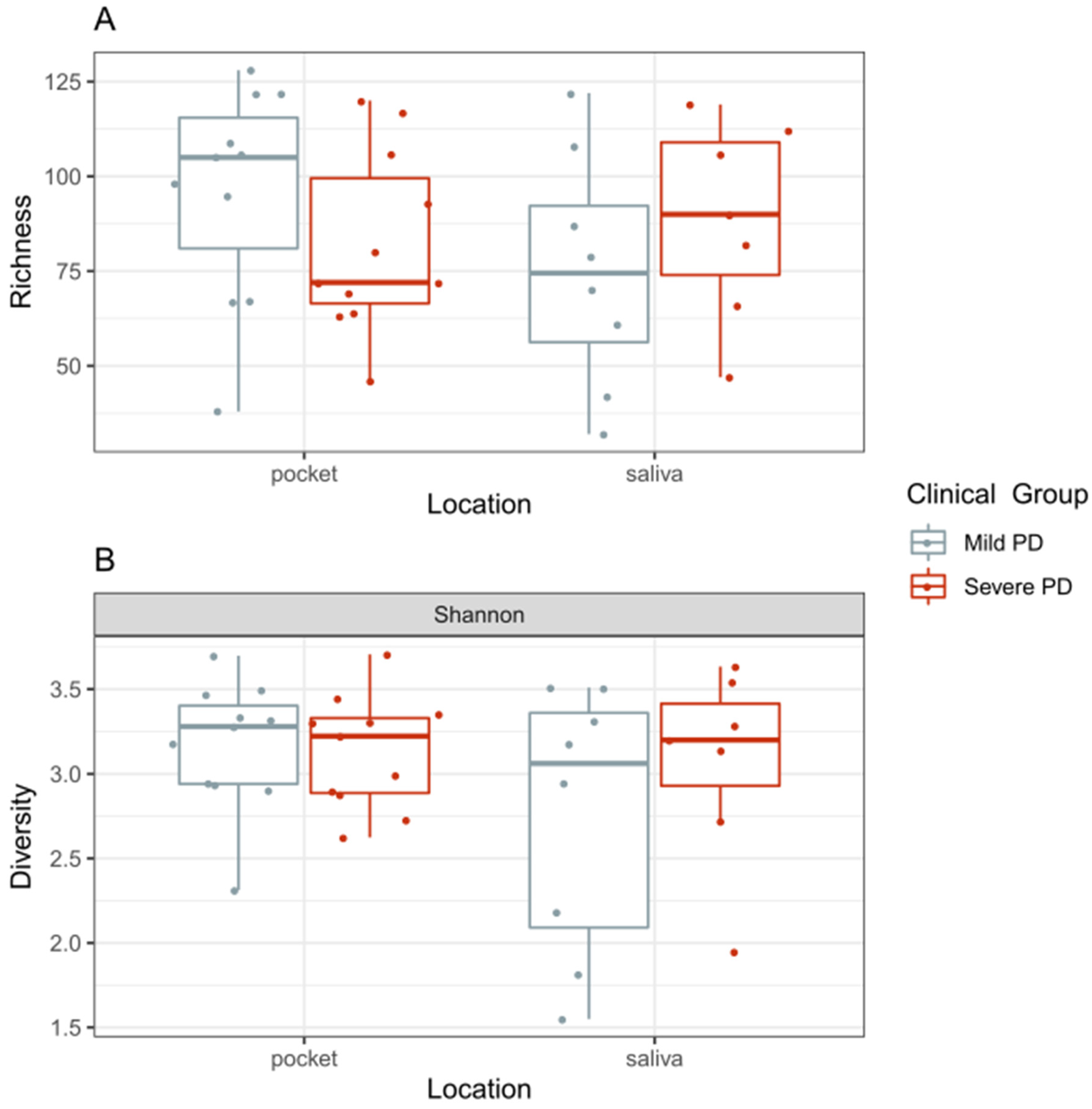
2.2.2. Diversity of the Microbiome and Its Association with PD Disease Severity
2.2.3. Individual Taxa and Metabolic Pathways Associated with Disease Severity
2.2.4. Identification of Bacteria and Bacterial Clusters Previously Reported in the Literature to Be Associated with PD and CVD
2.2.5. PD Bacteria and Bacterial Metabolic Pathways Associated with Systemic Inflammation
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Clinical Periodontal Examination
4.3. Markers of Systemic Inflammation
4.4. Periodontal Tissue Inflammation on [18F] FDG PET and Low-Dose CT Scanning
4.5. Demographic and Biomarker Comparison
4.6. Microbial Sample Collection
4.7. Metagenomic Shotgun Sequencing
4.8. Data Analysis and Statistics
4.9. Strain-Level Investigation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Periodontal Tissue Inflammation on [18F]FDG PET and Low-Dose CT Scanning
References
- Sanz, M.; Wimmer, G. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Slocum, C.; Genco, C.A. Immune dysregulation mediated by the oral microbiome: Potential link to chronic inflammation and atherosclerosis. J. Intern. Med. 2016, 280, 114–128. [Google Scholar] [CrossRef]
- Desvarieux, M.; Papapanou, P.N. Periodontal microbiota and carotid intima-media thickness: The oral infections and vascular disease epidemiology study (INVEST). Circulation 2005, 111, 576–582. [Google Scholar] [CrossRef]
- Demmer, R.T.; Papapanou, P.N. The subgingival microbiome, systemic inflammation and insulin resistance: The oral infections, glucose intolerance and insulin resistance study. J. Clin. Periodontol. 2017, 44, 255–265. [Google Scholar] [CrossRef]
- Socransky, S.S.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Leishman, S.J.; Ford, P.J. Cardiovascular disease and the role of oral bacteria. J. Oral Microbiol. 2010, 2. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Balan, P.; Ong, M.M.A.; Bostanci, N.; Belibasakis, G.N.; Seneviratne, C.J. Metagenomic sequencing provides new insights into the subgingival bacteriome and aetiopathology of periodontitis. J. Periodontal Res. 2021, 56, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, Y.; Lo, E.C.M.; McGrath, C.; Mei, M.L.; Dai, R. Using next-generation sequencing to detect oral microbiome change following periodontal interventions: A systematic review. Oral Dis. 2020. [Google Scholar] [CrossRef]
- Liu, B.; Amar, S. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE 2012, 7, e37919. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Li, H. Dynamic changes in the subgingival microbiome and their potential for diagnosis and prognosis of periodontitis. MBio 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Tonetti, M.S. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontol. 2018, 45, S149–S161. [Google Scholar] [CrossRef] [PubMed]
- e Silva Filho, W.S.; Gonçalves, R.B. Microbial diversity similarities in periodontal pockets and atheromatous plaques of cardiovascular disease patients. PLoS ONE 2014, 9, e109761. [Google Scholar] [CrossRef]
- Lourenço, T.G.; Colombo, A.P. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Fåk, F.; Bäckhed, F. Oral microbiota in patients with atherosclerosis. Atherosclerosis 2015, 243, 573–578. [Google Scholar] [CrossRef]
- Pérez-Chaparro, P.J.; Feres, M. Newly identified pathogens associated with periodontitis: A systematic review. J. Dent. Res. 2014, 93, 846–858. [Google Scholar] [CrossRef]
- Ai, D.; Xia, L.C. Integrated metagenomic data analysis demonstrates that a loss of diversity in oral microbiota is associated with periodontitis. BMC Genom. 2017, 18. [Google Scholar] [CrossRef]
- Ikeda, E.; Izumi, Y. Japanese subgingival microbiota in health vs disease and their roles in predicted functions associated with periodontitis. Odontology 2020, 108, 280–291. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontoly 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Lamont, R.J.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Koren, O.; Bäckhed, F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 4592–4598. [Google Scholar] [CrossRef]
- Hunter, M.C.; Pozhitkov, A.E.; Noble, P.A. Microbial signatures of oral dysbiosis, periodontitis and edentulism revealed by gene meter methodology. J. Microbiol. Methods 2016, 131, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J.; van Steenbergen, T.J.; de Graaff, J. Porphyromonas (Bacteroides) endodontalis: Its role in endodontal infections. J. Endod. 1992, 18, 431–434. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Mitroulis, I.; Chavakis, T. Trained innate immunity and its implications for mucosal immunity and inflammation. Adv. Exp. Med. Biol. 2019, 1197, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Givskov, M. In silico analyses of metagenomes from human atherosclerotic plaque samples. Microbiome 2015, 3. [Google Scholar] [CrossRef]
- Lindskog Jonsson, A.; Bergström, G. Bacterial profile in human atherosclerotic plaques. Atherosclerosis 2017, 263, 177–183. [Google Scholar] [CrossRef]
- Pietiäinen, M.; Pussinen, P.J. Mediators between oral dysbiosis and cardiovascular diseases. Eur. J. Oral Sci. 2018, 126, 26–36. [Google Scholar] [CrossRef]
- Takahashi, N. Oral microbiome metabolism: From “who are they?” to “what are they doing?”. J. Dent. Res. 2015, 94, 1628–1637. [Google Scholar] [CrossRef]
- Téllez, N.; Hernández, L. Arginine and glutamate levels in the gingival crevicular fluid from patients with chronic periodontitis. Braz. Dent. J. 2008, 19, 318–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griffiths, R.; Barbour, S. Lipoproteins and lipoprotein metabolism in periodontal disease. Clin. Lipidol. 2010, 5, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U. The Dutch periodontal screening index validation and its application in The Netherlands. J. Clin. Periodontol. 2009, 36, 1018–1024. [Google Scholar] [CrossRef]
- Zaki, H.A.; Scannapieco, F.A. Is radiologic assessment of alveolar crest height useful to monitor periodontal disease activity? Dent. Clin. N. Am. 2015, 59, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.; Lindner, O.; Sciagra, R.; Agostini, D.; Übleis, C.; Gimelli, A.; Hacker, M. Cardiovascular committee of the European Association of Nuclear Medicine (EANM). Position paper of the cardiovascular committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Holmstrup, P. Microbial profile comparisons of saliva, pooled and site-specific subgingival samples in periodontitis patients. PLoS ONE 2017, 12, e0182992. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Waldron, L.; Ballarini, A.; Jousson, O.; Huttenhower, C.; Narasimhan, V. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat. Methods 2012, 9, 811–814. [Google Scholar] [CrossRef]
- Franzosa, E.A.; McIver, L.J.; Rahnavard, G.; Thompson, L.R.; Schirmer, M.; Weingart, G.; Schwarzberg, K.; Knight, R.; Caporaso, J.G.; Segata, N.; et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 2018, 15, 962–968. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: A hub for protein information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.F.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package. Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 9 April 2021).
- Roger, B.J.; Curtis, J.T. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. Available online: www.jstor.org/stable/1942268 (accessed on 9 April 2021).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Mandal, S.; Davidov, O.; Peddada, S.D. Analysis of microbiome data in the presence of excess zeros. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gloor, G.B.; Macklaim, J.M.; Pawlowsky-Glahn, V.; Egozcue, J.J. Microbiome datasets are compositional: And this is not optional. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Truong, D.T.; Tett, A.; Pasolli, E.; Huttenhower, C.; Segata, N. Microbial strain-level population structure and genetic diversity from metagenomes. Genome Res. 2017, 27, 626–638. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed]


| Demographics | Mild PD (n = 13) | Severe PD (n = 12) |
|---|---|---|
| DPSI, 0–4 | 3.0 ± 0.0 | 4.0 ± 0.1 ** |
| Mean PPD, mm | 3.8 ± 0.5 | 5.3 ± 1.2 ** |
| Deepest PPD, mm | 4.7 ± 0.8 | 6.6 ± 1.6 ** |
| Age, years | 56 ± 9 | 63 ± 6 * |
| Sex, % men (n) | 62 (8) | 25 (3) |
| BMI, kg/m2 | 25.1 ± 3.5 | 28.4 ± 7.0 |
| Hypertension, % (n) | 46 (6) | 58 (7) |
| Current smoking, % (n) | 15 (2) | 8 (1) |
| Circulating inflammatory markers | ||
| WBC, 106/mL | 5.8 [4.5–6.2] | 5.7 [5.5–6.5] |
| IL-1Ra, pg/mL | 177 [157–236] | 254 [188–428] ^ |
| IL-6, pg/mL | 1.4 [1.0–1.8] | 2.0 [1.7–3.0] ^ |
| hsCRP, µg/mL | 0.7 [0.3–1.7] | 0.7 [0.6–1.2] |
| Periodontal inflammation | ||
| Periodontium, SUVmean | 1.5 ± 0.2 | 1.7 ± 0.3 ^ |
| Periodontium, SUVmax | 3.6 ± 0.5 | 3.6 ± 0.8 |
| Taxa | Associated with | Source | ANCOM W Pocket | Direction Pocket | ANCOM W Saliva | Direction Saliva |
|---|---|---|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | CVD-atherosclerotic plaques/aggressive PD | [13]/[14] | Low prevalence | - | Low prevalence | - |
| Anaeroglobus geminatus | CVD-atherosclerosis/PD | [13,14,15,16] | 0 | Increased | Structural | Increased |
| Bulleidia extructa | progressive PD | [17] | 35 | Increased | Structural | Increased |
| Capnocytophaga gingivales | CVD-blood lipid markers | [4]/[15] | 0 | Decreased | 0 | Increased |
| Capnocytophaga granulosa | CVD-blood lipid markers | [15] | 0 | Decreased | 0 | Increased |
| Capnocytophaga ochracea | Stable PD/CVD-blood lipid markers | [15]/[17] | 0 | Decreased | Structural | Increased |
| Capnocytophaga unclassified | CVD-blood lipid markers | [15] | 112 | Decreased | 0 | Increased |
| Catonella morbi | CVD-blood lipid markers/PD | [14,15] | 2 | Increased | Low prevalence | - |
| Eubacterium brachy | stable PD | [4,16,18] | 105 | Increased | 0 | Increased |
| Eubacterium infirmum | progressive PD | [17] | 3 | Increased | 0 | Increased |
| Filifactor alocis | progressive PD | [13,14,16,17] | 0 | Increased | 0 | Decreased |
| Olsenella uli | progressive PD | [17] | 0 | Increased | Structural | Increased |
| Parvimonas micra | CVD-uCRP/PD | [4]/[4,5,6,7,8,9,10,11,12,13,14,15,16] | 4 | Increased | Structural | Increased |
| Porphyromonas gingivalis | CVD-atherosclerotic plaques/PD | [13,14] /[16,19,20,21] | 0 | Increased | Structural | Increased |
| Propionibacterium propionicum | CVD-atherosclerotic plaques/progressive PD | [17]/[21] | 0 | Increased | 0 | Increased |
| Tannerella forsythia | CVD-atherosclerotic plaques/PD | [13,14] /[16,19] | 0 | Increased | 0 | Increased |
| Treponema denticola | PD | [14,16,19] | 0 | Increased | 0 | Increased |
| Streptococcus | CVD-HDL, ApoA1/PD | [21,22] | 0 | Increased | 0 | Decreased |
| Neisseria | CVD-negatively with HDL, ApoA1/PD | [21,22] | 0 | Increased | 0 | Increased |
| Fusobacterium | CVD-total cholesterol, LDL/PD | [14]/[16,21] | 0 | Increased | 0 | Increased |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plachokova, A.S.; Andreu-Sánchez, S.; Noz, M.P.; Fu, J.; Riksen, N.P. Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation. Int. J. Mol. Sci. 2021, 22, 5876. https://doi.org/10.3390/ijms22115876
Plachokova AS, Andreu-Sánchez S, Noz MP, Fu J, Riksen NP. Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation. International Journal of Molecular Sciences. 2021; 22(11):5876. https://doi.org/10.3390/ijms22115876
Chicago/Turabian StylePlachokova, Adelina S., Sergio Andreu-Sánchez, Marlies P. Noz, Jingyuan Fu, and Niels P. Riksen. 2021. "Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation" International Journal of Molecular Sciences 22, no. 11: 5876. https://doi.org/10.3390/ijms22115876
APA StylePlachokova, A. S., Andreu-Sánchez, S., Noz, M. P., Fu, J., & Riksen, N. P. (2021). Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation. International Journal of Molecular Sciences, 22(11), 5876. https://doi.org/10.3390/ijms22115876






