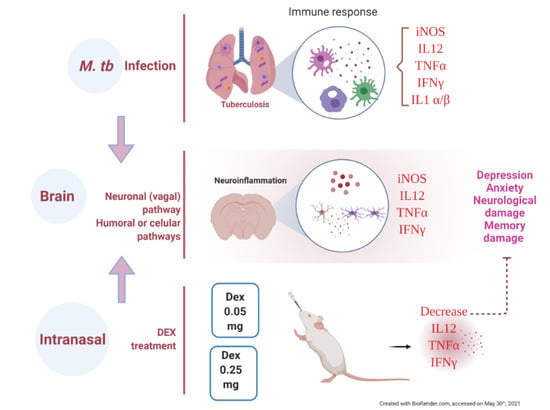
The Therapeutic Effect of Intranasal Administration of Dexamethasone in Neuroinflammation Induced by Experimental Pulmonary Tuberculosis
Abstract
1. Introduction
2. Results
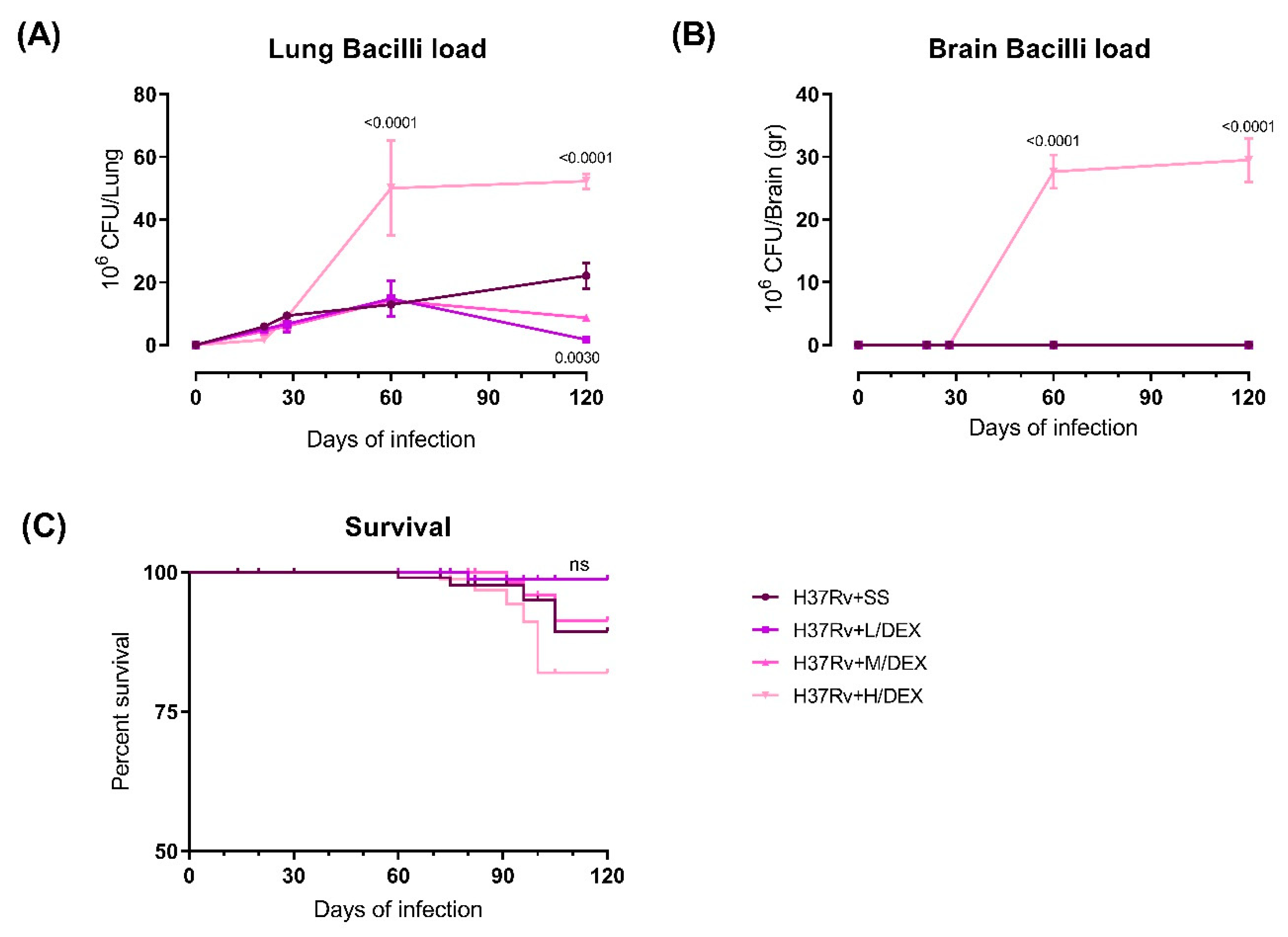
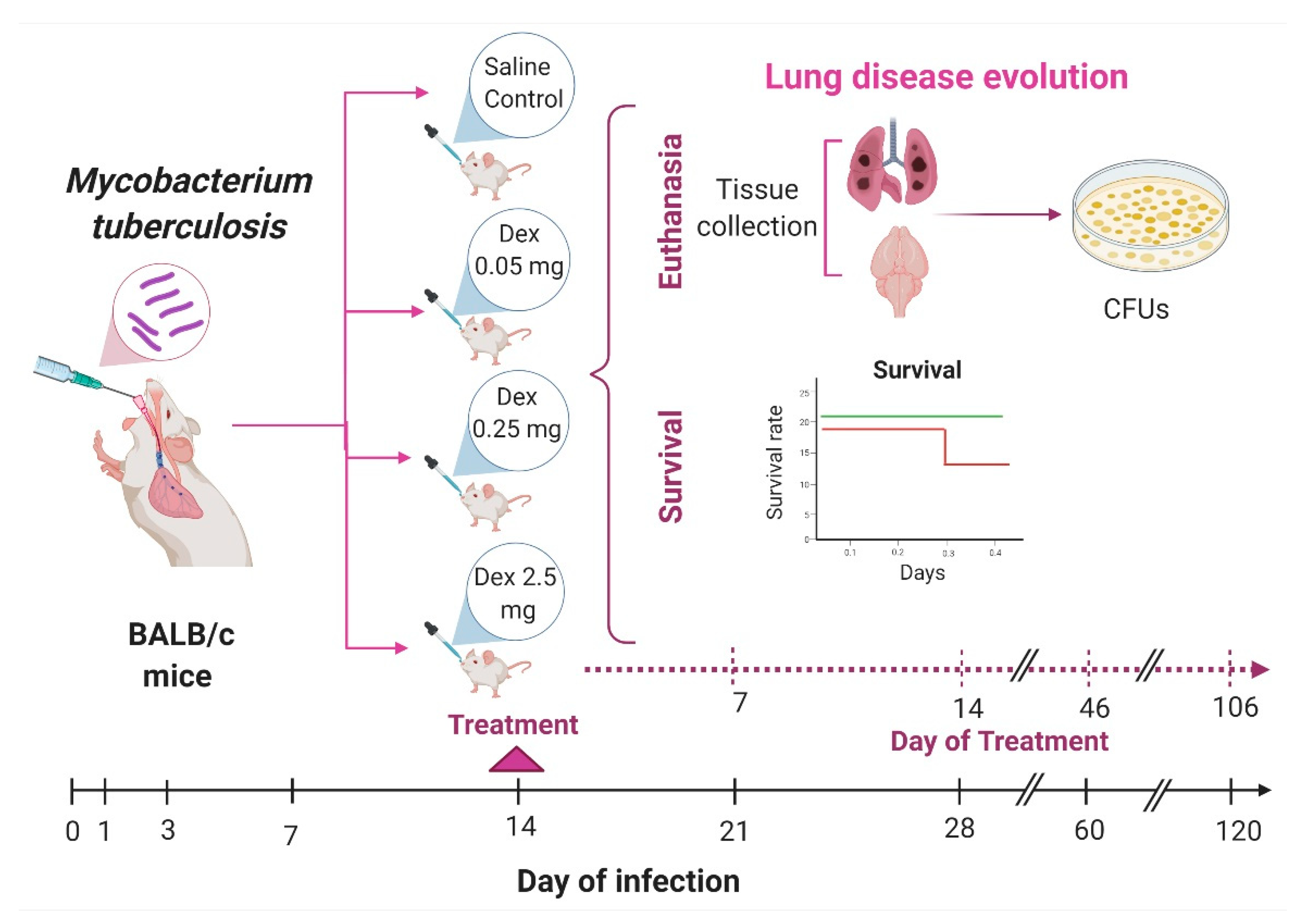
2.1. The Effect of Intranasal Dexamethasone (DEX) Treatment on Survival and Bacilli Loads in Experimental Pulmonary Tuberculosis
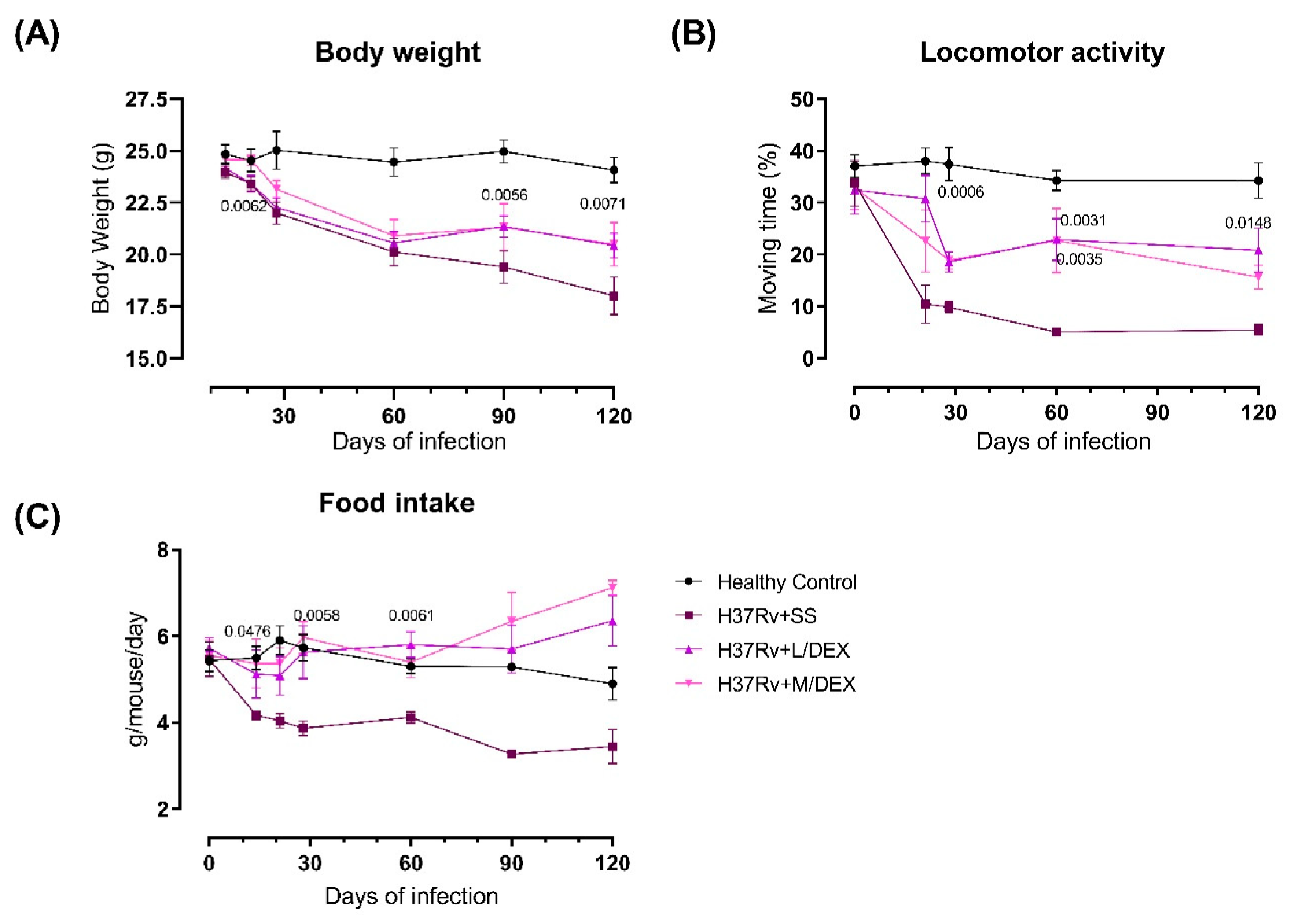
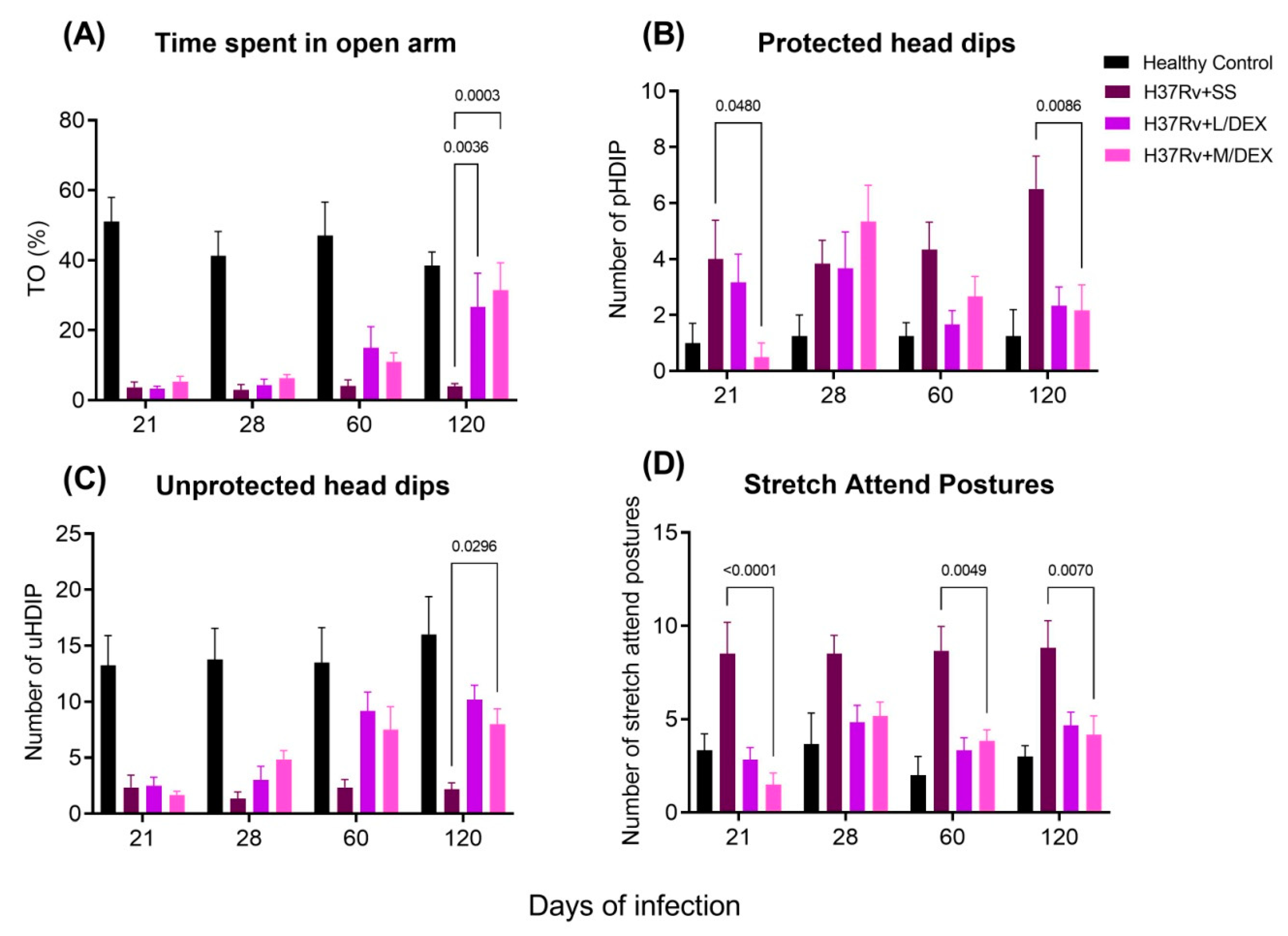
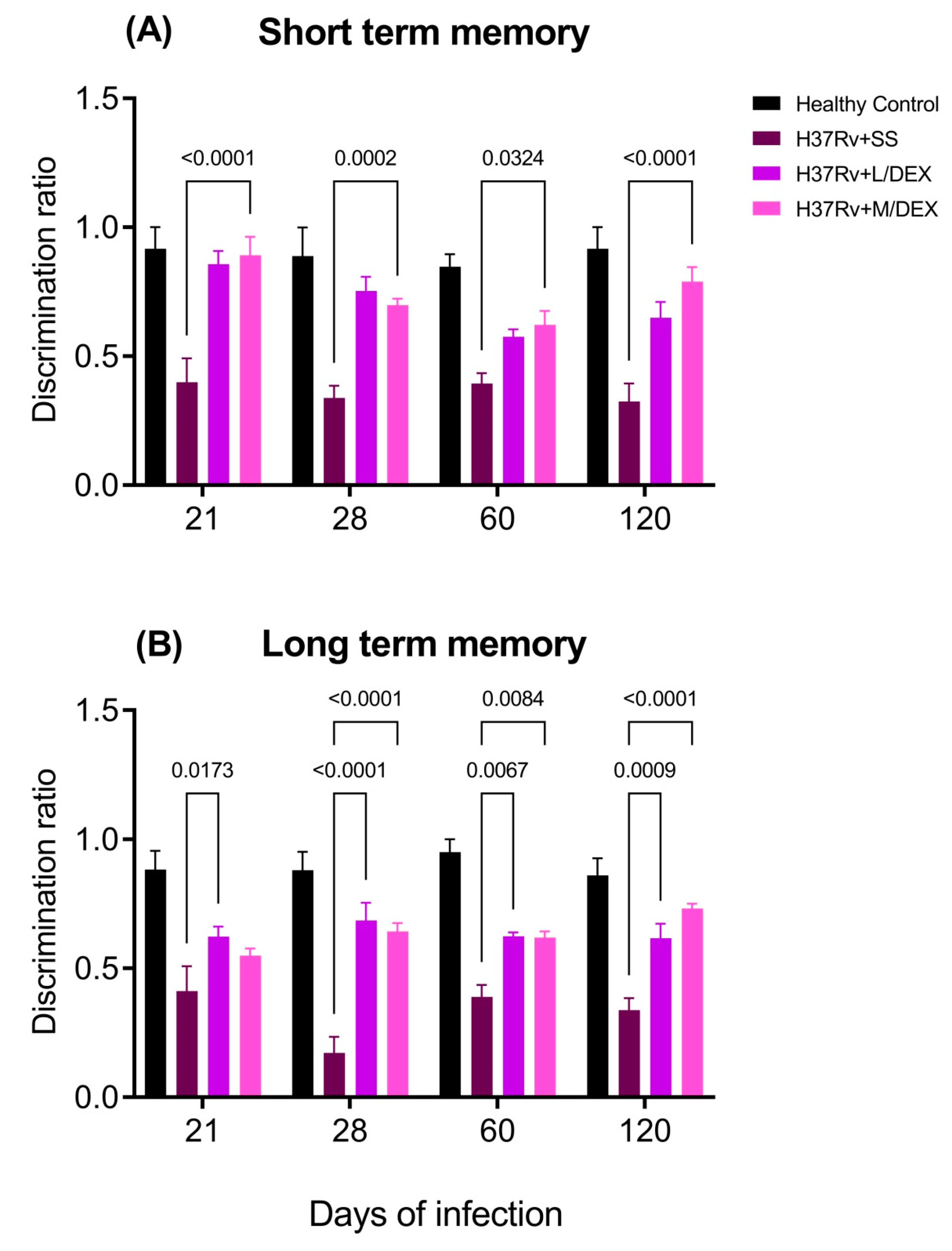
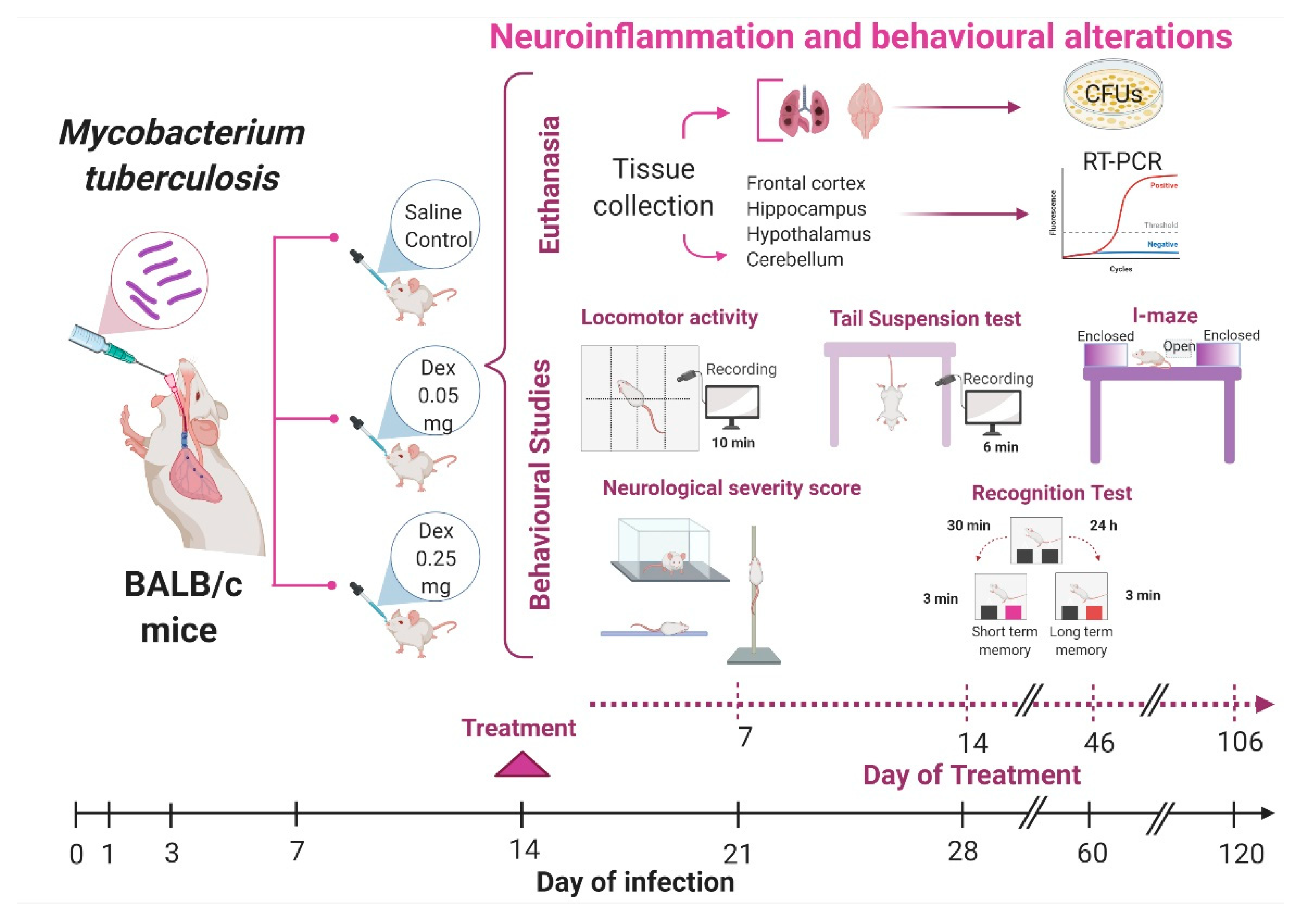
2.2. The Effect of Intranasal Low Dose DEX Treatment Since Early TB Infection on Diverse Behavioural Abnormalities
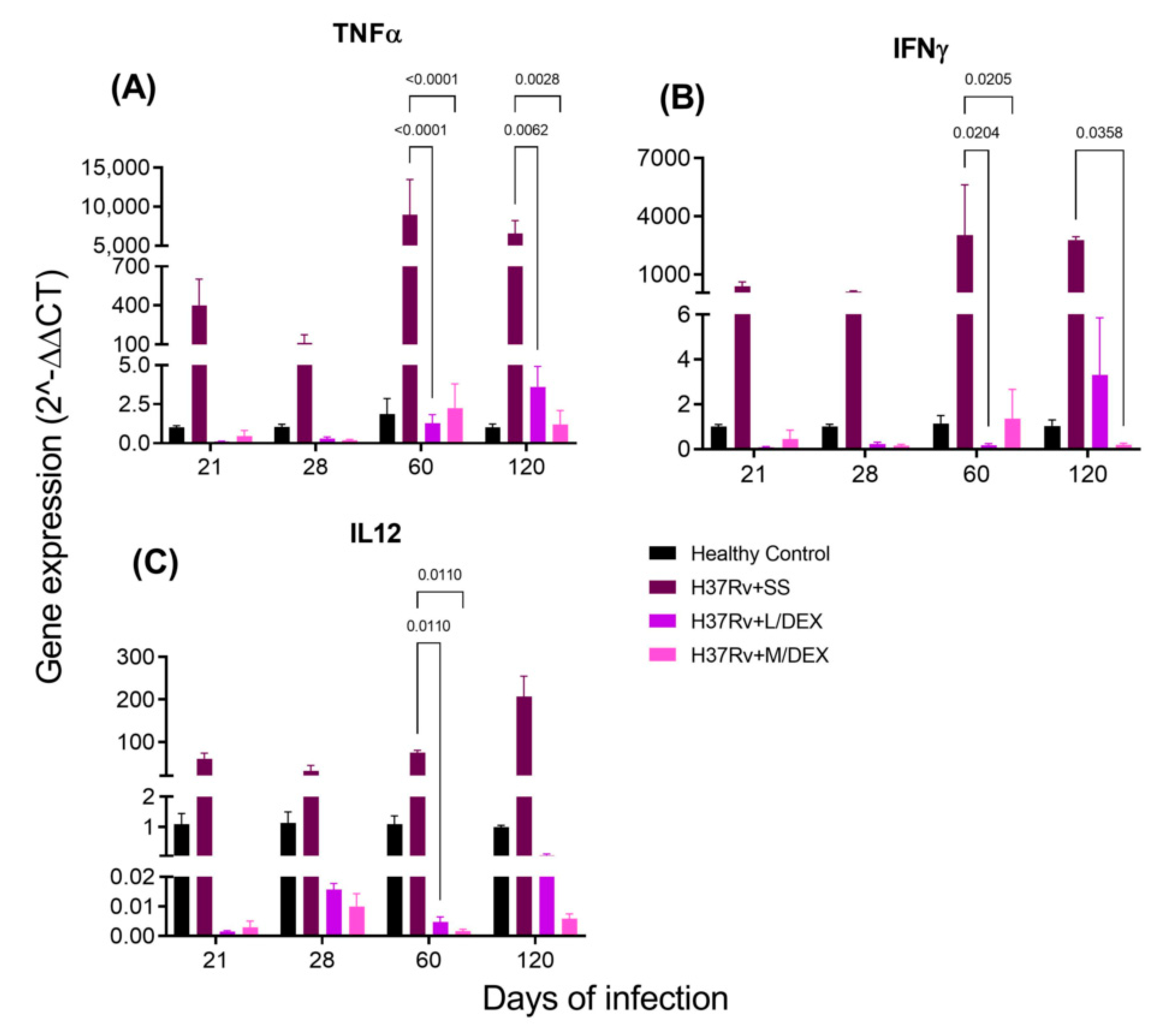
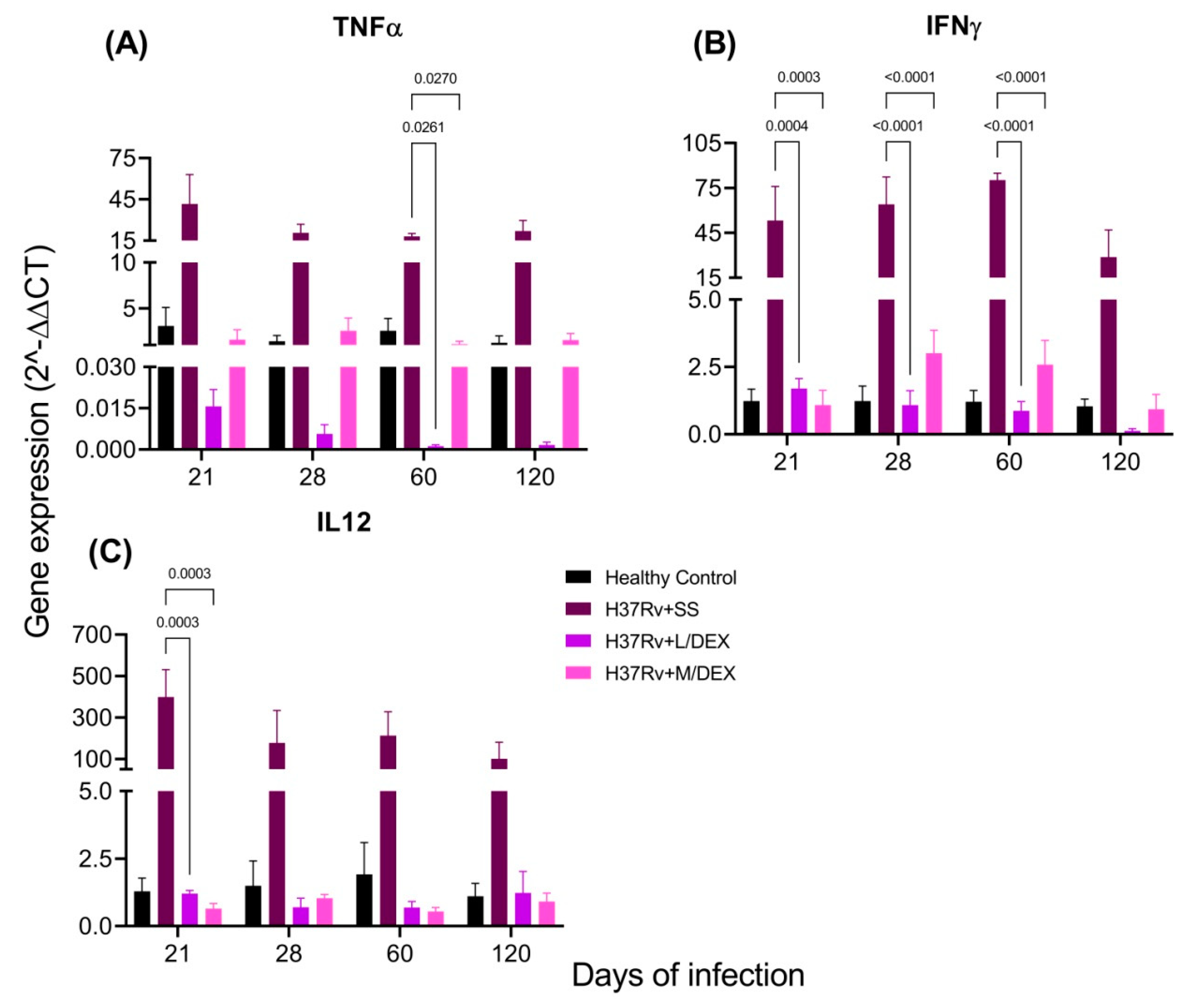
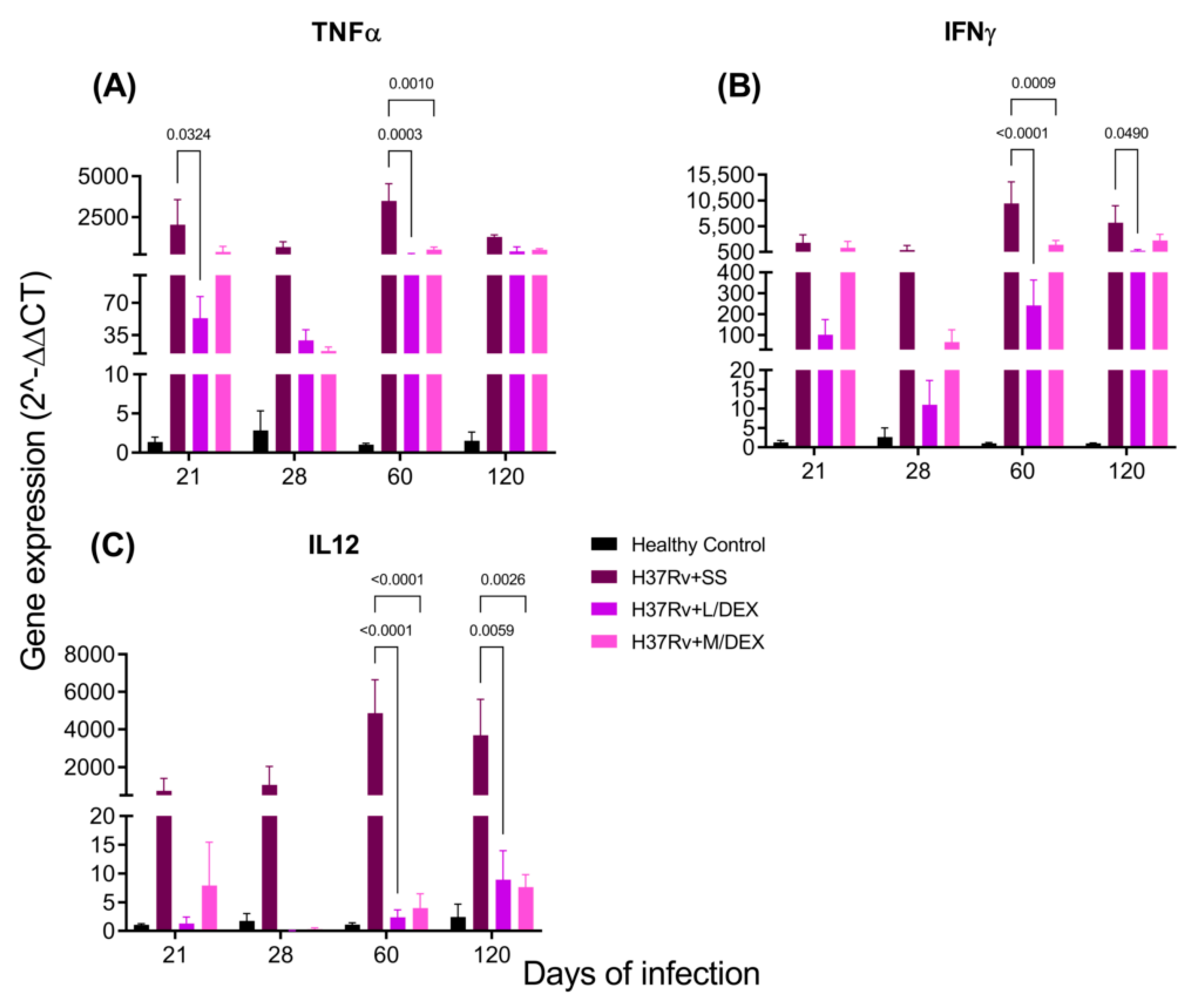
2.3. The Effect of Intranasal DEX Treatment on Cytokine Expression in Different Brain Areas of TB Mice
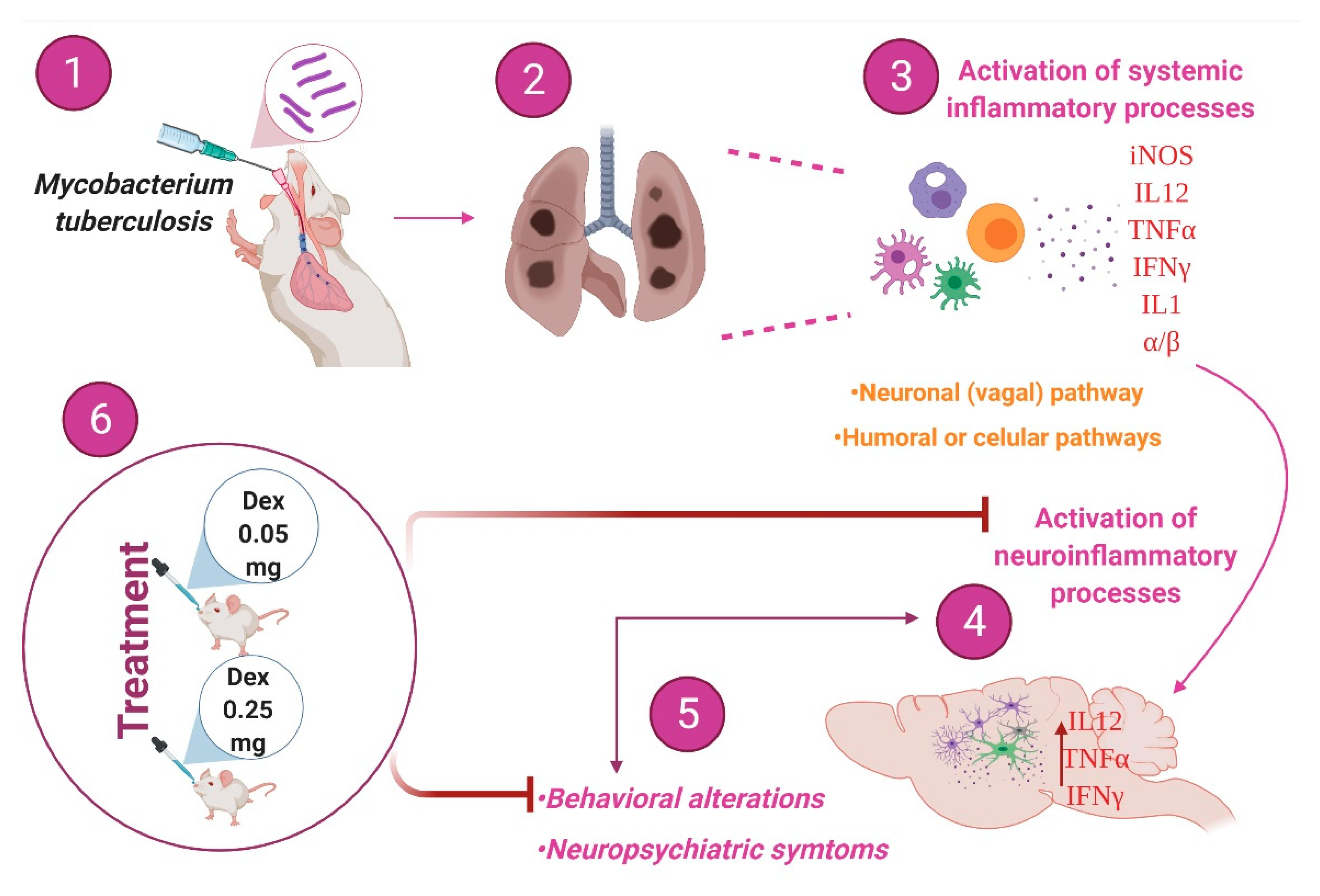
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. The Experimental Model of Pulmonary TB
4.4. Experimental Design
4.5. Dexamethasone Administration
4.6. Determination of Colony-Forming Units (CFU) in Infected Lungs and Brain
4.7. Expression of Cytokines by RT-PCR
4.8. Behaviour Tests
4.8.1. Sickness Behavior
4.8.2. Depression-Like Behavior
4.8.3. Anxiety-Like Behavior
4.8.4. Neurological Outcome
4.8.5. Memory Damage
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBB | Blood-brain barrier |
| BCG | Bacillus Calmette–Guérin |
| BW | Body weight |
| CFU | Colony-forming units |
| CNS | Central Nervous System |
| CMD | Common mental disorders |
| DEX | Dexamethasone |
| EAE | Experimental autoimmune encephalomyelitis |
| GCs | Glucocorticoids |
| GFAP | Glial fibrillary acidic protein |
| H | High |
| HPA | Hypothalamus-pituitary-adrenal axis |
| IL | Interleukin |
| IGCs | Inhaled GCs |
| IN | Intranasal delivery |
| IFNγ | Interferon-gamma |
| IV | Intravenously |
| L | Low |
| LMA | Locomotor activity |
| LPS | Lipopolysaccharide |
| M | Medium |
| MCAO | Middle cerebral artery occlusion |
| MDRTB | Multidrug-resistant TB |
| Mtb | Mycobacterium tuberculosis |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OADC | Oleic acid, albumin, dextrose and catalase |
| pHDIPS | Protected head dips |
| REML | Mixed-effects model |
| ROS | Reactive oxygen species |
| SAP | Stretched attend postures |
| SEM | The standard error of the mean |
| TO | Time spent by mice on the open arm |
| TB | Tuberculosis |
| TNFα | Tumour necrosis factor-alpha |
| uHDIPS | Unprotected head dips |
| WHO | World Health Organization |
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meneses, G.; Cárdenas, G.; Espinosa, A.; Rassy, D.; Pérez-Osorio, I.N.; Bárcena, B.; Fleury, A.; Besedovsky, H.; Fragoso, G.; Sciutto, E. Sepsis: Developing new alternatives to reduce neuroinflammation and attenuate brain injury. Ann. N. Y. Acad. Sci. 2019, 1437, 43–56. [Google Scholar] [CrossRef]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Klein, R.S.; Garber, C.; Howard, N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017, 18, 132–141. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neu-roinflammation Induces Neurodegeneration. J. Neurol. Neurosurg Spine 2016, 1, 1–15. [Google Scholar]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell. Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef]
- Ayroldi, E.; Cannarile, L.; Migliorati, G.; Nocentini, G.; Delfino, D.V.; Riccardi, C. Mechanisms of the anti-inflammatory effects of glucocorticoids: Genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 2012, 26, 4805–4820. [Google Scholar] [CrossRef]
- Cruz-Topete, D.; Cidlowski, J.A. One hormone, two actions: Anti- And pro-inflammatory effects of glucocorticoids. Neuroim-munomodulation 2014, 22, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Artelt, M.; Burnet, M.; Schluesener, H.J. Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol. 2007, 113, 675–682. [Google Scholar] [CrossRef]
- Tuckermann, J.P.; Kleiman, A.; McPherson, K.G.; Reichardt, H.M. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit. Rev. Clin. Lab. Sci. 2005, 42, 71–104. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Meneses, G.; Chavarría, A.; Mancilla, R.; Pedraza-Chaverri, J.; Fleury, A.; Bárcena, B.; Pérez-Osorio, I.N.; Be-sedovsky, H.; Arauz, A.; et al. Intranasal Dexamethasone Reduces Mortality and Brain Damage in a Mouse Experimental Is-chemic Stroke Model. Neurotherapeutics 2020, 17, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Ora, J.; Calzetta, L.; Matera, M.G.; Cazzola, M.; Rogliani, P. Advances with glucocorticoids in the treatment of asthma: State of the art. Expert Opin. Pharmacother. 2020, 21, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R. Systemic side effects of inhaled corticosteroids in patients with asthma. Respir. Med. 2006, 100, 1307–1317. [Google Scholar] [CrossRef]
- Meneses, G.; Gevorkian, G.; Florentino, A.; Bautista, M.A.; Espinosa, A.; Acero, G.; Díaz, G.; Fleury, A.; Pérez Osorio, I.N.; del Rey, A.; et al. Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clin. Exp. Immunol. 2017, 190, 304–314. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Messina, J.C.; Mahmoud, R.A. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther. Deliv. 2014, 5, 709–733. [Google Scholar] [CrossRef]
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076. [Google Scholar] [CrossRef]
- Stockdale, L.; Fletcher, H. The Future of Vaccines for Tuberculosis. Clin. Chest Med. 2019, 40, 849–856. [Google Scholar] [CrossRef]
- Zatarain-Barrón, Z.L.; Ramos-Espinosa, O.; Marquina-Castillo, B.; Barrios-Payán, J.; Cornejo-Granados, F.; Maya-Lucas, O.; López-Leal, G.; Molina-Romero, C.; Anthony, R.M.; Ochoa-Leyva, A.; et al. Evidence for the Effect of Vaccination on Host-Pathogen Interactions in a Murine Model of Pulmonary Tuberculosis by Mycobacterium tuberculosis. Front. Immunol. 2020, 11, 930. [Google Scholar] [CrossRef]
- Lara-Espinosa, J.V.; Hernández-Pando, R. Psychiatric Problems in Pulmonary Tuberculosis: Depression and Anxiety. J Tuberc Res. 2021, 9, 31–50. [Google Scholar] [CrossRef]
- Yen, Y.F.; Chung, M.S.; Hu, H.Y.; Lai, Y.J.; Huang, L.Y.; Lin, Y.S.; Chou, P.; Deng, C.Y. Association of pulmonary tuberculosis and ethambutol with incident depressive disorder: A nationwide, population-based cohort study. J. Clin. Psychiatry 2015, 76, e505–e511. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Mehreen, S.; Khan, M.A.; Ashiq, N.; Ihtesham, M. Depression and its Associated Factors with Multidrug-Resistant Tuberculosis at Baseline. J. Depress. Anxiety 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Mathai, P.J.; Ravindran, P.; Joshi, P.; Sundaram, P. Psychiatric morbidity in pulmonary tuberculosis—A clinical study. Indian J. Psychiatry 1981, 23, 66–68. [Google Scholar]
- Shyamala, K.K.; Sharadha Naveen, R.; Khatri, B. Depression: A neglected comorbidity in patients with tuberculosis. J. Assoc. Physicians India 2018, 66, 18–21. [Google Scholar] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Katsuura, G.; Arimura, A.; Koves, K.; Gottschall, P.E. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1β-induced ACTH release. Am. J. Physiol. Endocrinol. Metab. 1990, 258, E163–E171. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sci. 1995, 57, 1011–1026. [Google Scholar] [CrossRef]
- Khairova, R.A.; MacHado-Vieira, R.; Du, J.; Manji, H.K. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2009, 12, 561–578. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Neurol. Clin. 2006, 24, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, V.; Vernon, A.C. From early adversities to immune activation in psychiatric disorders: The role of the sympathetic nervous system. Clin. Exp. Immunol. 2019, 197, 319–328. [Google Scholar] [CrossRef]
- Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental pulmonary tuberculosis in the absence of detectable brain infection induces neuroinflammation and behavioural abnormalities in male balb/c mice. Int. J. Mol. Sci. 2020, 21, 9483. [Google Scholar] [CrossRef]
- Gilhotra, R.; Goel, S.; Gilhotra, N. Behavioral and biochemical characterisation of elevated “I-maze” as animal model of anxiety. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 214–224. [Google Scholar] [CrossRef][Green Version]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res. Int. 2014, 2014, 1–37. [Google Scholar] [CrossRef]
- Rassy, D.; Bárcena, B.; Nicolás Pérez-Osorio, I.; Espinosa, A.; Peón, A.N.; Terrazas, L.I.; Meneses, G.; Besedovsky, H.O.; Fra-goso, G.; Sciutto, E. Intranasal methylprednisolone effectively reduces neuroinflammation in mice with experimental auto-immune encephalitis. J. Neuropathol. Exp. Neurol. 2020, 79, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Fan, L.W.; Zheng, B.; Campbell, L.R.; Cai, Z.; Rhodes, P.G. Dexamethasone and betamethasone protect against lip-opolysaccharide-induced brain damage in neonatal rats. Pediatr. Res. 2012, 71, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, J.W.; Van Der Zee, M.; De Bruin, R.W.F.; Van Holten-Neelen, C.; Bastiaans, J.; Nagtzaam, N.M.A.; Ijzermans, J.N.M.; Benner, R.; Dik, W.A. Mild versus strong anti-inflammatory therapy during early sepsis in mice: A matter of life and death. Crit. Care Med. 2011, 39, 1275–1281. [Google Scholar] [CrossRef]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef]
- Chinenov, Y.; Rogatsky, I. Glucocorticoids and the innate immune system: Crosstalk with the Toll-like receptor signaling network. Mol. Cell. Endocrinol. 2007, 275, 30–42. [Google Scholar] [CrossRef]
- Busillo, J.M.; Azzams, K.M.; Cidlowski, J.A. Glucocorticoids sensitise the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011, 286, 38703–38713. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gao, Z.G.; Jacobson, K.A.; Suffredini, A.F. Dexamethasone enhances ATP-induced inflammatory responses in en-dothelial cells. J. Pharmacol. Exp. Ther. 2010, 335, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Ábrahám, I.M.; Meerlo, P.; Luiten, P.G. Concentration Dependent Actions of Glucocorticoids on Neuronal Viability and Sur-vival. Dose-Response 2006, 4, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, J.C.; Melo, F.B.S.; Albuquerque, M.F.P.M.; Montenegro, S.M.L.; Abath, F.G.C. Immune factors and immunoregulation in tuberculosis. Brazilian, J. Med. Biol. Res. 2006, 39, 1387–1397. [Google Scholar] [CrossRef]
- Holmin, S.; Mathiesen, T. Dexamethasone and colchicine reduce inflammation and delayed oedema following experimental brain contusion. Acta Neurochir. 1996, 138, 418–424. [Google Scholar] [CrossRef]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 35002. [Google Scholar] [CrossRef]
- Campolo, M.; Ahmad, A.; Crupi, R.; Impellizzeri, D.; Morabito, R.; Esposito, E.; Cuzzocrea, S. Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J. Endocrinol. 2013, 217, 291–301. [Google Scholar] [CrossRef]
- Bensch, G.W. Safety of intranasal corticosteroids. Ann. Allergy Asthma Immunol. 2016, 117, 601–605. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 2, 2637–2646. [Google Scholar] [CrossRef]
- Hernández-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavón, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar]
- Hernandez Pando, R.; Aguilar, D.; Cohen, I.; Guerrero, M.; Ribon, W.; Acosta, P.; Orozco, H.; Marquina, B.; Salinas, C.; Rembao, D.; et al. Specific bacterial genotypes of Mycobacterium tuberculosis cause extensive dissemination and brain infection in an experimental model. Tuberculosis 2010, 90, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013; Volume 246. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analysing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Stahel, P.F.; Shohami, E.; Younis, F.M.; Kariya, K.; Otto, V.I.; Lenzlinger, P.M.; Grosjean, M.B.; Eugster, H.P.; Trentz, O.; Kossmann, T.; et al. Experimental closed head injury: Analysis of neurological outcome, blood-brain barrier dysfunction, in-tracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000, 20, 369–380. [Google Scholar] [CrossRef]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef]
- GraphPad Software; Version 9.1.1.225; Software for Biostatistics. 2021. Available online: https://crackproduct.com/graphpad-prism/ (accessed on 20 May 2021).

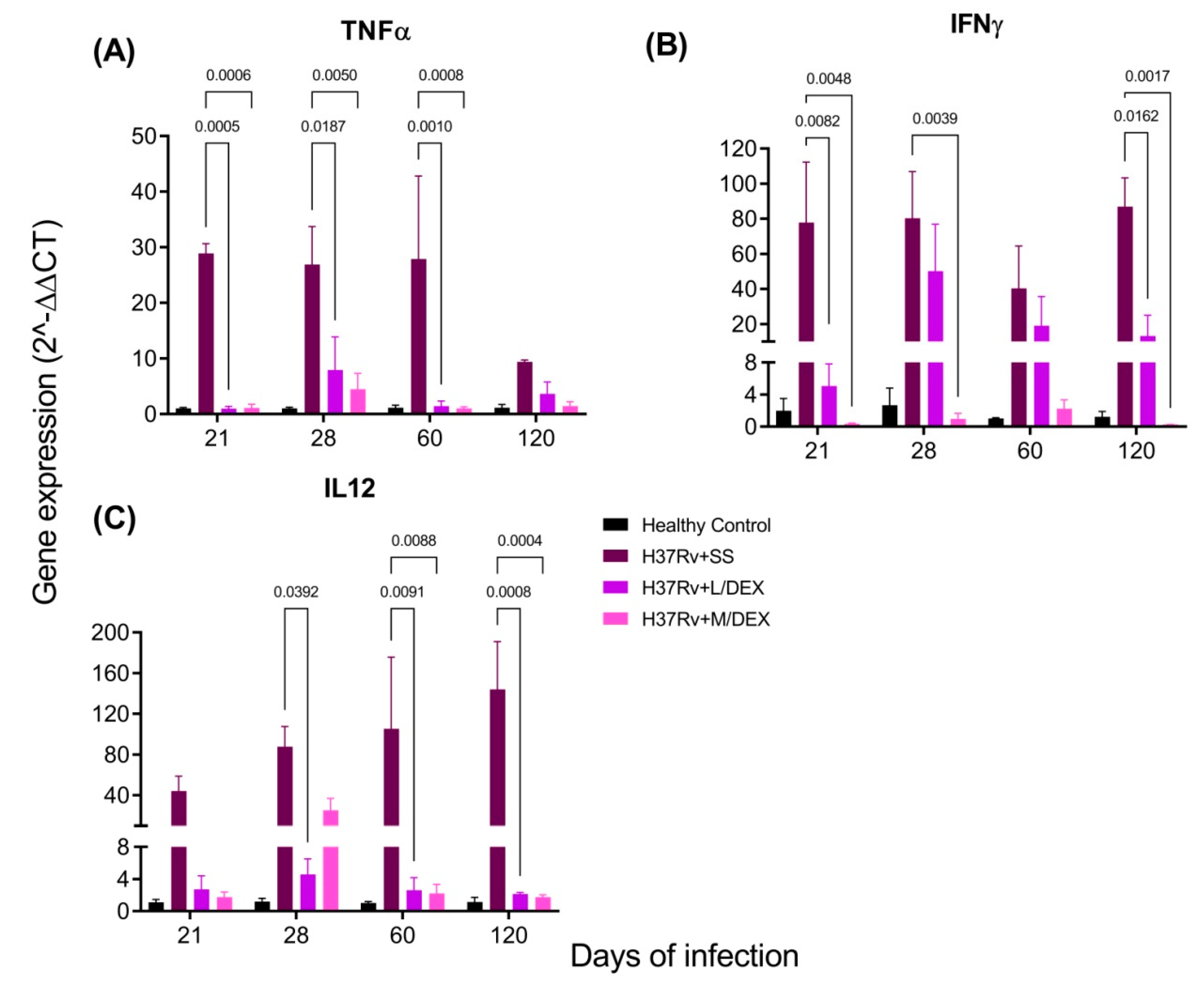
| Cytokine | TB | DEX |
|---|---|---|
| TNFα | Increases | Decreases |
| IFNγ | Increases | Decreases |
| IL12 | Increases | Decreases |
| iNOS | Increases | Decreases |
| IL1β | Increases | Decreases |
| IL6 | Increases | Decreases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Espinosa, J.V.; Arce-Aceves, M.F.; Mata-Espinosa, D.; Barrios-Payán, J.; Marquina-Castillo, B.; Hernández-Pando, R. The Therapeutic Effect of Intranasal Administration of Dexamethasone in Neuroinflammation Induced by Experimental Pulmonary Tuberculosis. Int. J. Mol. Sci. 2021, 22, 5997. https://doi.org/10.3390/ijms22115997
Lara-Espinosa JV, Arce-Aceves MF, Mata-Espinosa D, Barrios-Payán J, Marquina-Castillo B, Hernández-Pando R. The Therapeutic Effect of Intranasal Administration of Dexamethasone in Neuroinflammation Induced by Experimental Pulmonary Tuberculosis. International Journal of Molecular Sciences. 2021; 22(11):5997. https://doi.org/10.3390/ijms22115997
Chicago/Turabian StyleLara-Espinosa, Jacqueline V., María Fernanda Arce-Aceves, Dulce Mata-Espinosa, Jorge Barrios-Payán, Brenda Marquina-Castillo, and Rogelio Hernández-Pando. 2021. "The Therapeutic Effect of Intranasal Administration of Dexamethasone in Neuroinflammation Induced by Experimental Pulmonary Tuberculosis" International Journal of Molecular Sciences 22, no. 11: 5997. https://doi.org/10.3390/ijms22115997
APA StyleLara-Espinosa, J. V., Arce-Aceves, M. F., Mata-Espinosa, D., Barrios-Payán, J., Marquina-Castillo, B., & Hernández-Pando, R. (2021). The Therapeutic Effect of Intranasal Administration of Dexamethasone in Neuroinflammation Induced by Experimental Pulmonary Tuberculosis. International Journal of Molecular Sciences, 22(11), 5997. https://doi.org/10.3390/ijms22115997