Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes
Abstract
1. Introduction
2. Results
2.1. Effect of Compounds on Keratinocyte Viability
2.2. Effect of Compounds on Phagocytosis of FluoSphere Beads by Keratinocytes
2.3. Effect of Compounds on ADM and ET-1 Protein Levels in Keratinocytes
2.4. Effects of Compounds on Viability of HEMn-DP Cells
2.5. Effects of Compounds on Melanin Synthesis in HEMn-DP Cells
2.6. Effects of Compounds on Dendricity in HEMn-DP Cells
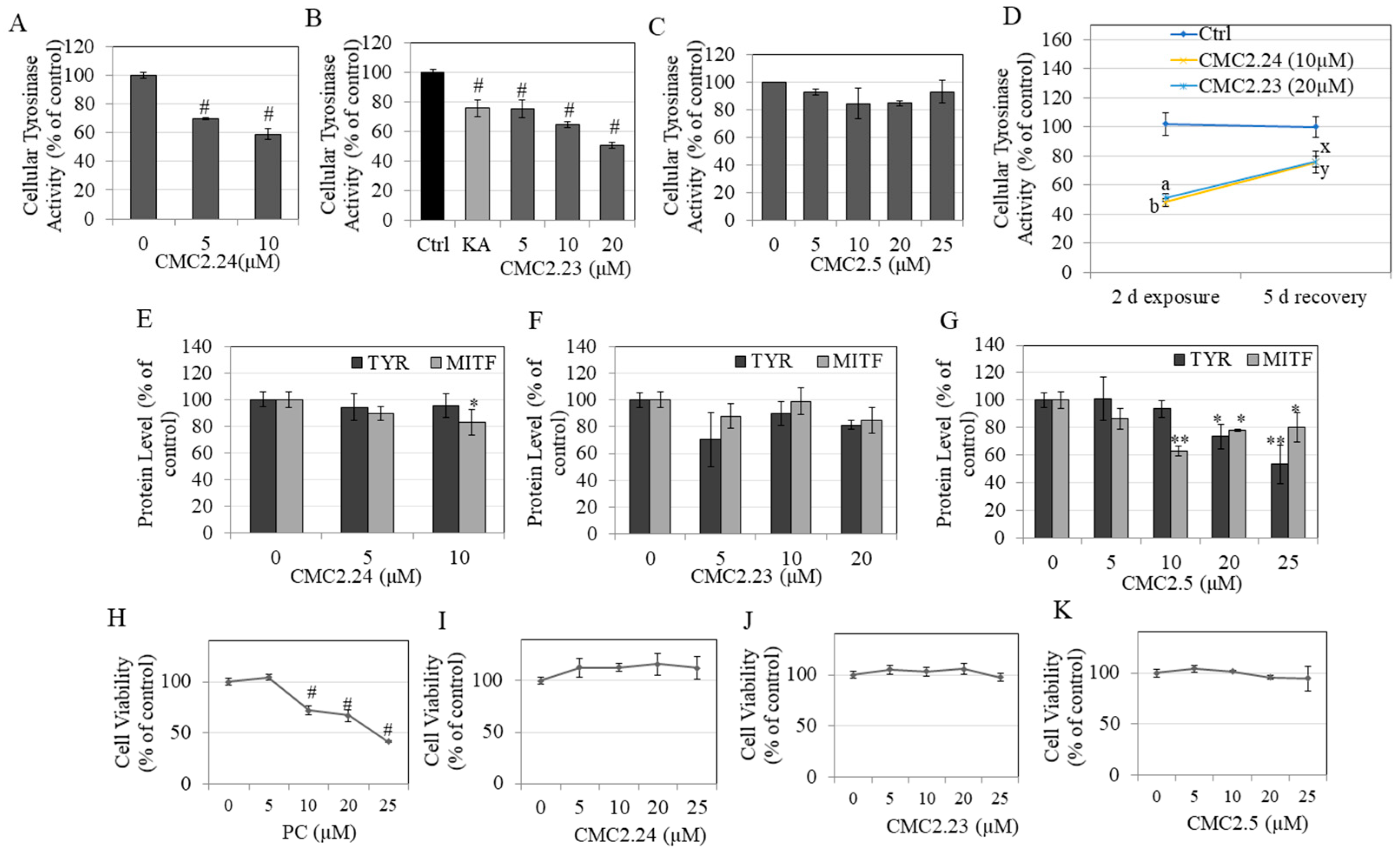
2.7. Effects of Compounds on Intracellular Tyrosinase Activity in HEMn-DP Cells
2.8. Recovery Study of Tyrosinase Activity in HEMn-DP Cells
2.9. Tyrosinase and MITF Protein Levels in HEMn-DP Cells
2.10. Effects of Compounds on Viability of Human Dermal Fibroblasts
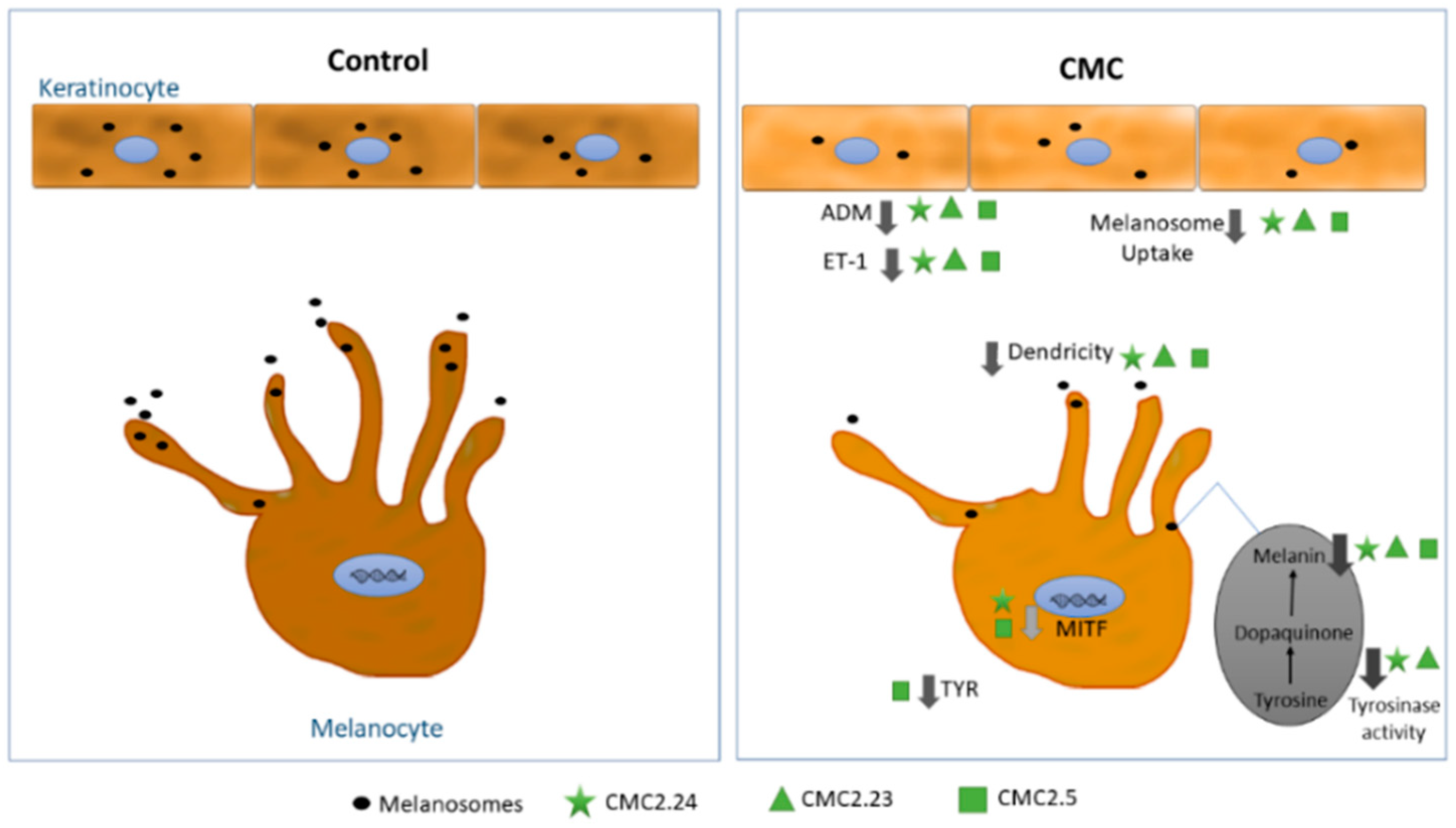
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTS Cytotoxicity Assay
4.4. Phagocytosis Assay in HaCaT Cells
4.5. Estimation of Adrenomedullin (ADM) Protein Levels in HaCaT Cells
4.6. Estimation of Endothelin-1 (ET-1) Protein Levels in HaCaT Cells
4.7. Estimation of Melanin Levels in HEMn-DP Cells
4.8. Quantitation of Dendricity in HEMn-DP Cells
4.9. Determination of Tyrosinase Activity in HEMn-DP Cells
4.10. Recovery of Tyrosinase Activity in HEMn-DP Cells
4.11. Estimation of MITF and TYR Protein Levels in HEMn-DP Cells
4.12. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hearing, V.J. Biochemical Control of Melanogenesis and Melanosomal Organization. J. Investig. Dermatol. Symp. Proc. 1999, 4, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Bae-Harboe, Y.-S.C.; Park, H.-Y. Tyrosinase: A Central Regulatory Protein for Cutaneous Pigmentation. J. Investig. Dermatol. 2012, 132, 2678–2680. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Breathnach, A.S. The Epidermal Melanin Unit System. Dermatol. Wochenschr. 1963, 147, 481–489. [Google Scholar] [PubMed]
- Ando, H.; Niki, Y.; Ito, M.; Akiyama, K.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J. Investig. Dermatol. 2012, 132, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hammer, J.A. Melanosome transfer: It is best to give and receive. Curr. Opin. Cell Biol. 2014, 29, 1–7. [Google Scholar] [CrossRef]
- Rao, G.V.; Gopalakrishnan, M.; Madhavi, M.; Mukhopadhyay, T.; Thanusu, J.; Ezhilarasi, M. Dendrite elongation inhibitor from artocarpus altilis parkinson. J. Pharm. Res. 2013, 7, 358–361. [Google Scholar] [CrossRef]
- Krishnamoorthy, J.; Ranjith, M.; Gokulshankar, S. Extract combinations of curcuma zedoaria and aloe vera inhibit melanin synthesis and dendrite formation in murine melanoma cells. J. Appl. Cosmetol. 2010, 28, 103–108. [Google Scholar]
- Kim, J.H.; Baek, E.J.; Lee, E.J.; Yeom, M.H.; Park, J.S.; Lee, K.W.; Kang, N.J. Ginsenoside F1 Attenuates hyperpigmentation in b16f10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp. Dermatol. 2015, 24, 150–152. [Google Scholar] [CrossRef]
- Ito, Y.; Kanamaru, A.; Tada, A. Centaureidin promotes dendrite retraction of melanocytes by activating Rho. Biochim. Biophys. Acta 2006, 1760, 487–494. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Cmt-308, a nonantimicrobial chemically-modified tetracycline, exhibits anti-melanogenic activity by suppression of melanosome export. Biomedicines 2020, 8, 411. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Inhibitory effects of the bioactive thermorubin isolated from the fungus thermoactinomyces antibioticus on melanogenesis. Cosmetics 2020, 7, 61. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Asoprisnil, A Selective progesterone receptor modulator (Sprm), inhibits melanosome export in B16f10 cells and Hemn-Dp melanocytes. Molecules 2020, 25, 3581. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocr. Rev. 2000, 21, 138–167. [Google Scholar] [CrossRef]
- Albertin, G.; Carraro, G.; Parnigotto, P.P.; Conconi, M.T.; Ziolkowska, A.; Malendowicz, L.K.; Nussdorfer, G.G. Human skin keratinocytes and fibroblasts express adrenomedullin and its receptors, and adrenomedullin enhances their growth in vitro by stimulating proliferation and inhibiting apoptosis. Int. J. Mol. Med. 2003, 11, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T.; Miwa, T.; Mochizuki, M.; Toritsuka, M.; Sakata, A.; Ito, M. Adrenomedullin: A novel melanocyte dendrite branching factor. J. Dermatol. Sci. 2015, 79, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef]
- Yohn, J.J.; Morelli, J.G.; Walchak, S.J.; Rundell, K.B.; Norris, D.A.; Zamora, M.R. Cultured human keratinocytes synthesize and secrete Endothelin-1. J. Investig. Dermatol. 1993, 100, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Yaar, M.; Gilchrest, B.A. Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J. Investig. Dermatol. 1995, 105, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Kobayashi, T.; Miyagishi, M.; Higashi, K.; Yada, Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment. Cell Res. 1997, 10, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Miyagishi, M.; Yada, Y. Endothelin-1 as a new Melanogen: Coordinated expression of its gene and the tyrosinase gene in Uvb-exposed Human epidermis. J. Investig. Dermatol. 1995, 105, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, W.; Yuan, X.; Li, D.; Gu, W.; Gao, T. Endothelin-1 enhances the melanogenesis via Mitf-Gpnmb pathway. BMB Rep. 2013, 46, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, A.; Kobayashi, A.; Yoshida, Y.; Kitahara, T.; Takema, Y.; Imokawa, G. Biphasic Expression of two paracrine melanogenic cytokines, stem cell factor and Endothelin-1, in ultraviolet B-Induced human melanogenesis. Am. J. Pathol. 2004, 165, 2099–2109. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Kimura, M. Signalling Mechanisms of endothelin-induced mitogenesis and melanogenesis in human melanocytes. Biochem. J. 1996, 314, 305–312. [Google Scholar] [CrossRef]
- Regazzetti, C.; De Donatis, G.M.; Ghorbel, H.H.; Cardot-Leccia, N.; Ambrosetti, D.; Bahadoran, P.; Chignon-Sicard, B.; Lacour, J.-P.; Ballotti, R.; Mahns, A. Endothelial cells promote pigmentation through endothelin receptor B activation. J. Investig. Dermatol. 2015, 135, 3096–3104. [Google Scholar] [CrossRef] [PubMed]
- Teraki, E.; Tajima, S.; Manaka, I.; Kawashima, M.; Miyaclshi, M.; Imokawa, G. Role of Endothelin-1 In hyperpigmentation in seborrhoeic keratosis. Br. J. Dermatol. 1996, 135, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Kadono, S.; Manaka, I.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J. Investig. Dermatol. 2001, 116, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Manaka, I.; Kadono, S.; Kawashima, M.; Kobayashi, T.; Imokawa, G. The mechanism of hyperpigmentation in seborrhoeic keratosis involves the high expression of Endothelin-converting Enzyme-1α And Tnf-A, which stimulate secretion of Endothelin 1. Br. J. Dermatol. 2001, 145, 895–903. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovansky, J. “Transcription Physiology” of pigment formation in melanocytes: Central role of Mitf. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Widlund, H.R.; Fisher, D.E. Microphthalamia-associated transcription factor: A critical regulator of pigment cell development and survival. Oncogene 2003, 22, 3035–3041. [Google Scholar] [CrossRef]
- Chiaverini, C.; Beuret, L.; Flori, E.; Busca, R.; Abbe, P.; Bille, K.; Bahadoran, P.; Ortonne, J.P.; Bertolotto, C.; Ballotti, R. Microphthalmia-associated transcription factor regulates Rab27a gene expression and controls melanosome transport. J. Biol. Chem. 2008, 283, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- García-Gavín, J.; González-Vilas, D.; Fernández-Redondo, V.; Toribio, J. Pigmented contact dermatitis due to Kojic acid. A Paradoxical side effect of a skin lightener. Contact Dermat. 2010, 62, 63–64. [Google Scholar] [CrossRef] [PubMed]
- Kooyers, T.; Westerhof, W. Toxicology and health risks of hydroquinone in skin lightening formulations. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 777–780. [Google Scholar] [CrossRef]
- Nistico, S.P.; Tolone, M.; Zingoni, T.; Tamburi, F.; Scali, E.; Bennardo, L.; Cannarozzo, G. A new 675 nm laser device in the treatment of melasma: Results of a prospective observational study. Photobiomodul. Photomed. Laser Surg. 2020, 38, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, M.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing boswellia serrata, curcuma longa, and vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, E12842. [Google Scholar] [CrossRef]
- Jang, J.Y.; Lee, J.H.; Jeong, S.Y.; Chung, K.T.; Choi, Y.H.; Choi, B.T. Partially purified curcuma longa inhibits alpha-melanocyte-stimulating hormone-stimulated melanogenesis through extracellular signal-regulated kinase or akt activation-mediated signalling In B16f10 cells. Exp. Dermatol. 2009, 18, 689–694. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.Y.; Park, C.; Kim, B.W.; Choi, Y.H.; Choi, B.T. Curcumin suppresses alpha-melanocyte stimulating hormone-stimulated melanogenesis in B16f10 cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar] [CrossRef]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Aromatic-turmerone inhibits Alpha-Msh and Ibmx-induced melanogenesis by inactivating Creb and Mitf signaling pathways. Arch. Dermatol. Res. 2011, 303, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Nakata, A.; Yamasaki, F.; Abas, F.; Shaari, K.; Lajis, N.H.; Morita, H. Curcumin-like diarylpentanoid analogues as melanogenesis inhibitors. J. Nat. Med. 2012, 66, 166–176. [Google Scholar] [CrossRef]
- Wolnicka-Glubisz, A.; Nogal, K.; Żądło, A.; Płonka, P.M. Curcumin does not switch melanin synthesis towards pheomelanin in B16f10 cells. Arch. Dermatol. Res. 2015, 307, 89–98. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Goenka, S.; Johnson, F.; Simon, S.R. Novel chemically modified curcumin (CMC) derivatives inhibit tyrosinase activity and melanin synthesis in B16f10 mouse melanoma cells. Biomolecules 2021, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viennet, C.; Robin, S.; Berthon, J.Y.; He, L.; Humbert, P. Precise role of dermal fibroblasts on melanocyte pigmentation. J. Dermatol. Sci. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: Impact of photo-aging. PLoS ONE 2014, 9, E114182. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Gerwat, W.; Batzer, J.; Eggers, K.; Scherner, C.; Wenck, H.; Stäb, F.; Hearing, V.J.; Röhm, K.-H.; Kolbe, L. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J. Investig. Dermatol. 2018, 138, 1601–1608. [Google Scholar] [CrossRef]
- Chawla, S.; Delong, M.A.; Visscher, M.O.; Wickett, R.R.; Manga, P.; Boissy, R.E. Mechanism of tyrosinase inhibition by deoxyarbutin and its second-generation derivatives. Br. J. Dermatol. 2008, 159, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Virador, V.M.; Muller, J.; Wu, X.; Abdel-Malek, Z.A.; Yu, Z.-X.; Ferrans, V.J.; Kobayashi, N.; Wakamatsu, K.; Ito, S.; Hammer, J.A. Influence Of A-Melanocyte-stimulating hormone and of ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Lorenz, P.; Petersen, R.; Heldermann, M.; Borchert, S. Skin-lightening agent with different pathways of action on melanogenesis. SOFW J. 2005, 131, 40. [Google Scholar]
- Tu, C.X.; Lin, M.; Lu, S.S.; Qi, X.Y.; Zhang, R.X.; Zhang, Y.Y. Curcumin inhibits melanogenesis in human melanocytes. Phytother. Res. 2012, 26, 174–179. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Ito, Y.; Kanamaru, A.; Tada, A. Effects of methylophiopogonanone B on melanosome transfer and dendrite retraction. J. Dermatol. Sci. 2006, 42, 68–70. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, T.; Takema, Y.; Imokawa, G. Biochemical Characterization of endothelin-converting Enzyme-1α in cultured skin-derived cells and its postulated role in the stimulation of melanogenesis in human epidermis. J. Biol. Chem. 2002, 277, 5395–5403. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Lee, H.M.; Hambardjieva, E.; Vrankova, K.; Golub, L.M.; Johnson, F. Design, synthesis and biological activity of new polyenolic inhibitors of matrix metalloproteinases: A focus on chemically-modified curcumins. Curr. Med. Chem. 2012, 19, 4348–4358. [Google Scholar] [CrossRef] [PubMed]
- Yajima, I.; Kumasaka, M.Y.; Iida, M.; Oshino, R.; Tanihata, H.; Al Hossain, A.; Ohgami, N.; Kato, M. Arsenic-mediated hyperpigmentation in skin via Nf-Kappa B/Endothelin-1 signaling in an originally developed hairless mouse model. Arch. Toxicol. 2017, 91, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Elburki, M.S.; Rossa, C.; Guimarães-Stabili, M.R.; Lee, H.-M.; Curylofo-Zotti, F.A.; Johnson, F.; Golub, L.M. A Chemically modified curcumin (Cmc 2.24) Inhibits nuclear factor κb activation and inflammatory bone loss in murine models of lps-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation 2017, 40, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-W.; Ni, Y.-J.; Tong, X.-Y.; Guo, X.; Wu, X.-P. Activation of Vegf receptors in response to Uvb promotes cell proliferation and melanogenesis of normal human melanocytes. Exp. Cell Res. 2020, 387, 111798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-W.; Ni, Y.-J.; Tong, X.-Y.; Guo, X.; Wu, X.-P.; Lu, Z.-F. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting Vegf receptors. Int. J. Med. Sci. 2020, 17, 903. [Google Scholar] [CrossRef]
- Zhu, J.-W.; Ni, Y.-J.; Tong, X.-Y.; Guo, X.; Wu, X.-P.; Lu, Z.-F. Inhibition of Vegf Production by Novel Chemically Modified Curcumin in Oral Squamous Cell Carcinoma. In Proceedings of the 42nd Annual Northeast Bioengineering Conference, Binghamton University, Vestal, NJ, USA, 5–7 April 2016. [Google Scholar]
- Kothari, M.; Simon, S.R. Chemically modified tetracyclines inhibit Vegf Secretion by breast cancer cell lines. Cytokine 2006, 35, 115–125. [Google Scholar] [CrossRef]
- Cardinali, G.; Bolasco, G.; Aspite, N.; Lucania, G.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J. Investig. Dermatol. 2008, 128, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Ceccarelli, S.; Kovacs, D.; Aspite, N.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Keratinocyte growth factor promotes melanosome transfer to keratinocytes. J. Investig. Dermatol. 2005, 125, 1190–1199. [Google Scholar] [CrossRef]
- Kondo, S.; Sauder, D.N.; Kono, T.; Galley, K.A.; Mckenzie, R.C. Differential modulation of Interleukin-1α (Il-1α) and interleukin-1β(Il-1β in human epidermal keratinocytes by Uvb. Exp. Dermatol. 1994, 3, 29–39. [Google Scholar] [CrossRef]
- Qin, Z.; Okubo, T.; Voorhees, J.J.; Fisher, G.J.; Quan, T. Elevated cysteine-rich protein 61 (Ccn1) promotes skin aging via upregulation of Il-1β in chronically sun-exposed human skin. Age 2014, 36, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Keller, M.; Niklaus, G.; Hohl, D.; Werner, S.; Beer, H.-D. The Inflammasome mediates Uvb-induced activation and secretion of interleukin-1β By keratinocytes. Curr. Biol. 2007, 17, 1140–1145. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goenka, S.; Simon, S.R. Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes. Int. J. Mol. Sci. 2021, 22, 6043. https://doi.org/10.3390/ijms22116043
Goenka S, Simon SR. Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes. International Journal of Molecular Sciences. 2021; 22(11):6043. https://doi.org/10.3390/ijms22116043
Chicago/Turabian StyleGoenka, Shilpi, and Sanford R. Simon. 2021. "Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes" International Journal of Molecular Sciences 22, no. 11: 6043. https://doi.org/10.3390/ijms22116043
APA StyleGoenka, S., & Simon, S. R. (2021). Novel Chemically Modified Curcumin (CMC) Analogs Exhibit Anti-Melanogenic Activity in Primary Human Melanocytes. International Journal of Molecular Sciences, 22(11), 6043. https://doi.org/10.3390/ijms22116043





