Colistin Resistance in Aeromonas spp.
Abstract
:1. Introduction
2. The Colistin Situation
2.1. Colistin Activity Spectrum
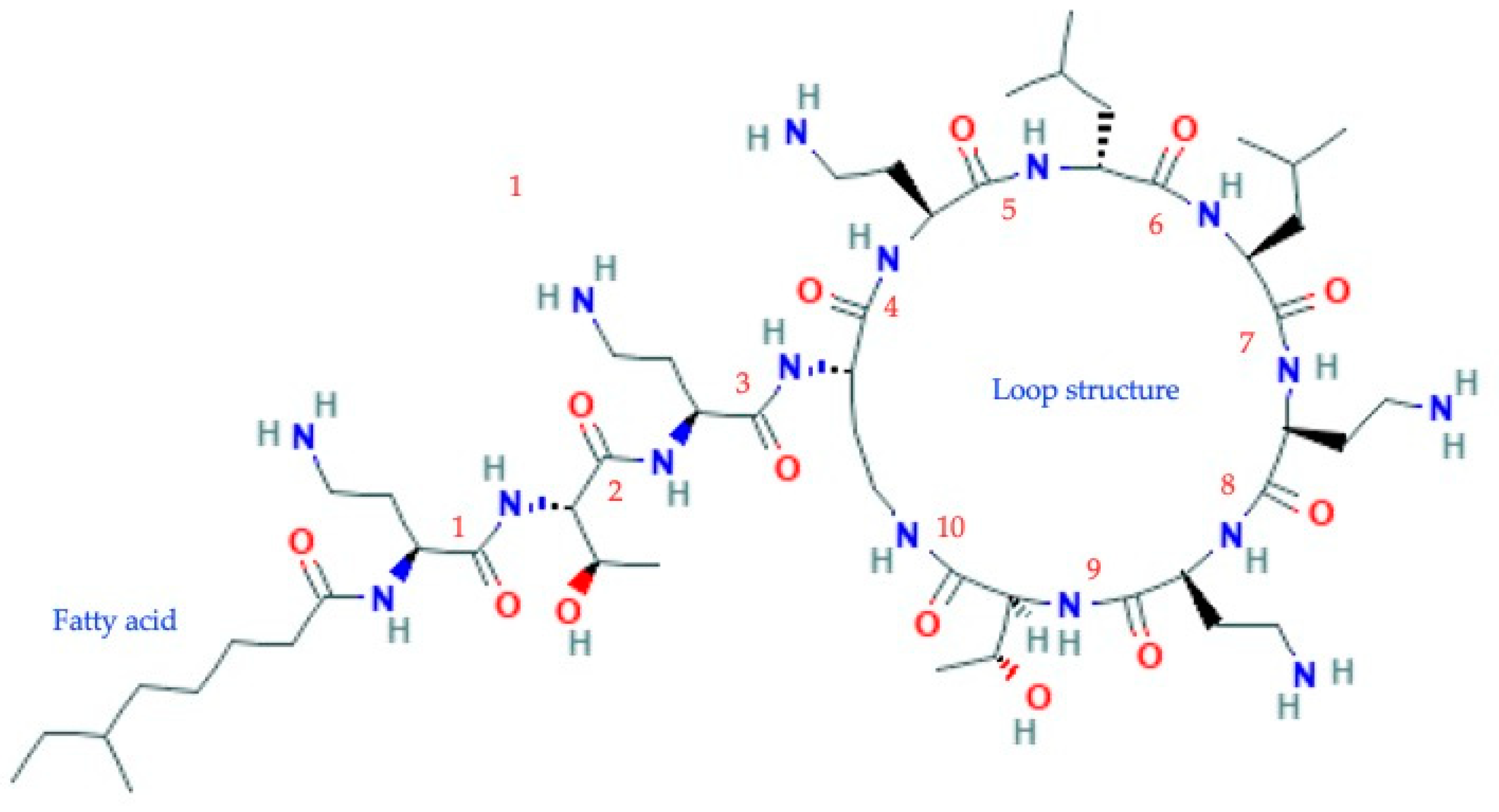
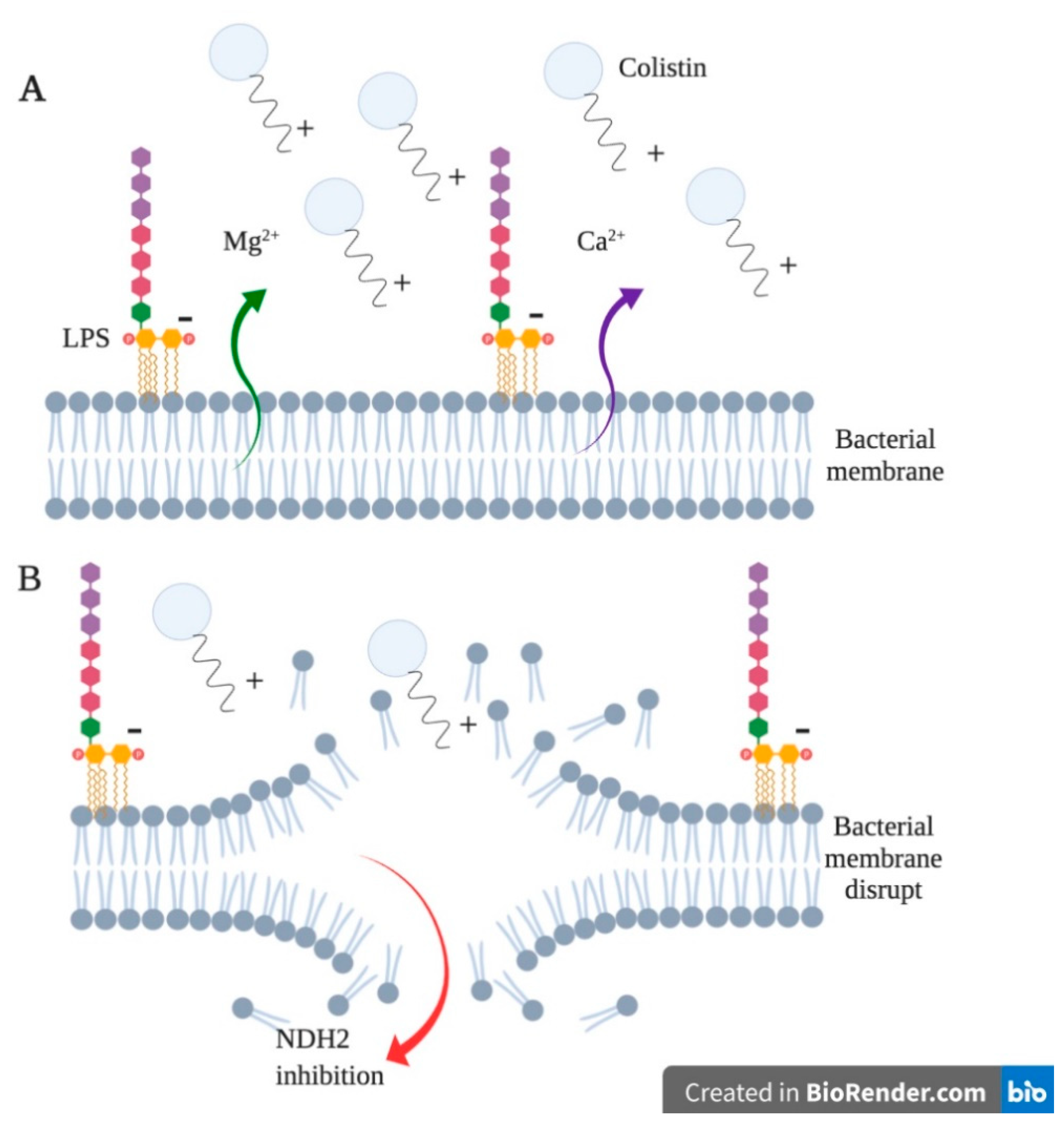
2.2. Colistin, Pharmacology and Application
3. Antimicrobial Resistance in Aeromonas
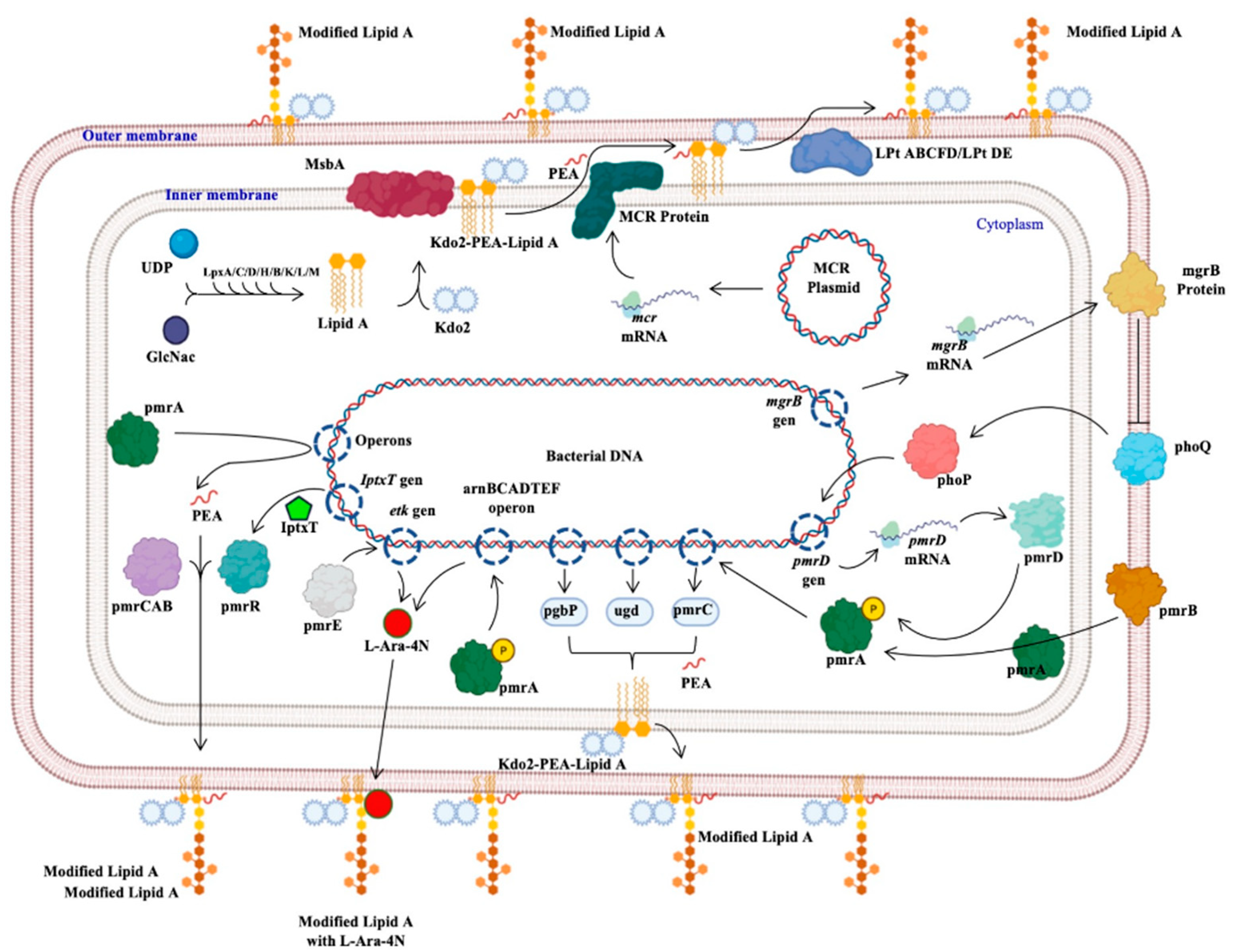
Colistin Resistance in Aeromonas
4. Detection Methods of Colistin Resistance
4.1. Elution Method in Broth
4.2. Plate Test Method
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Upert, G.; Luther, A.; Obrecht, D.; Ermert, P. Emerging peptide antibiotics with therapeutic potential. Med. Drug Discov. 2021, 9, 100078. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Yu, L.F. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Medina, J.; Paciel, D.; Noceti, O.; Rieppi, G. Actualización acerca de colistina (polimixina E): Aspectos clínicos, PK/PD y equivalencias. Rev. Méd. Uru. 2017, 33, 79–114. [Google Scholar] [CrossRef]
- Karaiskos, I.; Souli, M.; Galani, I.; Giamarellou, H. Colistin: Still a lifesaver for the 21st century? Expert Opin. Drug. Met. 2017, 13, 59–71. [Google Scholar] [CrossRef]
- Anandan, S.; Gopi, R.; Ragupathi, N.K.D.; Sethuvel, D.P.M.; Gunasekaran, P.; Walia, K.; Veeraraghavan, B. First report of blaOXA-181-mediated carbapenem resistance in Aeromonas caviae in association with pKP3-A: Threat for rapid dissemination. J. Glob. Antimicrob. Resist. 2017, 10, 310–314. [Google Scholar] [CrossRef]
- Jiménez-Pearson, M.A.; Galas, M.; Corso, A.; Hormazábal, J.C.; Duarte-Valderrama, C.; Salgado-Marcano, N.; Melano, R.G. Consenso latinoamericano para definir, categorizar y notificar patógenos multirresistentes, con resistencia extendida o panresistentes. Rev. Panam. Salud Púb. 2019, 43, e65. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Figueras, M.J.; Beaz-Hidalgo, R. Aeromonas infections in humans. In Aeromonas, 1st ed.; Graf, J., Ed.; Caister Academic Press: Pole, UK, 2015; Chapter 4; pp. 65–108. [Google Scholar]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Deris, Z.Z.; Swarbrick, J.D.; Roberts, K.D.; Azad, M.A.; Akter, J.; Horne, A.S.; Nation, R.L.; Rogers, K.L.; Thompson, P.E.; Velkov, T.; et al. Probing the penetration of antimicrobial polymyxin lipopeptides into gram-negative bacteria. Bioconjugate Chem. 2014, 25, 750–760. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Morand, S.; Rolain, J.M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial mechanisms of polymyxin and bacterial resistance. BioMed Res. Int. 2015, 679109. [Google Scholar] [CrossRef] [PubMed]
- Tietgen, M.; Semmler, T.; Riedel-Christ, S.; Kempf, V.A.; Molinaro, A.; Ewers, C.; Göttig, S. Impact of the colistin resistance gene mcr-1 on bacterial fitness. Internat. J. Antimicrob. Ag. 2018, 51, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Kaskhedikar, M.; Chhabra, D. Multiple drug resistance of Aeromonas hydrophila isolates from chicken samples collected from Mhow and Indore city of Madhyapradesh. World 2009, 2, 31–32. [Google Scholar]
- Kaskhedikar, M.; Chhabra, D. Multiple drug resistance in Aeromonas hydrophila isolates of fish. Food Microbiol. 2010, 28, 157–168. [Google Scholar]
- Bialvaei, A.Z.; Samadi-Kafil, H. Colistin, mechanisms and prevalence of resistance. Curr. Med. Res. Opin. 2015, 31, 707–721. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P. Aquaculture, exaptation, and the origin of mcr-positive colistin resistance. Antimicrob. Agents Chemother. 2018, 62, e01903-18. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhong, L.L.; Srinivas, S.; Sun, J.; Huang, M.; Paterson, D.L.; Lei, S.; Lin, J.; Li, X.; Tang, Z.; et al. Spread of MCR-3 colistin resistance in China: An epidemiological, genomic and mechanistic study. EbioMedicine 2018, 34, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Tekedar, H.C.; Kumru, S.; Blom, J.; Perkins, A.D.; Griffin, M.J.; Abdelhamed, H.; Karsi, A.; Lawrence, M.L. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, e0221018. [Google Scholar] [CrossRef] [Green Version]
- Esteve, C.; Alcaide, E.; Giménez, M.J. Multidrug-resistant (MDR) Aeromonas recovered from the metropolitan area of Valencia (Spain): Diseases spectrum and prevalence in the environment. Eur. J. Clin. Microbiol. 2015, 34, 137–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, L.; Nan, Z.; Zhang, P.; Kan, B.; Yan, D.; Su, J. Taxonomy, virulence genes and antimicrobial resistance of Aeromonas isolated from extra-intestinal and intestinal infections. BMC Infect. Dis. 2019, 19, 158. [Google Scholar] [CrossRef] [Green Version]
- Tansarli, G.S.; Papaparaskevas, J.; Balaska, M.; Samarkos, M.; Pantazatou, A.; Markogiannakis, A.; Daikos, G.L. Colistin resistance in carbapenemase-producing Klebsiella pneumoniae bloodstream isolates: Evolution over 15 years and temporal association with colistin use by time series analysis. Int. J. Antimicrob. Agents 2018, 52, 397–403. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Hindler, J.F.; Reller, L.B.; Weinstein, M.P. New consensus guidelines from the Clinical and Laboratory Standards Institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin. Infect. Dis. 2007, 44, 280–286. [Google Scholar] [CrossRef] [Green Version]
- Bravo-Fariñas, L.; Cabrera-Rodríguez, L.E.; Margarita-Ramírez, M.; Llop-Hernández, A.; Verdecía-Pérez, J.; Borrego-Hernández, G.; Fernández-Abreu, A. Resistencia antimicrobiana en cepas de Aeromonas spp. aisladas de pacientes con bacteriemia. Rev. Biomédica 2007, 18, 176–181. [Google Scholar]
- Fosse, T.; Giraud-Morin, C.; Madinier, I. Induced colistin resistance as an identifying marker for Aeromonas phenospecies groups. Lett. Appl. Microbiol. 2003, 36, 25–29. [Google Scholar] [CrossRef]
- Ling, Z.; Yin, W.; Li, H.; Zhang, Q.; Wang, X.; Wang, Z.; Shen, J. Chromosome-mediated mcr-3 variants in Aeromonas veronii from chicken meat. Antimicrob. Agents Chemother. 2017, 61, e01272-17. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, I.; Feudi, C.; Wang, Y.; Kaspar, H.; Feßler, A.T.; Lübke-Becker, A.; Michael, G.B.; Shen, J.; Schwarz, S. Identification of novel variants of the colistin resistance gene mcr-3 in Aeromonas spp. from the national resistance monitoring programme GE RM-Vet and from diagnostic submissions. J. Antimicrob. Chemother. 2018, 73, 1217–1221. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, C.; Sun, Q.; Schwarz, S.; Ou, Y.; Yang, L.; Zhang, R. Prevalence and genetic analysis of mcr-3-positive Aeromonas species from humans, retail meat, and environmental water samples. Antimicrob. Agents Chemother. 2018, 62, e00404-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Sun, C.; Hulth, A.; Li, J.; Nilsson, L.E.; Zhou, Y.; Wang, Y. Mobile colistin resistance gene mcr-5 in porcine Aeromonas hydrophila. J. Antimicrob. Chemother. 2018, 73, 1777–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghapour, Z.; Gholizadeh, P.; Ganbarov, K.; Bialvaei, A.Z.; Mahmood, S.S.; Tanomand, A.; Yousefi, M.; Asgharzadeh, M.; Yousefi, B.; Kafil, H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019, 12, 965–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trebosc, V.; Gartenmann, S.; Tötzl, M.; Lucchini, V.; Schellhorn, B.; Pieren, M.; Lociuro, S.; Gitzinger, M.; Tigges, M.; Bumann, D.; et al. Dissecting Colistin Resistance Mechanisms in Extensively Drug-Resistant Acinetobacter baumannii Clinical Isolates. mBio 2019, 10, e01083-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, H.; Henningsen, M.L.; Begg, S.L. Antimicrobial resistance in healthcare, agriculture and the environment: The biochemistry behind the headlines. Essays Biochem. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and Characteristics of Mobile Colistin Resistance (mcr) Gene-Containing Isolates from the Environment: A Review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standars Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 29th ed.; CLSI supplement M100; Clinical and Laboratory Standars Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Kavanagh, A.; Ramu, S.; Gong, Y.; Cooper, M.A.; Blaskovich, M.A. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrob. Agents Chemother. 2019, 63, e01760-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simner, P.J.; Bergman, Y.; Trejo, M.; Roberts, A.A.; Marayan, R.; Tekle, T.; Tamma, P.D. Two-site evaluation of the colistin broth disk elution test to determine colistin in vitro activity against Gram-negative bacilli. J. Clin. Microbiol. 2019, 57, e01163-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmolin, T.V.; Mazzetti, A.; Ávila, H.; Kranich, J.; Carneiro, G.I.B.; Arend, L.N.V.S.; Pillonetto, M. Elution methods to evaluate colistin susceptibility of Gram-negative rods. Diagn. Microbiol. Infect. Dis. 2020, 96, 114910. [Google Scholar] [CrossRef] [PubMed]
| Sensitive | Resistance | Variables |
|---|---|---|
| Escherichia coli * Klebsiella pneumoniae * Pseudomonas aeruginosa * Acinetobacter baumannii * Salmonella spp. Shigella spp. Legionella pneumophila Haemophilus influenzae Bordetella pertussis Prevotella spp.a Fusobacterium spp.a | Proteus spp. Burkholderia spp. Helicobacter pylori Neisseria meningitidis Bacteroides fragilis a | Stenotrophomonas maltophilia Moraxella catarrhalis Vibrio spp. Aeromonas spp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Avila, L.U.; Loyola-Cruz, M.A.; Hernández-Cortez, C.; Bello-López, J.M.; Castro-Escarpulli, G. Colistin Resistance in Aeromonas spp. Int. J. Mol. Sci. 2021, 22, 5974. https://doi.org/10.3390/ijms22115974
Gonzalez-Avila LU, Loyola-Cruz MA, Hernández-Cortez C, Bello-López JM, Castro-Escarpulli G. Colistin Resistance in Aeromonas spp. International Journal of Molecular Sciences. 2021; 22(11):5974. https://doi.org/10.3390/ijms22115974
Chicago/Turabian StyleGonzalez-Avila, Luis Uriel, Miguel Angel Loyola-Cruz, Cecilia Hernández-Cortez, Juan Manuel Bello-López, and Graciela Castro-Escarpulli. 2021. "Colistin Resistance in Aeromonas spp." International Journal of Molecular Sciences 22, no. 11: 5974. https://doi.org/10.3390/ijms22115974
APA StyleGonzalez-Avila, L. U., Loyola-Cruz, M. A., Hernández-Cortez, C., Bello-López, J. M., & Castro-Escarpulli, G. (2021). Colistin Resistance in Aeromonas spp. International Journal of Molecular Sciences, 22(11), 5974. https://doi.org/10.3390/ijms22115974










