Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis
Abstract
1. Introduction
2. Results
2.1. Effect of FT+DM on the Inflammatory Microenvironment
2.2. Effect of FT+DM on Endometriotic Lesions
2.3. Effect of FT+DM on Histological Score and Hyperproliferation
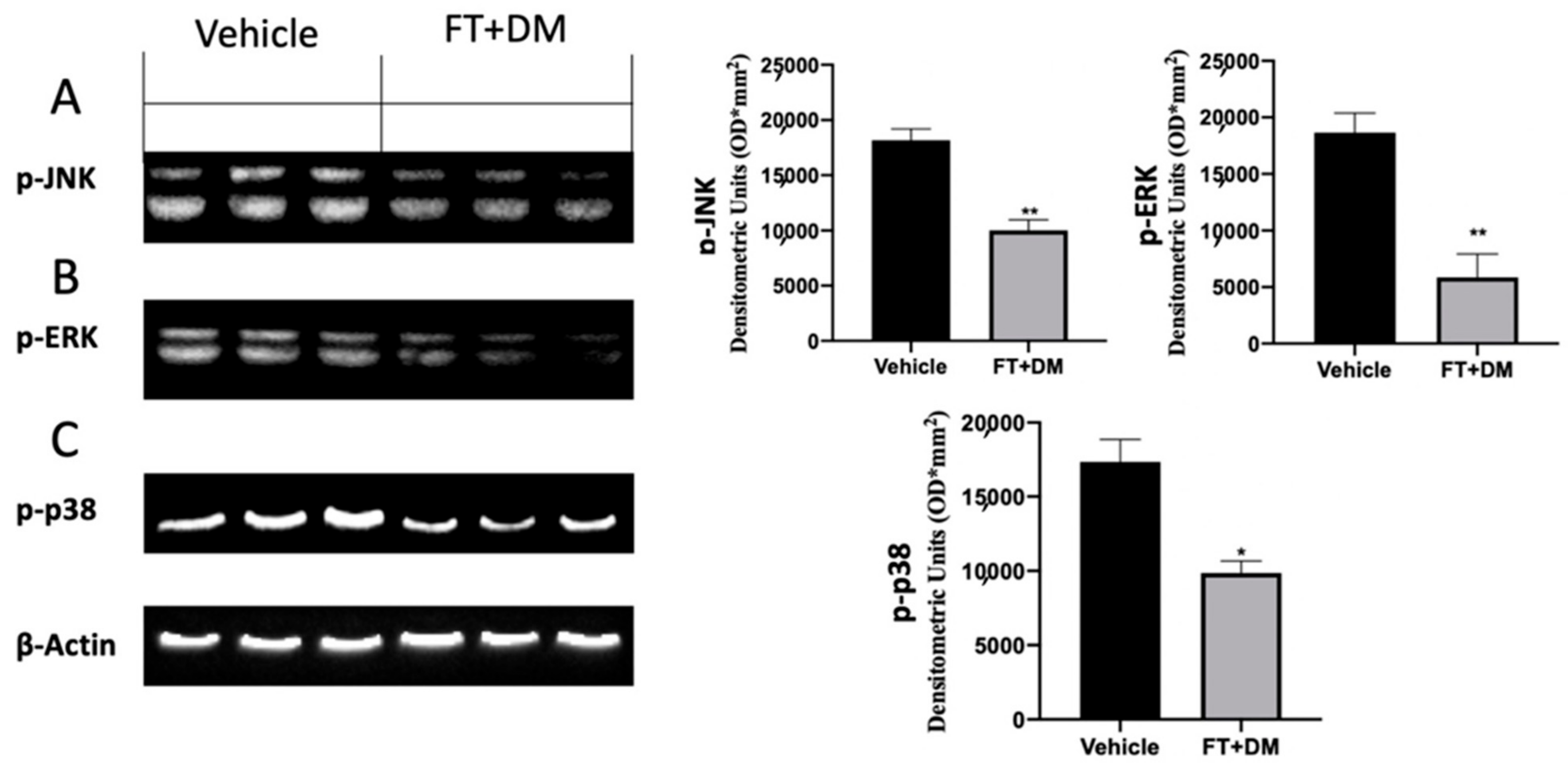
2.4. Effect of FT+DM on the MAPK Pathway
2.5. Effect of FT+DM on NFκB and COX-2 Expression
2.6. Effect of FT+DM on Apoptosis
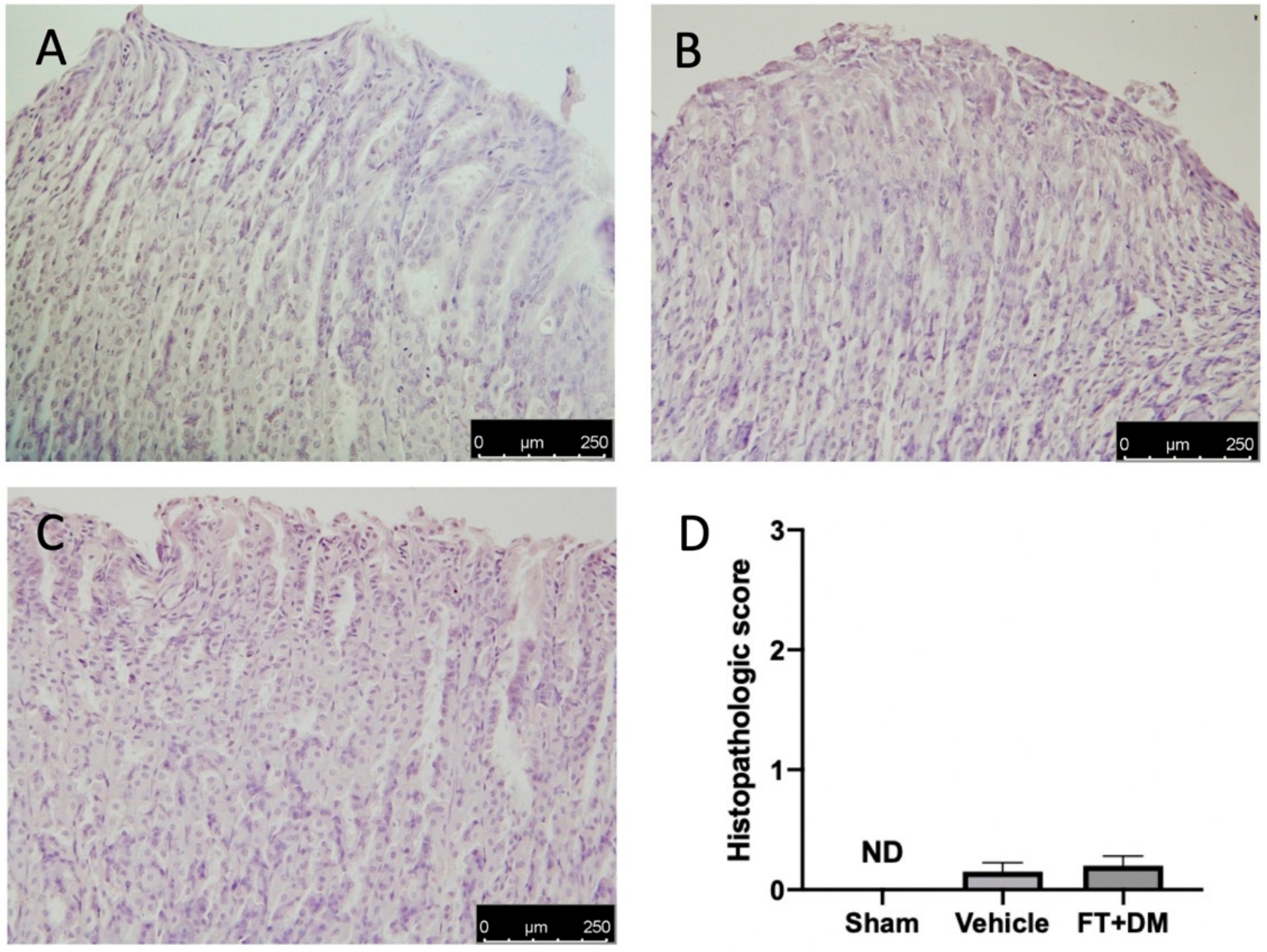
2.7. Effect of FT+DM on Gastric Homeostasis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Experimental Groups
- (1)
- Vehicle group: Rats were subjected to experimental endometriosis as described above, and the vehicle (saline) was administered by gavage on the seventh day and for the next 7 days.
- (2)
- FM group: Rats were subjected to experimental endometriosis as described above, and FM (10 mg/kg i.p. fotemustine (single dose)) was administered on the seventh day.
- (3)
- DM group: Rats were subjected to experimental endometriosis as described above, and DM (0.1 mg/kg/day i.p. dexamethasone) was administered on the seventh day and for the next 7 days.
- (4)
- FM+DM group: Rats were subjected to experimental endometriosis as described above, and FM+DM (10 mg/kg i.p. fotemustine (single dose) and 0.1 mg/kg/day i.p. dexamethasone) was administered on the seventh day and for the next 7 days.
- (5)
- Sham group: Rats were injected intraperitoneally with 500 μL of PBS without endometrial tissue and the vehicle (saline) was administered.
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Histological Examination
4.6. Immunohistochemical Analysis
4.7. Western Blot Analysis
4.8. Terminal Deoxynucleotidyl Nick-End Labeling (TUNEL) Assay
4.9. Statistical Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Jana, S.; Chatterjee, K.; Ray, A.K.; DasMahapatra, P.; Swarnakar, S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS ONE 2016, 11, e0163540. [Google Scholar] [CrossRef]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392. [Google Scholar] [CrossRef] [PubMed]
- Beliard, A.; Noel, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hopwood, D.; Levison, D.A. Atrophy and apoptosis in the cyclical human endometrium. J. Pathol. 1976, 119, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kokawa, K.; Shikone, T.; Nakano, R. Apoptosis in the human uterine endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 1996, 81, 4144–4147. [Google Scholar] [CrossRef][Green Version]
- Shikone, T.; Yamoto, M.; Kokawa, K.; Yamashita, K.; Nishimori, K.; Nakano, R. Apoptosis of human corpora lutea during cyclic luteal regression and early pregnancy. J. Clin. Endocrinol. Metab. 1996, 81, 2376–2380. [Google Scholar] [CrossRef] [PubMed]
- Gebel, H.M.; Braun, D.P.; Tambur, A.; Frame, D.; Rana, N.; Dmowski, W.P. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertil. Steril. 1998, 69, 1042–1047. [Google Scholar] [CrossRef]
- Harada, A.; Kimura, Y.; Kojima, C.; Kono, K. Effective tolerance to serum proteins of head-tail type polycation vectors by PEGylation at the periphery of the head block. Biomacromolecules 2010, 11, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Nasu, K.; Ueda, T.; Fukuda, J.; Takai, N.; Miyakawa, I. Endometriotic cells are resistant to interferon-gamma-induced cell growth inhibition and apoptosis: A possible mechanism involved in the pathogenesis of endometriosis. Mol. Hum. Reprod. 2005, 11, 29–34. [Google Scholar] [CrossRef]
- Vaskivuo, T.E.; Stenback, F.; Karhumaa, P.; Risteli, J.; Dunkel, L.; Tapanainen, J.S. Apoptosis and apoptosis-related proteins in human endometrium. Mol. Cell. Endocrinol. 2000, 165, 75–83. [Google Scholar] [CrossRef]
- Zhuang, M.; Cao, Y.; Shi, Y.; Yu, L.; Niu, Y.; Zhang, T.; Sun, Z. Caulis Sargentodoxae Prescription Plays a Therapeutic Role with Decreased Inflammatory Cytokines in Peritoneal Fluid in the Rat Endometriosis Model. Evid. Based Complement. Altern. Med. 2020, 2020, 9627907. [Google Scholar] [CrossRef]
- Malutan, A.M.; Drugan, T.; Costin, N.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol. 2015, 40, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Fusco, R. The Methyl Ester of 2-Cyano-3, 12-Dioxooleana-1, 9-Dien-28-Oic Acid Reduces Endometrial Lesions Development by Modulating the NFkB and Nrf2 Pathways. Int. J. Mol. Sci. 2021, 22, 3991. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, J.Y.; Kim, M.R.; Hwang, K.J.; Chang, D.Y.; Jeon, M.K. Tumor necrosis factor-alpha-induced cyclooxygenase-2 overexpression in eutopic endometrium of women with endometriosis by stromal cell culture through nuclear factor-kappaB activation. J. Reprod. Med. 2009, 54, 625–630. [Google Scholar] [PubMed]
- Chen, D.B.; Yang, Z.M.; Hilsenrath, R.; Le, S.P.; Harper, M.J. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum. Reprod. 1995, 10, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, A.; Rossi, L.; Laudisi, A.; Sini, V.; Toppo, L.; Marchesi, F.; Tortorelli, G.; Leti, M.; Turriziani, M.; Aquino, A.; et al. Focus on Fotemustine. J. Exp. Clin. Cancer Res. 2006, 25, 461–468. [Google Scholar] [PubMed]
- Kula, M.; Tanriverdi, G.; Oksuz, E.; Bilir, A.; Shahzadi, A.; Yazici, Z. Simvastatin and dexamethasone potentiate antitumor activity of fotemustine. Int. J. Pharmacol. 2014, 10, 267–274. [Google Scholar] [CrossRef]
- Paoletti, P.; Butti, G.; Knerich, R.; Gaetani, P.; Assietti, R. Chemotherapy for malignant gliomas of the brain: A review of ten-years experience. Acta Neurochir. 1990, 103, 35–46. [Google Scholar] [CrossRef]
- Amano, Y.; Lee, S.W.; Allison, A.C. Inhibition by glucocorticoids of the formation of interleukin-1 alpha, interleukin-1 beta, and interleukin-6: Mediation by decreased mRNA stability. Mol. Pharm. 1993, 43, 176–182. [Google Scholar]
- Crinelli, R.; Antonelli, A.; Bianchi, M.; Gentilini, L.; Scaramucci, S.; Magnani, M. Selective inhibition of NF-kB activation and TNF-alpha production in macrophages by red blood cell-mediated delivery of dexamethasone. Blood Cells Mol. Dis. 2000, 26, 211–222. [Google Scholar] [CrossRef]
- Portnow, J.; Suleman, S.; Grossman, S.A.; Eller, S.; Carson, K. A cyclooxygenase-2 (COX-2) inhibitor compared with dexamethasone in a survival study of rats with intracerebral 9L gliosarcomas. Neuro Oncol. 2002, 4, 22–25. [Google Scholar] [CrossRef]
- Ozaki, T.; Habara, K.; Matsui, K.; Kaibori, M.; Kwon, A.H.; Ito, S.; Nishizawa, M.; Okumura, T. Dexamethasone inhibits the induction of iNOS gene expression through destabilization of its mRNA in proinflammatory cytokine-stimulated hepatocytes. Shock 2010, 33, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Almawi, W.Y.; Melemedjian, O.K. Molecular mechanisms of glucocorticoid antiproliferative effects: Antagonism of transcription factor activity by glucocorticoid receptor. J. Leukoc. Biol. 2002, 71, 9–15. [Google Scholar]
- Kassel, O.; Sancono, A.; Kratzschmar, J.; Kreft, B.; Stassen, M.; Cato, A.C. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001, 20, 7108–7116. [Google Scholar] [CrossRef]
- Garcia-Gomez, E.; Vazquez-Martinez, E.R.; Reyes-Mayoral, C.; Cruz-Orozco, O.P.; Camacho-Arroyo, I.; Cerbon, M. Regulation of Inflammation Pathways and Inflammasome by Sex Steroid Hormones in Endometriosis. Front. Endocrinol. 2019, 10, 935. [Google Scholar] [CrossRef]
- Haney, A.F.; Jenkins, S.; Weinberg, J.B. The stimulus responsible for the peritoneal fluid inflammation observed in infertile women with endometriosis. Fertil. Steril. 1991, 56, 408–413. [Google Scholar] [CrossRef]
- Haney, A.F.; Weinberg, J.B. Reduction of the intraperitoneal inflammation associated with endometriosis by treatment with medroxyprogesterone acetate. Am. J. Obstet. Gynecol. 1988, 159, 450–454. [Google Scholar] [CrossRef]
- Cao, X.; Yang, D.; Song, M.; Murphy, A.; Parthasarathy, S. The presence of endometrial cells in the peritoneal cavity enhances monocyte recruitment and induces inflammatory cytokines in mice: Implications for endometriosis. Fertil. Steril. 2004, 82 (Suppl. 3), 999–1007. [Google Scholar] [CrossRef]
- Fusco, R.; Siracusa, R.; D’Amico, R.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Melatonin Plus Folic Acid Treatment Ameliorates Reserpine-Induced Fibromyalgia: An Evaluation of Pain, Oxidative Stress, and Inflammation. Antioxidants 2019, 8, 628. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Peditto, M.; Oteri, G.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. Treatment With a Flavonoid-Rich Fraction of Bergamot Juice Improved Lipopolysaccharide-Induced Periodontitis in Rats. Front. Pharm. 2018, 9, 1563. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Crupi, R.; Genovese, T.; Impellizzeri, D.; Cuzzocrea, S.; et al. Ultramicronized Palmitoylethanolamide and Paracetamol, a New Association to Relieve Hyperalgesia and Pain in a Sciatic Nerve Injury Model in Rat. Int. J. Mol. Sci. 2020, 21, 3509. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, M.; Macpherson, A.; Healy, D.L.; Rogers, P.A. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril. 1995, 64, 340–346. [Google Scholar] [CrossRef]
- Duchrow, M.; Schluter, C.; Wohlenberg, C.; Flad, H.D.; Gerdes, J. Molecular characterization of the gene locus of the human cell proliferation-associated nuclear protein defined by monoclonal antibody Ki-67. Cell Prolif. 1996, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.C.; Hall, P.A.; Fletcher, C.D.; Camplejohn, R.S.; Waseem, N.H.; Lane, D.P.; Levison, D.A. Haemangiopericytomas: The prognostic value of immunohistochemical staining with a monoclonal antibody to proliferating cell nuclear antigen (PCNA). Histopathology 1991, 19, 29–33. [Google Scholar] [CrossRef]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Gugliandolo, E.; Peritore, A.F.; Di Paola, R.; Cuzzocrea, S. Topical application of adelmidrol + trans-traumatic acid enhances skin wound healing in a streptozotocin-induced diabetic mouse model. Front. Pharmacol. 2018, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Bosma, A.J.; Weigelt, B.; Lambrechts, A.C.; Verhagen, O.J.; Pruntel, R.; Hart, A.A.; Rodenhuis, S.; van ‘t Veer, L.J. Detection of circulating breast tumor cells by differential expression of marker genes. Clin. Cancer Res. 2002, 8, 1871–1877. [Google Scholar]
- Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Crupi, R.; Esposito, E.; Petrosino, S.; Cuzzocrea, S. Anti-inflammatory and neuroprotective effects of co-ultraPEALut in a mouse model of vascular dementia. Front. Neurol. 2017, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Shackney, S.E.; McCormack, G.W.; Cuchural, G.J., Jr. Growth rate patterns of solid tumors and their relation to responsiveness to therapy: An analytical review. Ann. Intern. Med. 1978, 89, 107–121. [Google Scholar] [CrossRef]
- Valeriote, F.; van Putten, L. Proliferation-dependent cytotoxicity of anticancer agents: A review. Cancer Res. 1975, 35, 2619–2630. [Google Scholar]
- Drewinko, B.; Patchen, M.; Yang, L.Y.; Barlogie, B. Differential killing efficacy of twenty antitumor drugs on proliferating and nonproliferating human tumor cells. Cancer Res. 1981, 41, 2328–2333. [Google Scholar]
- Mattern, J.; Wayss, K.; Volm, M. Effect of five antineoplastic agents on tumor xenografts with different growth rates. J. Natl. Cancer Inst. 1984, 72, 1335–1339. [Google Scholar]
- Leconte, M.; Nicco, C.; Ngo, C.; Chereau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.; Nicco, C.; Leconte, M.; Chereau, C.; Arkwright, S.; Vacher-Lavenu, M.C.; Weill, B.; Chapron, C.; Batteux, F. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J. Pathol. 2010, 222, 148–157. [Google Scholar] [CrossRef]
- Yoshino, O.; Osuga, Y.; Hirota, Y.; Koga, K.; Hirata, T.; Harada, M.; Morimoto, C.; Yano, T.; Nishii, O.; Tsutsumi, O.; et al. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am. J. Reprod. Immunol. 2004, 52, 306–311. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Z.; Hirota, Y.; Osuga, Y.; Hamasaki, K.; Hasegawa, A.; Tajima, T.; Hirata, T.; Koga, K.; Yoshino, O.; Harada, M.; et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am. J. Pathol. 2008, 173, 463–469. [Google Scholar] [CrossRef]
- Hirata, T.; Osuga, Y.; Hamasaki, K.; Yoshino, O.; Ito, M.; Hasegawa, A.; Takemura, Y.; Hirota, Y.; Nose, E.; Morimoto, C.; et al. Interleukin (IL)-17A stimulates IL-8 secretion, cyclooxygensase-2 expression, and cell proliferation of endometriotic stromal cells. Endocrinology 2008, 149, 1260–1267. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Vermeulen, L.; De Wilde, G.; Van Damme, P.; Vanden Berghe, W.; Haegeman, G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003, 22, 1313–1324. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Impellizzeri, D.; Cordaro, M.; Britti, D.; Morittu, V.M.; Evangelista, M.; Cuzzocrea, S. Adelmidrol, in combination with hyaluronic acid, displays increased anti-inflammatory and analgesic effects against monosodium iodoacetate-induced osteoarthritis in rats. Arthritis Res. Ther. 2016, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Travelli, C.; Aprile, S.; Rahimian, R.; Grolla, A.A.; Rogati, F.; Bertolotti, M.; Malagnino, F.; Di Paola, R.; Impellizzeri, D.; Fusco, R.; et al. Identification of Novel Triazole-Based Nicotinamide Phosphoribosyltransferase (NAMPT) Inhibitors Endowed with Antiproliferative and Antiinflammatory Activity. J. Med. Chem. 2017, 60, 1768–1792. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’ Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C.; et al. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Gugliandolo, E.; Biundo, F.; Campolo, M.; Di Paola, R.; Cuzzocrea, S. Inhibition of inflammasome activation improves lung acute injury induced by carrageenan in a mouse model of pleurisy. FASEB J. 2017, 31, 3497–3511. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Impellizzeri, D.; Siracusa, R.; Gugliandolo, E.; Fusco, R.; Inferrera, A.; Esposito, E.; Di Paola, R.; Cuzzocrea, S. Effects of a co-micronized composite containing palmitoylethanolamide and polydatin in an experimental model of benign prostatic hyperplasia. Toxicol. Appl. Pharm. 2017, 329, 231–240. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Neuroprotective Effect of Artesunate in Experimental Model of Traumatic Brain Injury. Front. Neurol. 2018, 9, 590. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; D’Amico, R.; Militi, A.; Oteri, G.; Wallace, J.L.; Di Paola, R.; Cuzzocrea, S. Anti-inflammatory effect of ATB-352, a H2S-releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats. Pharm. Res. 2018, 132, 220–231. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’Amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Cuzzocrea, S.; Di Paola, R. Effects of a new compound containing Palmitoylethanolamide and Baicalein in myocardial ischaemia/reperfusion injury in vivo. Phytomedicine 2019, 54, 27–42. [Google Scholar] [CrossRef]
- Fusco, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. N-Palmitoylethanolamide-Oxazoline Protects against Middle Cerebral Artery Occlusion Injury in Diabetic Rats by Regulating the SIRT1 Pathway. Int. J. Mol. Sci. 2019, 20, 4845. [Google Scholar] [CrossRef]
- Lai, Z.Z.; Yang, H.L.; Ha, S.Y.; Chang, K.K.; Mei, J.; Zhou, W.J.; Qiu, X.M.; Wang, X.Q.; Zhu, R.; Li, D.J.; et al. Cyclooxygenase-2 in Endometriosis. Int. J. Biol. Sci. 2019, 15, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Kudo, I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog. Lipid Res. 2004, 43, 3–35. [Google Scholar] [CrossRef]
- Harada, T.; Kaponis, A.; Iwabe, T.; Taniguchi, F.; Makrydimas, G.; Sofikitis, N.; Paschopoulos, M.; Paraskevaidis, E.; Terakawa, N. Apoptosis in human endometrium and endometriosis. Hum. Reprod. Update 2004, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Narum, S.; Westergren, T.; Klemp, M. Corticosteroids and risk of gastrointestinal bleeding: A systematic review and meta-analysis. BMJ Open 2014, 4, e004587. [Google Scholar] [CrossRef]
- Filep, J.G.; Herman, F.; Foldes-Filep, E.; Schneider, F.; Braquet, P. Dexamethasone-induced gastric mucosal damage in the rat: Possible role of platelet-activating factor. Br. J. Pharm. 1992, 105, 912–918. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genovese, T.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Trovato Salinaro, A.; Raffone, E.; Impellizzeri, D.; et al. Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis. Int. J. Mol. Sci. 2021, 22, 5998. https://doi.org/10.3390/ijms22115998
Genovese T, Siracusa R, D’Amico R, Cordaro M, Peritore AF, Gugliandolo E, Crupi R, Trovato Salinaro A, Raffone E, Impellizzeri D, et al. Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis. International Journal of Molecular Sciences. 2021; 22(11):5998. https://doi.org/10.3390/ijms22115998
Chicago/Turabian StyleGenovese, Tiziana, Rosalba Siracusa, Ramona D’Amico, Marika Cordaro, Alessio Filippo Peritore, Enrico Gugliandolo, Rosalia Crupi, Angela Trovato Salinaro, Emanuela Raffone, Daniela Impellizzeri, and et al. 2021. "Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis" International Journal of Molecular Sciences 22, no. 11: 5998. https://doi.org/10.3390/ijms22115998
APA StyleGenovese, T., Siracusa, R., D’Amico, R., Cordaro, M., Peritore, A. F., Gugliandolo, E., Crupi, R., Trovato Salinaro, A., Raffone, E., Impellizzeri, D., Cuzzocrea, S., Fusco, R., & Di Paola, R. (2021). Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis. International Journal of Molecular Sciences, 22(11), 5998. https://doi.org/10.3390/ijms22115998












