MAPKs Are Highly Abundant but Do Not Contribute to α1-Adrenergic Contraction of Rat Saphenous Arteries in the Early Postnatal Period
Abstract
1. Introduction
2. Results
3. Discussion
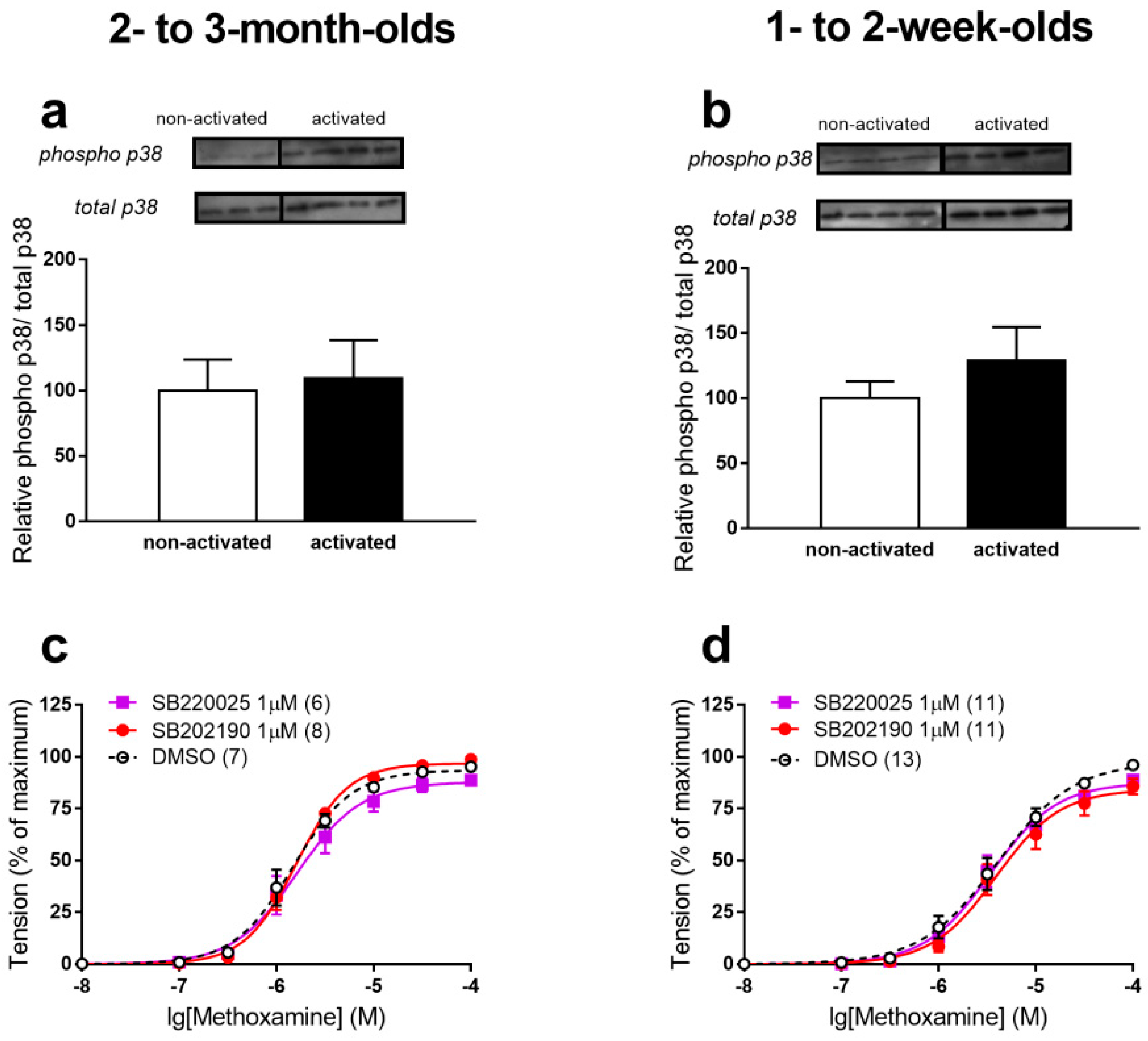
3.1. p38 MAPK Does Not Regulate α1-Adrenergic Contractions of Saphenous Arteries at Both Ages
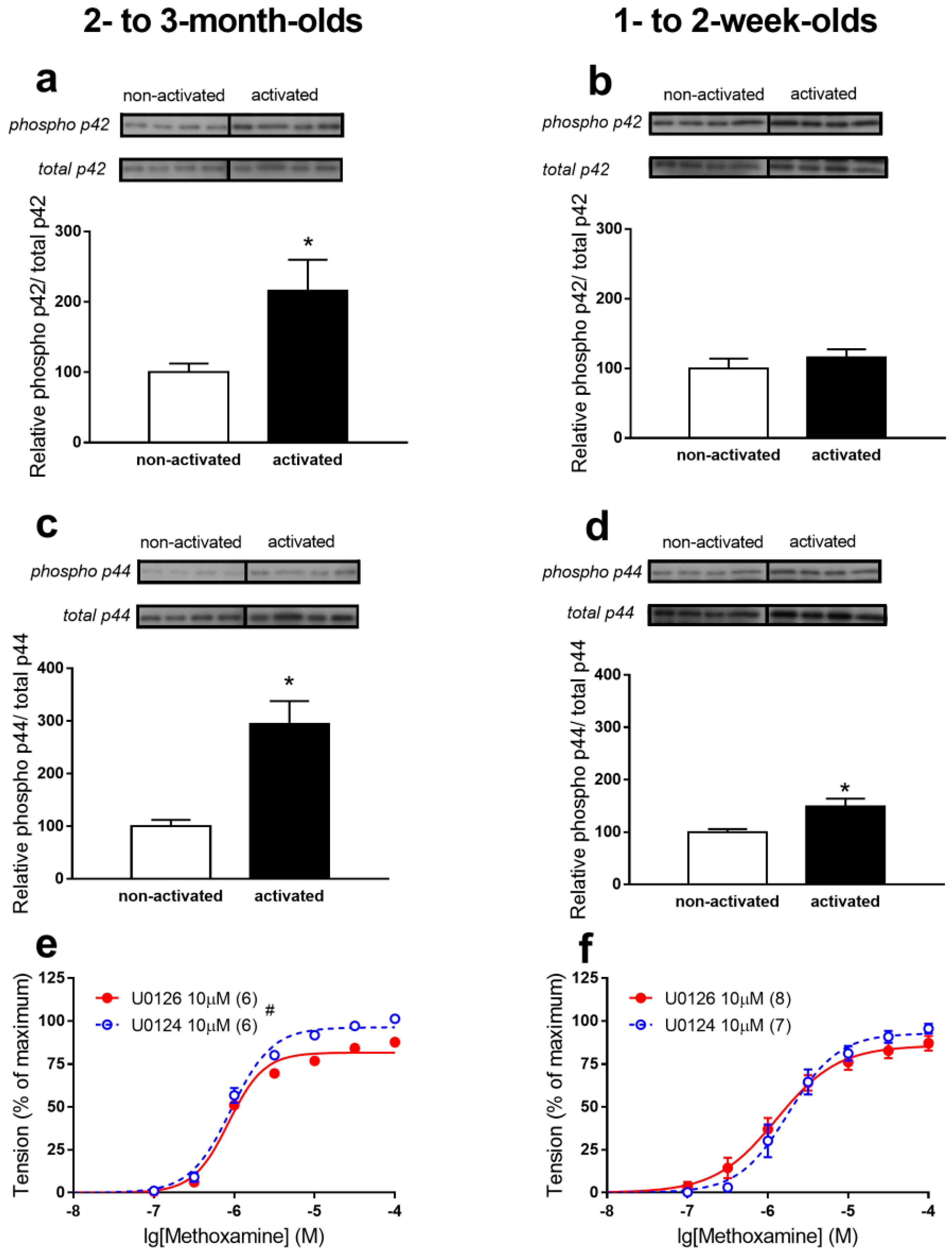
3.2. p42/44 MAPK Regulates α1-Adrenergic Contractile Responses of Saphenous Arteries of 2- to 3-Month-Old, but Not 1- to 2-Week-Old Rats
4. Materials and Methods
4.1. Animals
4.2. Wire Myography
4.3. Western Blotting
4.4. Solutions
- (1)
- Solution for vessel isolation, in mM: 145 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1 MgSO4, 0.1 CaCl2, 0.025 EDTA and 5 HEPES.
- (2)
- Solution for myograph experiments, in mM: 120 NaCl, 26 NaHCO3, 4.5 KCl, 1.2 NaH2PO4, 1.0 MgSO4, 1.6 CaCl2, 5.5 glucose, 0.025 EDTA and 5 HEPES; equilibrated with 5% CO2 in 95% O2.
- (3)
- SDS-buffer: 10% water-free glycerin, 1% SDS, 0.0625 mM tris-HCl, 5% β-mercaptoethanol and 0.05% bromphenol blue.
- (4)
- TBST: 20 mM tris-HCl, 20 mM NaCl, 0.1% Tween 20, pH 7.6.
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sofronova, S.I.; Borzykh, A.A.; Gaynullina, D.K.; Kuzmin, I.V.; Shvetsova, A.A.; Lukoshkova, E.V.; Tarasova, O.S. Endothelial nitric oxide weakens arterial contractile responses and reduces blood pressure during early postnatal development in rats. Nitric. Oxide Biol. Chem. 2016, 55–56, 1–9. [Google Scholar] [CrossRef]
- Kasparov, S.; Paton, J. Changes in baroreceptor vagal reflex performance in the developing rat. Pflügers Arch. Eur. J. Physiol. 1997, 434, 438–444. [Google Scholar] [CrossRef]
- Puzdrova, V.A.; Kudryashova, T.V.; Gaynullina, D.K.; Mochalov, S.V.; Aalkjaer, C.; Nilsson, H.; Vorotnikov, A.V.; Schubert, R.; Tarasova, O.S. Trophic action of sympathetic nerves reduces arterial smooth muscle Ca2+ sensitivity during early post-natal development in rats. Acta Physiol. 2014, 212, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Gaynullina, D.; Lubomirov, L.T.; Sofronova, S.I.; Kalenchuk, V.U.; Gloe, T.; Pfitzer, G.; Tarasova, O.S.; Schubert, R. Functional remodelling of arterial endothelium during early postnatal development in rats. Cardiovasc. Res. 2013, 99, 612–621. [Google Scholar] [CrossRef]
- Akopov, S.E.; Zhang, L.; Pearce, W.J. Maturation alters the contractile role of calcium in ovine basilar arteries. Pediatr. Res. 1998, 44, 154–160. [Google Scholar] [CrossRef]
- Akopov, S.E.; Zhang, L.; Pearce, W.J. Regulation of Ca2+ sensitization by PKC and rho proteins in ovine cerebral arteries: Effects of artery size and age. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Klemke, R.L.; Cai, S.; Giannini, A.L.; Gallagher, P.J.; De Lanerolle, P.; Cheresh, D.A. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 1997, 137, 481–492. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Bae, Y.M.; Cho, S.I.; Kwon, S.C.; Jung, J.Y.; Park, J.C.; Ahn, H.Y. p38 Mitogen-Activated Protein Kinase Contributes to the Diminished Aortic Contraction by Endothelin-1 in DOCA-Salt Hypertensive Rats. Hypertension 2004, 43, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Dessy, C.; Kim, I.; Sougnez, C.L.; Laporte, R.; Morgan, K.G. A role for MAP kinase in differentiated smooth muscle contraction evoked by α-adrenoceptor stimulation. Am. J. Physiol. Cell Physiol. 1998, 275, 1081–1086. [Google Scholar] [CrossRef]
- Ihara, E.; Moffat, L.; Ostrander, J.; Walsh, M.P.; MacDonald, J.A. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, 699–710. [Google Scholar] [CrossRef]
- Kwon, S.; Fang, L.H.; Kim, B.; Ha, T.S.; Lee, S.J.; Ahn, H.Y. P38 Mitogen-Activated Protein Kinase Regulates Vasoconstriction in Spontaneously Hypertensive Rats. J. Pharmacol. Sci. 2004, 95, 267–272. [Google Scholar] [CrossRef]
- Watanabe, S.; Matsumoto, T.; Ando, M.; Adachi, T.; Kobayashi, S.; Iguchi, M.; Takeuchi, M.; Taguchi, K.; Kobayashi, T. Multiple activation mechanisms of serotonin-mediated contraction in the carotid arteries obtained from spontaneously hypertensive rats. Pflugers Arch. Eur. J. Physiol. 2016, 468, 1271–1282. [Google Scholar] [CrossRef]
- Matsumoto, T.; Watanabe, S.; Taguchi, K.; Kobayashi, T. Mechanisms underlying increased serotonin-induced contraction in carotid arteries from chronic type 2 diabetic Goto-Kakizaki rats. Pharmacol. Res. 2014, 87, 123–132. [Google Scholar] [CrossRef]
- Santiago, E.; Contreras, C.; García-Sacristán, A.; Sánchez, A.; Rivera, L.; Climent, B.; Prieto, D. Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic. Biol. Med. 2013, 60, 136–146. [Google Scholar] [CrossRef]
- Srinivasan, R.; Forman, S.; Quinlan, R.A.; Ohanian, J.; Ohanian, V. Regulation of contractility by Hsp27 and Hic-5 in rat mesenteric small arteries. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Zaluski, J.; Banes-Berceli, A. Molecular interactions of serotonin (5-HT) and endothelin-1 in vascular smooth muscle cells: In vitro and ex vivo analyses. Am. J. Physiol. Cell Physiol. 2014, 306, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.M.; Hayashi, Y.; Nguyen, S.V.; Nguyen, T.M.; Purdy, R.E. Hindlimb unweighting induces changes in the p38MAPK contractile pathway of the rat abdominal aorta. J. Appl. Physiol. 2009, 107, 121–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spurrell, B.E.; Murphy, T.V.; Hill, M.A. Intraluminal pressure stimulates MAPK phosphorylation in arterioles: Temporal dissociation from myogenic contractile response. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, 1764–1773. [Google Scholar] [CrossRef]
- Pearce, W.J.; Williams, J.M.; Chang, M.M.; Gerthoffer, W.T. ERK inhibition attenuates 5-HT-induced contractions in fetal and adult ovine carotid arteries. Arch. Physiol. Biochem. 2003, 111, 36–44. [Google Scholar] [CrossRef]
- Goyal, R.; Mittal, A.; Chu, N.; Zhang, L.; Longo, L.D. A1-Adrenergic Receptor Subtype Function in Fetal and Adult Cerebral Arteries. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Long, W.; Zhang, L.; Longo, L.D. Extracellular signal-regulated kinases and contractile responses in ovine adult and fetal cerebral arteries. J. Physiol. 2003, 551, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Bellik, L.; Vinci, M.C.; Filippi, S.; Ledda, F.; Parenti, A. Intracellular pathways triggered by the selective FLT-1-agonist placental growth factor in vascular smooth muscle cells exposed to hypoxia. Br. J. Pharmacol. 2005, 146, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Kanaji, N.; Nelson, A.; Allen-Gipson, D.S.; Sato, T.; Nakanishi, M.; Wang, X.; Li, Y.; Basma, H.; Michalski, J.; Farid, M.; et al. The p38 mitogen-activated protein kinases modulate endothelial cell survival and tissue repair. Inflamm. Res. 2012, 61, 233–244. [Google Scholar] [CrossRef]
- Meloche, S.; Landry, J.; Huot, J.; Houle, F.; Marceau, F.; Giasson, E. p38 MAP kinase pathway regulates angiotensin II-induced contraction of rat vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, 741–751. [Google Scholar] [CrossRef]
- Massett, M.P.; Ungvari, Z.; Csiszar, A.; Kaley, G.; Koller, A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, 2282–2287. [Google Scholar] [CrossRef][Green Version]
- Mochalov, S.V.; Tarasova, N.V.; Kudryashova, T.V.; Gaynullina, D.K.; Kalenchuk, V.U.; Borovik, A.S.; Vorotnikov, A.V.; Tarasova, O.S.; Schubert, R. Higher Ca2+-sensitivity of arterial contraction in 1-week-old rats is due to a greater Rho-kinase activity. Acta Physiol. 2018, 223, 1–15. [Google Scholar] [CrossRef]
- Mulvany, M.J.; Halpern, W. Contractile Properties of Small Arterial Resistance Vessels in Spontaneously Hypertensive and Normotensive Rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef]
- Ricard, N.; Zhang, J.; Zhuang, Z.W.; Simons, M. Isoform-Specific Roles of ERK1 and ERK2 in Arteriogenesis. Cells 2019, 9, 38. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaynullina, D.K.; Kudryashova, T.V.; Vorotnikov, A.V.; Schubert, R.; Tarasova, O.S. MAPKs Are Highly Abundant but Do Not Contribute to α1-Adrenergic Contraction of Rat Saphenous Arteries in the Early Postnatal Period. Int. J. Mol. Sci. 2021, 22, 6037. https://doi.org/10.3390/ijms22116037
Gaynullina DK, Kudryashova TV, Vorotnikov AV, Schubert R, Tarasova OS. MAPKs Are Highly Abundant but Do Not Contribute to α1-Adrenergic Contraction of Rat Saphenous Arteries in the Early Postnatal Period. International Journal of Molecular Sciences. 2021; 22(11):6037. https://doi.org/10.3390/ijms22116037
Chicago/Turabian StyleGaynullina, Dina K., Tatiana V. Kudryashova, Alexander V. Vorotnikov, Rudolf Schubert, and Olga S. Tarasova. 2021. "MAPKs Are Highly Abundant but Do Not Contribute to α1-Adrenergic Contraction of Rat Saphenous Arteries in the Early Postnatal Period" International Journal of Molecular Sciences 22, no. 11: 6037. https://doi.org/10.3390/ijms22116037
APA StyleGaynullina, D. K., Kudryashova, T. V., Vorotnikov, A. V., Schubert, R., & Tarasova, O. S. (2021). MAPKs Are Highly Abundant but Do Not Contribute to α1-Adrenergic Contraction of Rat Saphenous Arteries in the Early Postnatal Period. International Journal of Molecular Sciences, 22(11), 6037. https://doi.org/10.3390/ijms22116037





