Development of a Highly Sensitive β-Glucan Detection System Using Scanning Single-Molecule Counting Method
Abstract
1. Introduction
2. Results
2.1. SSMC Method for BG Detection Using 16BGM and S-BGRP
2.2. Comparison of BG Detection by LAL and SSMC
2.3. Correlation between LAL Method and SSMC Method
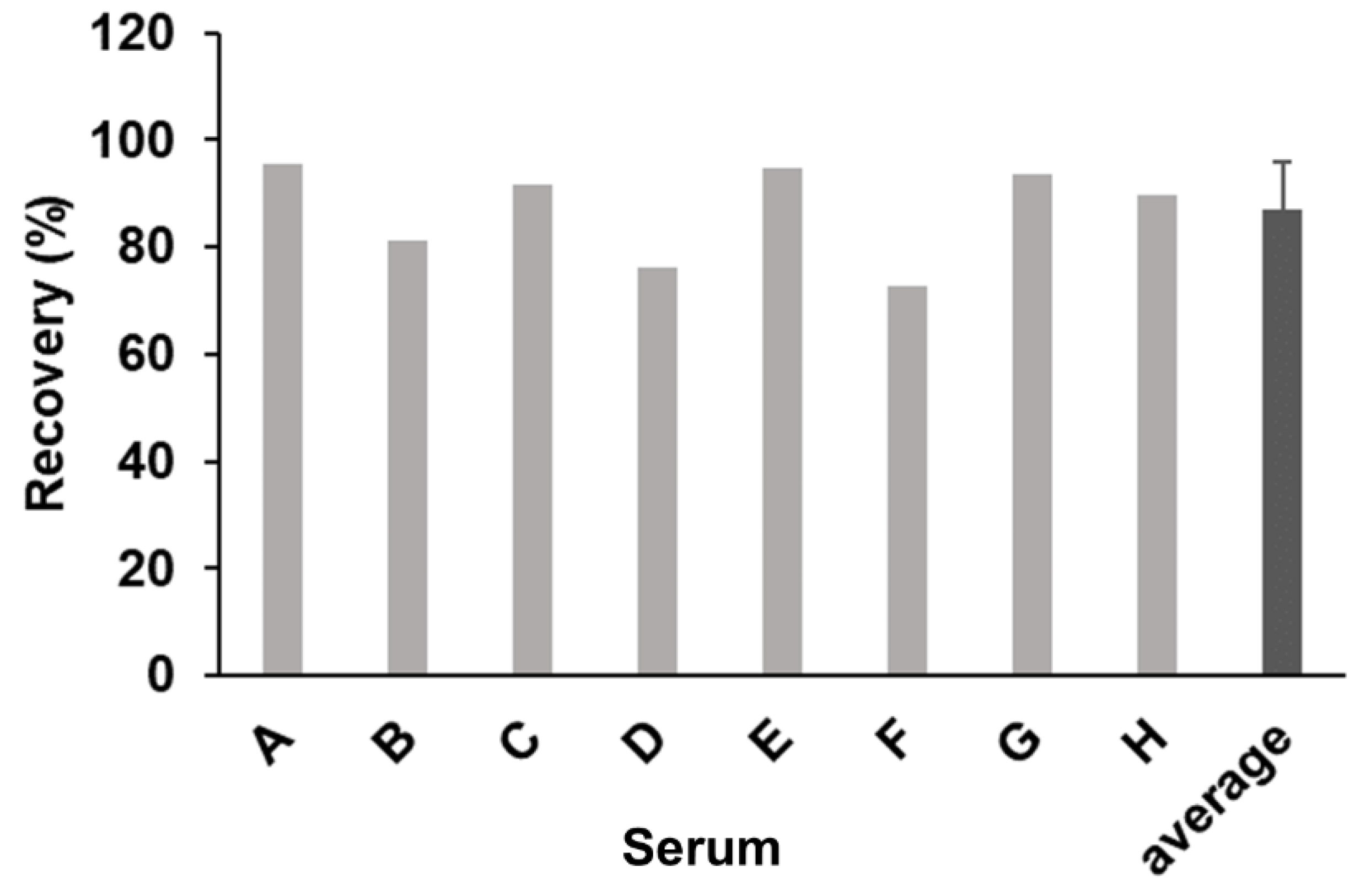
2.4. Effect of Human Sera on the Reaction with CSBG in the SSMC Method
2.5. Comparison of Reactivity to Immunoglobulin Preparations
3. Discussion
4. Materials and Methods
4.1. Fungal Culture
4.2. Reagents
4.3. Preparation of Candida Albicans-Derived Soluble Beta-Glucan
4.4. Preparation of Aspergillus Cell Wall Glucans
4.5. Purification of Pollen BG Using BGRP-Assisted Affinity Chromatography
4.6. Preparation of S-BGRP and 16BGM
4.7. Basic Assay Method for SSMC
4.8. SSMC
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enoch, D.A.; Yang, H.; Aliyu, S.H.; Micallef, C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017, 1508, 17–65. [Google Scholar]
- Obayashi, T.; Yoshida, M.; Mori, T.; Goto, H.; Yasuoka, A.; Iwasaki, H.; Teshima, H.; Kohno, S.; Horiuchi, A.; Ito, A.; et al. Plasma (1-->3)-beta-D-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 1995, 345, 17–20. [Google Scholar] [CrossRef]
- Japan Ministry of Health, Labor and Welfare, Statistics by Social Medical Practices. Available online: https://www.mhlw.go.jp/toukei/list/26-19c.html (accessed on 27 June 2019).
- Nakao, A.; Yasui, M.; Kawagoe, T.; Tamura, H.; Tanaka, S.; Takagi, H. False-positive endotoxemia derives from gauze glucan after hepatectomy for hepatocellular carcinoma with cirrhosis. Hepatogastroenterology 1997, 44, 1413–1418. [Google Scholar]
- Kato, A.; Takita, T.; Furuhashi, M.; Takahashi, T.; Maruyama, Y.; Hishida, A. Elevation of blood (1-->3)-beta-D-glucan concentrations in hemodialysis patients. Nephron 2001, 89, 15–19. [Google Scholar] [CrossRef]
- Nagasawa, K.; Yano, T.; Kitabayashi, G.; Morimoto, H.; Yamada, Y.; Ohata, A.; Usami, M.; Horiuchi, T. Experimental proof of contamination of blood components by (1-->3)-beta-D-glucan caused by filtration with cellulose filters in the manufacturing process. J. Artif. Organs 2003, 6, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Kim, C.; Yamanaka, D.; Ishibashi, K.I.; Tanaka, H.; Ohno, N.; Adachi, Y. Possibility of Japanese cedar pollen causing false positives in the deep mycosis test. Int. J. Mol. Sci. 2021, 22, 2135. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Farkas, V.; Lahnstein, J.; Fincher, G.B. A Barley xyloglucan xyloglucosyl transferase covalently links xyloglucan, cellulosic substrates, and (1,3;1,4)-beta-D-glucans. J. Biol. Chem. 2007, 282, 12951–12962. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Ochiai, M.; Ashida, M. Purification of a beta-1,3-glucan recognition protein in the prophenoloxidase activating system from hemolymph of the silkworm, Bombyx mori. J. Biol. Chem. 1988, 263, 12056–12062. [Google Scholar] [CrossRef]
- Ochiai, M.; Ashida, M. A pattern-recognition protein for beta-1,3-glucan. The binding domain and the cDNA cloning of beta-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem. 2000, 275, 4995–5002. [Google Scholar] [CrossRef]
- Takahasi, K.; Ochiai, M.; Horiuchi, M.; Kumeta, H.; Ogura, K.; Ashida, M.; Inagaki, F. Solution structure of the silkworm betaGRP/GNBP3 N-terminal domain reveals the mechanism for beta-1,3-glucan-specific recognition. Proc. Natl. Acad. Sci. USA 2009, 106, 11679–11684. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Ishii, M.; Kanno, T.; Tetsui, J.; Ishibashi, K.-I.; Yamanaka, D.; Miura, N.; Ohno, N. N-Terminal (1-->3)-beta-D-glucan recognition proteins from insects recognize the difference in ultra-structures of (1-->3)-beta-D-glucan. Int. J. Mol. Sci. 2019, 20, 3498. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Kurokawa, T. A sensitive sandwich ELISA to measure (1-->3)-beta-D-glucan levels in blood. J. Immunol. Methods 2011, 365, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Kanno, T.; Ishibashi, K.-i.; Yamanaka, D.; Motoi, A.; Motoi, M.; Ohno, N. Binding specificity of a new artificial beta-glucan recognition protein and its application to beta-glucan detection in mushroom extracts. Int. J. Med. Mushrooms 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Takatsu, K.; Kimura, M.; Swamydas, M.; Ohnishi, H.; Umeyama, T.; Oyama, F.; Lionakis, M.S.; Ohno, N. Development of a novel β-1,6-glucan-specific detection system using functionally-modified recombinant endo-β-1,6-glucanase. J. Biol. Chem. 2020, 295, 5362–5376. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, D.; Kurita, S.; Hanayama, Y.; Adachi, Y. Split enzyme-based biosensors for structural characterization of soluble and insoluble β-glucans. Int. J. Mol. Sci. 2021, 22, 1576. [Google Scholar] [CrossRef]
- Obayashi, T. The plasma (1->3)-beta-D-glucan assay, a Japanese contribution to the diagnosis of invasive fungal infection. Med. Mycol. J. 2017, 58, J141–J147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moro, H.; Koshio, N.; Bamba, Y.; Koizumi, T.; Cho, H.; Aoki, N.; Hayashi, M.; Tsubata, C.; Sakagami, A.; Sato, M.; et al. The effect of intravenous gamma-globulin reagents on the measurement results of (1->3)-β-D-glucan. Kansenshogaku Zasshi 2017, 91, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Buchacher, A.; Krause, D.; Wiry, G.; Weinberger, J. Elevated endotoxin levels in human intravenous immunoglobulin concentrates caused by (1->3)-beta-D-glucans. PDA J. Pharm. Sci. Technol. 2010, 64, 536–544. [Google Scholar]
- Ogawa, M.; Hori, H.; Niiguchi, S.; Azuma, E.; Komada, Y. False-positive plasma (1-->3)-beta-D-glucan test following immunoglobulin product replacement in an adult bone marrow recipient. Int. J. Hematol. 2004, 80, 97–98. [Google Scholar] [CrossRef]
- Yoshida, K.; Niki, Y.; Mitekura, H.; Nakajima, M.; Kawane, H.; Matsushima, T. A discrepancy in the values of serum (1-3)-beta-D-glucan measured by two kits using different methods. Nihon Ishinkin Gakkai Zasshi 2001, 42, 237–242. [Google Scholar] [CrossRef][Green Version]
- Kato, K.; Onoda, S.; Asano, J.; Fukaya, S.; Yoshida, S. Evaluation of the clinical cutoff level of serum (1 --> 3)-beta-D-glucan in patients with connective tissue diseases complicated by deep fungal infections. Mod. Rheumatol. 2010, 20, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Shibakami, M.; Tsubouchi, G.; Nakamura, M.; Hayashi, M. Polysaccharide nanofiber made from euglenoid alga. Carbohydr. Polym. 2013, 93, 499–505. [Google Scholar] [CrossRef]
- Rylander, R.; Fogelmark, B.; McWilliam, A.; Currie, A. (1-->3)-beta-D-glucan may contribute to pollen sensitivity. Clin. Exp. Immunol. 1999, 115, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.C.; Lemmon, B.E.; Stone, B.A.; Olsen, O.A. Cell wall (1-->3)- and (1-->3, 1-->4)-beta-glucans during early grain development in rice (Oryza sativa L.). Planta 1997, 202, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Sander, I.; Fleischer, C.; Borowitzki, G.; Brüning, T.; Raulf-Heimsoth, M. Development of a two-site enzyme immunoassay based on monoclonal antibodies to measure airborne exposure to (1-->3)-beta-D-glucan. J. Immunol. Methods 2008, 337, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, E.-i.; Iwasaki, M.; Shiina, S.; Kitahara, S.-i.; Yotani, T.; Manabe, M.; Miyazaki, O. A novel enzyme immunoassay for the measurement of plasma (1-->3)-beta-D-glucan levels. J. Immunol. Methods 2020, 487, 112872. [Google Scholar] [CrossRef]
- Meikle, P.J.; Bonig, I.; Hoogenraad, N.J.; Clarke, A.E.; Stone, B.A. The location of (1→3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta 1991, 185, 1–8. [Google Scholar] [CrossRef]
- Hanami, T.; Tanabe, T.; Hanashi, T.; Yamaguchi, M.; Nakata, H.; Mitani, Y.; Kimura, Y.; Soma, T.; Usui, K.; Isobe, M.; et al. Scanning single-molecule counting system for Eprobe with highly simple and effective approach. PLoS ONE 2020, 15, e0243319. [Google Scholar] [CrossRef]
- Anderson, G.P.; Nerurkar, N.L. Improved fluoroimmunoassays using the dye Alexa Fluor 647 with the RAPTOR, a fiber optic biosensor. J. Immunol. Methods 2002, 271, 17–24. [Google Scholar] [CrossRef]
- Shepherd, M.G.; Sullivan, P.A. The production and growth characteristics of yeast and mycelial forms of Candida albicans in continuous culture. J. Gen. Microbiol. 1976, 93, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Uchiyama, M.; Tsuzuki, A.; Tokunaka, K.; Miura, N.N.; Adachi, Y.; Aizawa, M.W.; Tamura, H.; Tanaka, S.; Yadomae, T. Solubilization of yeast cell-wall beta-(1->3)-D-glucan by sodium hypochlorite oxidation and dimethyl sulfoxide extraction. Carbohydr. Res. 1999, 316, 161–172. [Google Scholar]

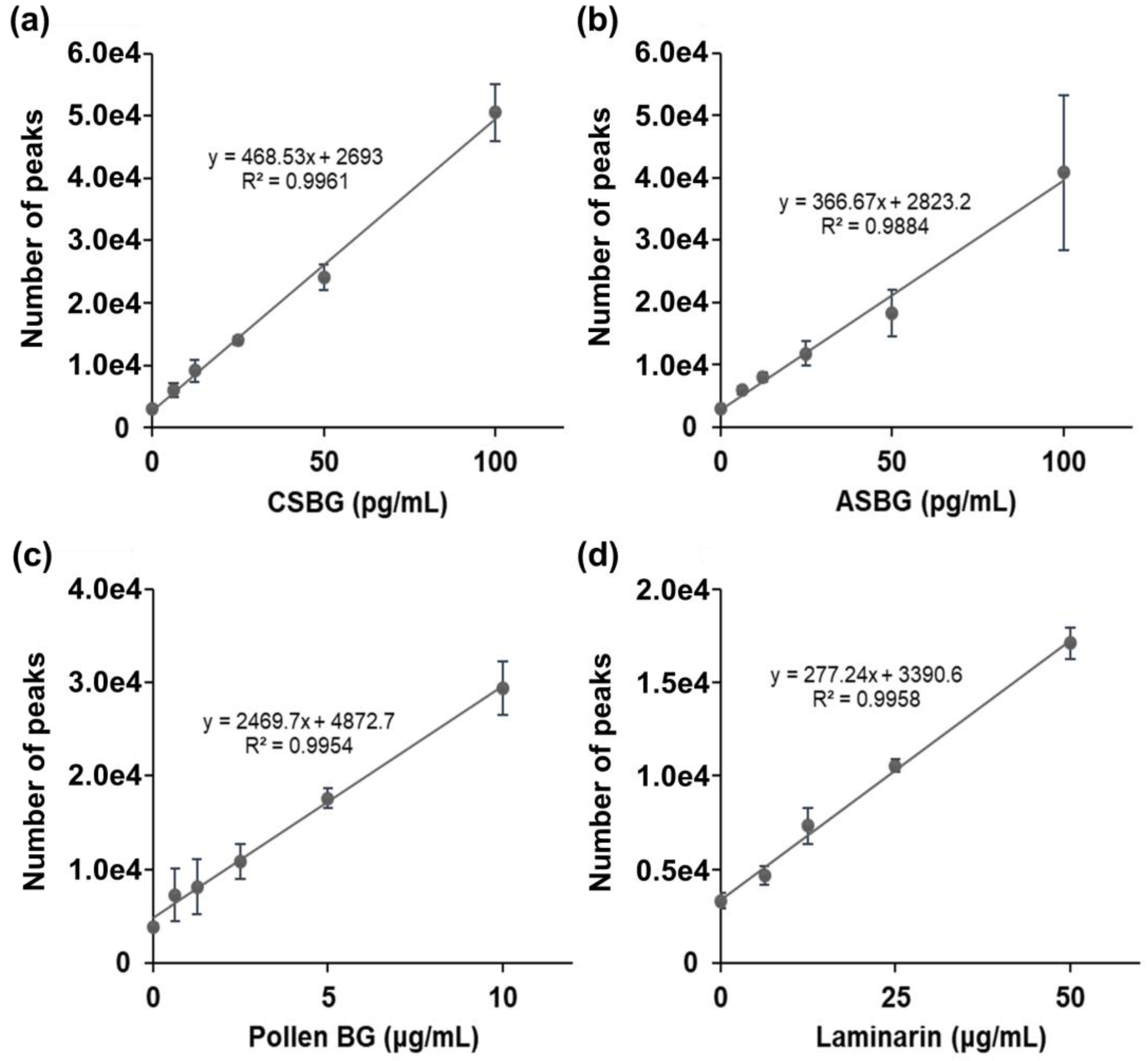

| BGs (10 ng/mL) | LAL (pg/mL) (Pachyman Equivalents) |
|---|---|
| CSBG | 490 ± 48 |
| ASBG | 819 ± 17 |
| Pollen BG | 51.6 ± 0.9 |
| Laminarin | 338 ± 2 |
| Intravenous Immunoglobulin (IVIG) | LAL (pg/mL) (Pachyman Equivalents) | SSMC (pg/mL) (CSBG Equivalents) |
|---|---|---|
| Venilon-I | <5.0 | <32.4 |
| Venoglobulin IH 5% | 48.4 | <32.4 |
| Gammagard | 36.8 | <32.4 |
| Glovenin-I | 73.9 | <32.4 |
| Polyglobin-N | 60.6 | 41.1 |
| Sanglopor | 1570 | <32.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adachi, Y.; Nakata, H.; Tanabe, T.; Yamanaka, D.; Kanno, T.; Ishibashi, K.-i.; Ohno, N. Development of a Highly Sensitive β-Glucan Detection System Using Scanning Single-Molecule Counting Method. Int. J. Mol. Sci. 2021, 22, 5977. https://doi.org/10.3390/ijms22115977
Adachi Y, Nakata H, Tanabe T, Yamanaka D, Kanno T, Ishibashi K-i, Ohno N. Development of a Highly Sensitive β-Glucan Detection System Using Scanning Single-Molecule Counting Method. International Journal of Molecular Sciences. 2021; 22(11):5977. https://doi.org/10.3390/ijms22115977
Chicago/Turabian StyleAdachi, Yoshiyuki, Hidetaka Nakata, Tetsuya Tanabe, Daisuke Yamanaka, Takashi Kanno, Ken-ichi Ishibashi, and Naohito Ohno. 2021. "Development of a Highly Sensitive β-Glucan Detection System Using Scanning Single-Molecule Counting Method" International Journal of Molecular Sciences 22, no. 11: 5977. https://doi.org/10.3390/ijms22115977
APA StyleAdachi, Y., Nakata, H., Tanabe, T., Yamanaka, D., Kanno, T., Ishibashi, K.-i., & Ohno, N. (2021). Development of a Highly Sensitive β-Glucan Detection System Using Scanning Single-Molecule Counting Method. International Journal of Molecular Sciences, 22(11), 5977. https://doi.org/10.3390/ijms22115977






