Association of IgG1 Antibody Clearance with FcγRIIA Polymorphism and Platelet Count in Infliximab-Treated Patients
Abstract
1. Introduction
2. Results
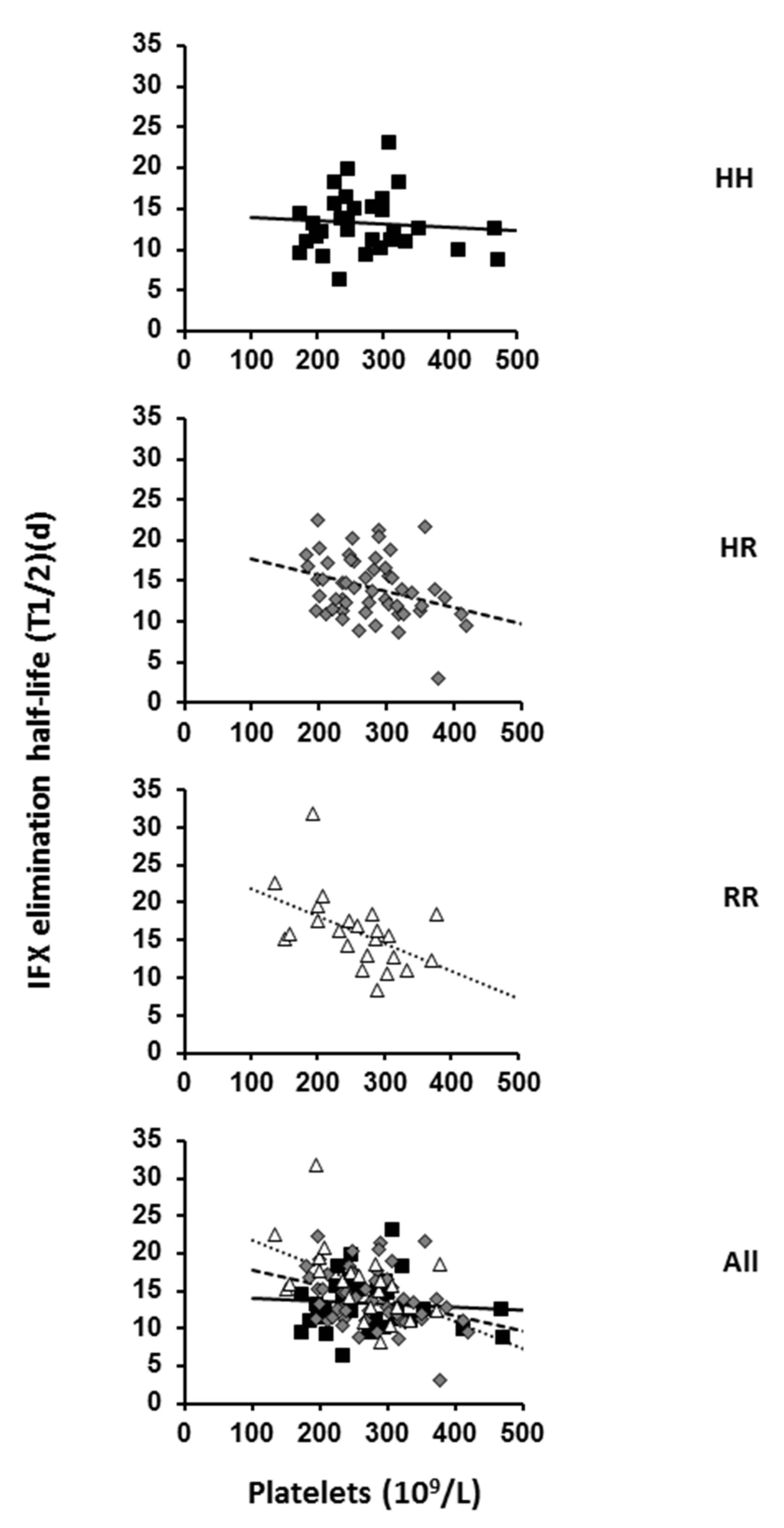
2.1. Effect of FcγRIIA Polymorphism and Platelet Count on IFX Pharmacokinetics
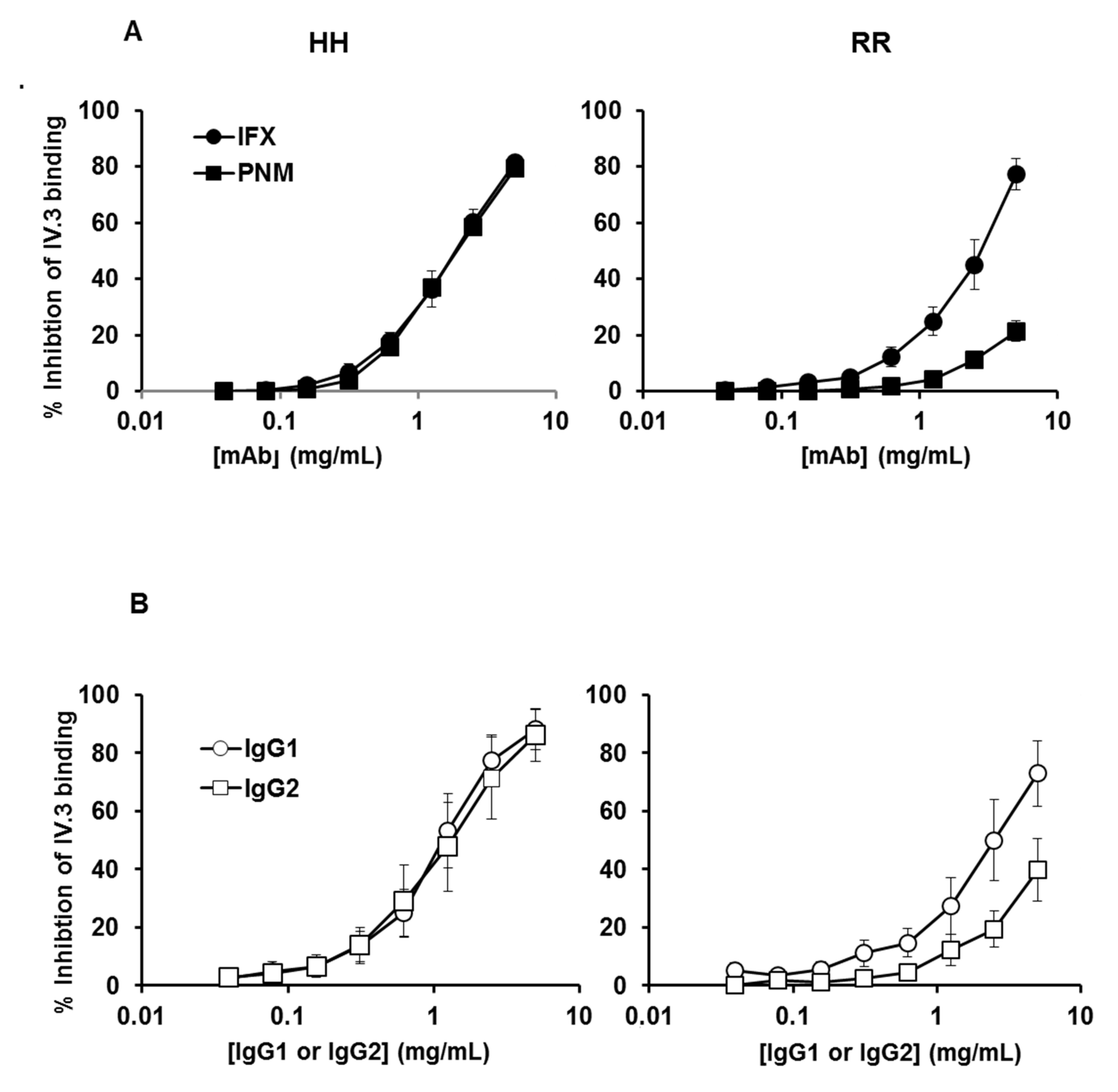
2.2. Binding of IFX, Panitumumab, or Myeloma IgG1 and IG2 to Platelet FcγRIIA-131H and FcγRIIA-131R
3. Discussion
4. Materials and Methods
4.1. Patients and Biological Analyses
4.2. Pharmacokinetics Analysis
4.3. Antibodies
4.4. Washed Platelet Preparation
4.5. Flow Cytometry Assay of mAb or Myeloma IgG Binding to Platelet FcγRIIA
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simister, N.E.; Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nat. Cell Biol. 1989, 337, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Morell, A.; Terry, W.D.; Waldmann, T.A. Metabolic properties of IgG subclasses in man. J. Clin. Investig. 1970, 49, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, N.M.; Einarsdóttir, H.K.; Stemerding, A.M.; Vidarsson, G. The multiple facets of FcRn in immunity. Immunol. Rev. 2015, 268, 253–268. [Google Scholar] [CrossRef]
- Stapleton, N.M.; Andersen, J.T.; Stemerding, A.M.; Bjarnarson, S.P.; Verheul, R.C.; Gerritsen, J.; Zhao, Y.; Kleijer, M.; Sandlie, I.; de Haas, M.; et al. Competition for FcRn mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2011, 2, 599–608. [Google Scholar] [CrossRef]
- Ghetie, V.; Ward, E.S. Transcytosis and Catabolism of Antibody. Immunol. Res. 2002, 25, 097–114. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Ward, E.S.; Ober, R.J. Chapter 4 Multitasking by Exploitation of Intracellular Transport Functions: The many faces of FcRn. Adv. Immunol. 2009, 103, 77–115. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Brambell, F.W.; Hemmings, W.A.; Morris, I.G. A theoretical model of gamma-globulin catabolism. Nature 1964, 203, 1352–1354. [Google Scholar] [CrossRef]
- Ghetie, V.; Hubbard, J.G.; Kim, J.K.; Tsen, M.F.; Lee, Y.; Ward, E.S. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 1996, 26, 690–696. [Google Scholar] [CrossRef]
- Junghans, R.P.; Anderson, C.L. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Challa, D.K.; Wang, X.; Montoyo, H.P.; Velmurugan, R.; Ober, R.J.; Ward, E.S. Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis. mAbs 2019, 11, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, N.M.; Brinkhaus, M.; Armour, K.L.; Bentlage, A.E.H.; De Taeye, S.W.; Temming, A.R.; Mok, J.Y.; Brasser, G.; Maas, M.; Van Esch, W.J.E.; et al. Reduced FcRn-mediated transcytosis of IgG2 due to a missing Glycine in its lower hinge. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.; Brachet, G.; Watier, H. Evolutionary Story of the Low/Medium-Affinity IgG Fc Receptor Gene Cluster. Front. Immunol. 2019, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, S.L.; Looney, R.J.; Leddy, J.P.; Phipps, D.C.; Abraham, G.N.; Anderson, C.L. Human Platelet Fc Receptor for Im-munoglobulin G Identification as a 40,000-Molecular-Weight Membrane Protein Shared by Monocytes. J. Clin. Investig. 1985, 76, 2317–2322. [Google Scholar] [CrossRef] [PubMed]
- Unkeless, J.C. Function and heterogeneity of human Fc receptors for immunoglobulin G. J. Clin. Investig. 1989, 83, 355–361. [Google Scholar] [CrossRef]
- Pan, L.F.; Kreisle, R.A.; Shi, Y.D. Detection of Fcgamma receptors on human endothelial cells stimulated with cytokines tumour necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma). Clin. Exp. Immunol. 1998, 112, 533–538. [Google Scholar] [CrossRef]
- Devaraj, S.; Du Clos, T.W.; Jialal, I. Binding and Internalization of C-Reactive Protein by Fcgamma Receptors on Human Aortic Endothelial Cells Mediates Biological Effects. Arter. Thromb. Vasc. Biol. 2005, 25, 1359–1363. [Google Scholar] [CrossRef]
- Raaz-Schrauder, D.; Ekici, A.B.; Klinghammer, L.; Stumpf, C.; Achenbach, S.; Herrmann, M.; Reis, A.; Garlichs, C.D. The proinflammatory effect of C-reactive protein on human endothelial cells depends on the FcγRIIa genotype. Thromb. Res. 2014, 133, 426–432. [Google Scholar] [CrossRef]
- Strauss, O.; Phillips, A.; Ruggiero, K.; Bartlett, A.; Dunbar, P.R. Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci. Rep. 2017, 7, 44356. [Google Scholar] [CrossRef]
- Berlacher, M.D.; Vieth, J.A.; Heflin, B.C.; Gay, S.R.; Antczak, A.J.; Tasma, B.E.; Boardman, H.J.; Singh, N.; Montel, A.H.; Kahaleh, M.B.; et al. FcγRIIa Ligation Induces Platelet Hypersensitivity to Thrombotic Stimuli. Am. J. Pathol. 2013, 182, 244–254. [Google Scholar] [CrossRef]
- Kerntke, C.; Nimmerjahn, F.; Biburger, M. There Is (Scientific) Strength in Numbers: A Comprehensive Quantitation of Fc Gamma Receptor Numbers on Human and Murine Peripheral Blood Leukocytes. Front. Immunol. 2020, 11, 118. [Google Scholar] [CrossRef]
- Armour, K.L.; van de Winkel, J.G.; Williamson, L.M.; Clark, M.R. Differential binding to human FcgammaRIIa and Fcgam-maRIIb receptors by human IgG wildtype and mutant antibodies. Mol. Immunol. 2003, 40, 585–593. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daëron, M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Clark, M.R.; Clarkson, S.B.; Ory, P.A.; Stollman, N.; Goldstein, I.M. Molecular basis for a polymorphism involving Fc receptor II on human monocytes. J. Immunol. 1989, 143, 1731–1734. [Google Scholar] [PubMed]
- Warmerdam, P.A.; Van De Winkel, J.G.; Vlug, A.; Westerdaal, N.A.; Capel, P.J. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J. Immunol. 1991, 147, 1338–1343. [Google Scholar]
- Parren, P.W.; Warmerdam, P.A.; Boeije, L.C.; Arts, J.; Westerdaal, N.A.; Vlug, A.; Capel, P.J.; Aarden, L.A.; Van De Winkel, J.G. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J. Clin. Investig. 1992, 90, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Worth, R.G.; Chien, C.D.; Chien, P.; Reilly, M.P.; McKenzie, S.E.; Schreiber, A.D. Platelet FcγRIIA binds and internalizes IgG-containing complexes. Exp. Hematol. 2006, 34, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Y.; Chien, P.; Indik, Z.K.; Schreiber, A.D. Human platelet FcγRIIA and phagocytes in immune-complex clearance. Mol. Immunol. 2011, 48, 691–696. [Google Scholar] [CrossRef]
- Rollin, J.; Pouplard, C.; Sung, H.C.; Leroux, D.; Saada, A.; Gouilleux-Gruart, V.; Thibault, G.; Gruel, Y. Increased risk of thrombosis in FcγRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood 2015, 125, 2397–2404. [Google Scholar] [CrossRef]
- Mager, D.E.; Jusko, W.J. General Pharmacokinetic Model for Drugs Exhibiting Target-Mediated Drug Disposition. J. Pharmacokinet. Pharmacodyn. 2001, 28, 507–532. [Google Scholar] [CrossRef]
- Gibiansky, L.; Gibiansky, E. Target-mediated drug disposition model: Relationships with indirect response models and application to population PK–PD analysis. J. Pharmacokinet. Pharmacodyn. 2009, 36, 341–351. [Google Scholar] [CrossRef]
- Louis, E.; Mary, J.Y.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Dupas, J.L.; Pillant, H.; Picon, L.; Veyrac, M.; et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012, 142, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.; Thibault, G.; Ternant, D.; Cartron, G.; Watier, H.; Ohresser, M. Evidence for Linkage Disequilibrium Between FcγRIIIa-V158F and FcγRIIa-H131R Polymorphisms in White Patients, and for an FcγRIIIa-Restricted Influence on the Response to Therapeutic Antibodies. J. Clin. Oncol. 2008, 26, 5489–5491. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Berkane, Z.; Picon, L.; Gouilleux-Gruart, V.; Colombel, J.-F.; Allez, M.; Louis, E.; Paintaud, G. Assessment of the Influence of Inflammation and FCGR3A Genotype on Infliximab Pharmacokinetics and Time to Relapse in Patients with Crohn’s Disease. Clin. Pharmacokinet. 2014, 54, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Vollertsen, R.S.; McDuffie, F.C.; Bowie, E.J. Interaction of human platelets with particle-adherent aggregated IgG: Description of the experimental system and role of C1q and monomeric IgG. Clin. Exp. Immunol. 1983, 52, 423–429. [Google Scholar]
- Van Mirre, E.; Teeling, J.L.; van der Meer, J.W.; Bleeker, W.K.; Hack, C.E. Monomeric IgG in intravenous Ig preparations is a functional antagonist of FcgammaRII and FcgammaRIIIb. J. Immunol. 2004, 173, 332–339. [Google Scholar] [CrossRef]
- Quach, M.E.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521. [Google Scholar] [CrossRef]
- Nagelkerke, S.Q.; Dekkers, G.; Kustiawan, I.; Van De Bovenkamp, F.S.; Geissler, J.; Plomp, R.; Wuhrer, M.; Vidarsson, G.; Rispens, T.; Berg, T.K.V.D.; et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood 2014, 124, 3709–3718. [Google Scholar] [CrossRef]
- Liu, X.; Lu, L.; Yang, Z.; Palaniyandi, S.; Zeng, R.; Gao, L.-Y.; Mosser, D.M.; Roopenian, D.C.; Zhu, X. The Neonatal FcR-Mediated Presentation of Immune-Complexed Antigen Is Associated with Endosomal and Phagosomal pH and Antigen Stability in Macrophages and Dendritic Cells. J. Immunol. 2011, 186, 4674–4686. [Google Scholar] [CrossRef]
- Vidarsson, G.; Stemerding, A.M.; Stapleton, N.M.; Spliethoff, S.E.; Janssen, H.; Rebers, F.E.; De Haas, M.; Van De Winkel, J.G. FcRn: An IgG receptor on phagocytes with a novel role in phagocytosis. Blood 2006, 108, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.J.; Pyzik, M.; Rath, T.; Kozicky, L.K.; Sand, K.M.; Gandhi, A.K.; Grevys, A.; Foss, S.; Menzies, S.C.; Glickman, J.N.; et al. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex–driven autoimmunity. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Mulleman, D.; Degenne, D.; Willot, S.; Guillaumin, J.-M.; Watier, H.; Goupille, P.; Paintaud, G. An Enzyme-Linked Immunosorbent Assay for Therapeutic Drug Monitoring of Infliximab. Ther. Drug Monit. 2006, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ozzo, S.; Tartas, S.; Paintaud, G.; Cartron, G.; Colombat, P.; Bardos, P.; Watier, H.; Thibault, G. Rituximab-dependent cytotoxicity by natural killer cells: Influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res. 2004, 64, 4664–4669. [Google Scholar] [CrossRef] [PubMed]
| FCGR2A Genotype | ||||
|---|---|---|---|---|
| Characteristics | Total | HH | HR | RR |
| Number of patients 1 | 107 | 31 | 53 | 23 |
| Sex, women † [n (%)] | 47 (42) | 13 (42) | 22 (42) | 12 (52) |
| Age †, years | 31 (25–39) | 29 (25–35) | 33 (27–43) | 30 (26–37) |
| Body weight †, kg | 67 (57–75) | 68 (63–79) | 62 (56–75) | 72 (58–78) |
| Surgery † [n (%)] | 22 (21) | 5 (16) | 14 (26) | 3 (13) |
| CDAI † | 36 (17–60) | 24 (6–54) | 36 (21–61) | 40 (17–53) |
| CDEIS † | 0.7 (0.0–3.0) | 0.8 (0.0–3.0) | 0.4 (0.0–2.0) | 1.8 (0.1–3.4) |
| HsCRP †, mg/L | 2.2 (0.8–4.8) | 2.6 (1.5–4.7) | 1.7 (0.7–4.2) | 2.0 (0.9–5.2) |
| IgG †, mg/mL (range) | 12.6 (6.6–17.8) | 11.9 (8.6–17.4) | 13.0 (6.6–17.8) | 12.6 (8–16.7) |
| IgG1 †, mg/mL (range) | 6.0 (3.3–12.7) | 5.6 (3.6–8.6) | 6.2 (3.3–12.2) | 6.0 (3.6–9.8) |
| IgG2 †, mg/mL (range) | 5.0 (1.8–8.7) | 4.9 (2.5–8.2) | 5.0 (1.8–8.7) | 4.9 (2.9–8.7) |
| Platelets †, 109/L (range) | 272 (135–471) | 255 (135–379) | 274 (181–420) | 266 (174–471) |
| Infliximab * t½, d | 14.2 (12.2–16.4) | 13.2 (11.3–16.0) | 14.4 (12.4–16.3) | 15.6 (13.6–17.3) |
| Factor 1 | Parameter | Value (d) | p-Value |
|---|---|---|---|
| (Intercept) | β0 | 17.3714 | <0.00001 |
| CRP serum concentration (CRP) | β1 | −0.4646 | 0.0367 |
| IgG1 serum concentration (IgG1) | β2 | −0.6766 | 0.0269 |
| FCGR2A R alleles (F2A) | β3 | 5.7478 | 0.00003 |
| Interaction between platelet count and FCGR2A polymorphism (Pl*F2A) | γ1 | −0.0170 | 0.0004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thibault, G.; Paintaud, G.; Sung, H.C.; Lajoie, L.; Louis, E.; the GETAID; Desvignes, C.; Watier, H.; Gouilleux-Gruart, V.; Ternant, D. Association of IgG1 Antibody Clearance with FcγRIIA Polymorphism and Platelet Count in Infliximab-Treated Patients. Int. J. Mol. Sci. 2021, 22, 6051. https://doi.org/10.3390/ijms22116051
Thibault G, Paintaud G, Sung HC, Lajoie L, Louis E, the GETAID, Desvignes C, Watier H, Gouilleux-Gruart V, Ternant D. Association of IgG1 Antibody Clearance with FcγRIIA Polymorphism and Platelet Count in Infliximab-Treated Patients. International Journal of Molecular Sciences. 2021; 22(11):6051. https://doi.org/10.3390/ijms22116051
Chicago/Turabian StyleThibault, Gilles, Gilles Paintaud, Hsueh Cheng Sung, Laurie Lajoie, Edouard Louis, the GETAID, Celine Desvignes, Hervé Watier, Valérie Gouilleux-Gruart, and David Ternant. 2021. "Association of IgG1 Antibody Clearance with FcγRIIA Polymorphism and Platelet Count in Infliximab-Treated Patients" International Journal of Molecular Sciences 22, no. 11: 6051. https://doi.org/10.3390/ijms22116051
APA StyleThibault, G., Paintaud, G., Sung, H. C., Lajoie, L., Louis, E., the GETAID, Desvignes, C., Watier, H., Gouilleux-Gruart, V., & Ternant, D. (2021). Association of IgG1 Antibody Clearance with FcγRIIA Polymorphism and Platelet Count in Infliximab-Treated Patients. International Journal of Molecular Sciences, 22(11), 6051. https://doi.org/10.3390/ijms22116051







