Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment
Abstract
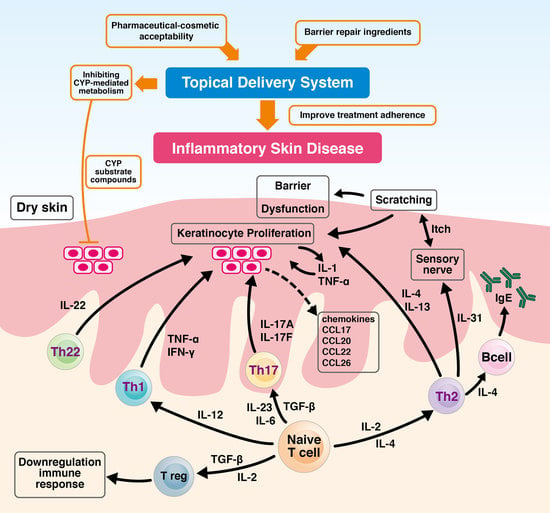
:1. Introduction
2. Inflammatory Skin Diseases
2.1. Inflammatory Skin Diseases, Skin Barrier, and T-Cells Immune System
2.2. T Cells-Mediated Inflammatory Skin Diseases
2.2.1. Vitiligo
2.2.2. Atopic Dermatitis
2.2.3. Psoriasis
2.2.4. Melanoma
2.3. T-Cell-Involved Inflammatory Skin Barrier
2.4. Topical Treatment and Inflammatory Skin Diseases
3. Skin Microbiota
4. Topical Formulation Development Strategies
4.1. Physicochemical Properties of the Active Compound
4.2. The Effect of Topical Pharmaceutical Formulation Compositions
4.3. Skin Absorption Routes and Skin Deposition
4.4. Inflammatory Skin and Irritation Test
4.5. Therapeutic Adherence, Cosmetic Acceptability Formulation and Barrier Repair Therapy
5. Future Concept for Topical Formulation Development
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nemes, Z.; Steinert, P.M. Bricks and mortar of the epidermal barrier. Exp. Mol. Med. 1999, 31, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; Jacques, C. Epidermal barrier function and systemic diseases. Clin. Dermatol. 2012, 30, 277–279. [Google Scholar] [CrossRef]
- Elias, P.M.; Menon, G.K.; Grayson, S.; Brown, B.E.; Rehfeld, S.J. Avian sebokeratocytes and marine mammal lipokeratinocytes: Structural, lipid biochemical, and functional considerations. Am. J. Anat. 1987, 180, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y. Molecular Mechanism of Epidermal Barrier Dysfunction as Primary Abnormalities. Int. J. Mol. Sci. 2020, 21, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Steinhoff, M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J. Investig. Dermatol. 2008, 128, 1067–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Wood, L.C.; Feingold, K.R. Epidermal pathogenesis of inflammatory dermatoses. Am. J. Contact Dermat. 1999, 10, 119–126. [Google Scholar] [PubMed]
- Proksch, E.; Brasch, J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin. Dermatol. 2012, 30, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, F.; Morales, J. Limitations and Opportunities in Topical Drug Delivery: Interaction Between Silica Nanoparticles and Skin Barrier. Curr. Pharm. Des. 2019, 25, 455–466. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Dainichi, T.; Hanakawa, S.; Kabashima, K. Classification of inflammatory skin diseases: A proposal based on the disorders of the three-layered defense systems, barrier, innate immunity and acquired immunity. J. Dermatol. Sci. 2014, 76, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Justiz Vaillant, A.A.; Sabir, S.; Jan, A. Physiology, Immune Response. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ho, A.W.; Kupper, T.S. T cells and the skin: From protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019, 19, 490–502. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Docke, W.D.; Ghoreschi, K. T cell pathology in skin inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Seneschal, J.; Boniface, K.; D’Arino, A.; Picardo, M. An update on Vitiligo pathogenesis. Pigment Cell Melanoma Res. 2021, 34, 236–243. [Google Scholar] [CrossRef]
- Martins, C.; Darrigade, A.S.; Jacquemin, C.; Barnetche, T.; Taieb, A.; Ezzedine, K.; Boniface, K.; Seneschal, J. Phenotype and function of circulating memory T cells in human vitiligo. Br. J. Dermatol. 2020, 183, 899–908. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, Y.; Chen, S.; Wang, L.; Jiang, M.; Xiang, L. Circulating CCL20: A potential biomarker for active vitiligo together with the number of Th1/17 cells. J. Dermatol. Sci. 2019, 93, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Man, W.Y.; Lv, C.Z.; Song, S.P.; Shi, Y.J.; Elias, P.M.; Man, M.Q. Epidermal permeability barrier recovery is delayed in vitiligo-involved sites. Skin Pharm. Physiol. 2010, 23, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Bishnoi, A.; Parsad, D. Clinical and Molecular Aspects of Vitiligo Treatments. Int. J. Mol. Sci. 2018, 19, 1509. [Google Scholar] [CrossRef] [Green Version]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. Vitiligo: An update on systemic treatments. Clin. Exp. Dermatol. 2021, 46, 248–258. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Frazier, W.; Bhardwaj, N. Atopic Dermatitis: Diagnosis and Treatment. Am. Fam. Phys. 2020, 101, 590–598. [Google Scholar]
- Boehncke, W.H.; Schon, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [Green Version]
- Schon, M.P.; Boehncke, W.H. Psoriasis. N. Engl. J. Med. 2005, 352, 1899–1912. [Google Scholar] [CrossRef]
- Kormeili, T.; Lowe, N.J.; Yamauchi, P.S. Psoriasis: Immunopathogenesis and evolving immunomodulators and systemic therapies; U.S. experiences. Br. J. Dermatol. 2004, 151, 3–15. [Google Scholar] [CrossRef]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A. The possibilities and principles of methotrexate treatment of psoriasis—The updated knowledge. Postepy. Dermatol. Alergol. 2014, 31, 392–400. [Google Scholar] [CrossRef]
- Gudjonsson, J.E.; Elder, J.T. Psoriasis. In Fitzpatrick’s Dermatology in General Medicine; Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffell, D.J., Wolff, K., Eds.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Fletcher, J.M.; Moran, B.; Petrasca, A.; Smith, C.M. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin. Exp. Immunol. 2020, 201, 121–134. [Google Scholar] [CrossRef]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Mignogna, C.; Scali, E.; Camastra, C.; Presta, I.; Zeppa, P.; Barni, T.; Donato, G.; Bottoni, U.; Di Vito, A. Innate immunity in cutaneous melanoma. Clin. Exp. Dermatol. 2017, 42, 243–250. [Google Scholar] [CrossRef]
- Marzagalli, M.; Ebelt, N.D.; Manuel, E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin. Cancer Biol. 2019, 59, 236–250. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, T.; Guo, Q.S.; Dong, X.N.; Qin, M.J.; Xiao, N.W.; Han, Z.Z.; Wei, M. Effect of Chrysanthemum indicum on absorption characteristics of Cd and its effect on quality of medicinal materials. Zhongguo Zhong Yao Za Zhi 2019, 44, 641–647. [Google Scholar] [CrossRef]
- Stene, M.A.; Babajanians, M.; Bhuta, S.; Cochran, A.J. Quantitative alterations in cutaneous Langerhans cells during the evolution of malignant melanoma of the skin. J. Investig. Dermatol. 1988, 91, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer. 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Boyer, M.; Cayrefourcq, L.; Dereure, O.; Meunier, L.; Becquart, O.; Alix-Panabieres, C. Clinical Relevance of Liquid Biopsy in Melanoma and Merkel Cell Carcinoma. Cancers 2020, 12, 960. [Google Scholar] [CrossRef] [Green Version]
- Shabaneh, T.B.; Molodtsov, A.K.; Steinberg, S.M.; Zhang, P.; Torres, G.M.; Mohamed, G.A.; Boni, A.; Curiel, T.J.; Angeles, C.V.; Turk, M.J. Oncogenic BRAF(V600E) Governs Regulatory T-cell Recruitment during Melanoma Tumorigenesis. Cancer Res. 2018, 78, 5038–5049. [Google Scholar] [CrossRef] [Green Version]
- Zoschke, C.; Ulrich, M.; Sochorova, M.; Wolff, C.; Vavrova, K.; Ma, N.; Ulrich, C.; Brandner, J.M.; Schafer-Korting, M. The barrier function of organotypic non-melanoma skin cancer models. J. Control Release 2016, 233, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, L. Retinoids, methotrexate and cyclosporine. Curr. Probl. Dermatol. 2009, 38, 79–94. [Google Scholar] [CrossRef]
- Francesco, B.; Paolo, G.; Giampiero, G. A dermatologist perspective in the pharmacological treatment of patients with psoriasis and psoriatic arthritis. Expert Rev. Clin. Pharm. 2020, 5, 481–491. [Google Scholar] [CrossRef]
- Rendon, A.; Schakel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montaudie, H.; Sbidian, E.; Paul, C.; Maza, A.; Gallini, A.; Aractingi, S.; Aubin, F.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Methotrexate in psoriasis: A systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J. Eur. Acad. Dermatol. Venereol. 2011, 25 (Suppl. S2), 12–18. [Google Scholar] [CrossRef] [PubMed]
- McClure, S.L.; Valentine, J.; Gordon, K.B. Comparative tolerability of systemic treatments for plaque-type psoriasis. Drug. Saf. 2002, 25, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Aickara, D.; Bashyam, A.M.; Pichardo, R.O.; Feldman, S.R. Topical methotrexate in dermatology: A review of the literature. J. Dermatolog. Treat. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, A.; Sahin, D.; Impellizzieri, D.; Nguyen, T.T.; Hafner, J.; Yawalkar, N.; Kurzbach, D.; Tan, G.; Akdis, C.A.; Nilsson, J.; et al. Nanoparticle-Coupled Topical Methotrexate Can Normalize Immune Responses and Induce Tissue Remodeling in Psoriasis. J. Investig. Dermatol. 2020, 140, 1003–1014.e1008. [Google Scholar] [CrossRef]
- Aguilera, A.C.; Dagher, I.A.; Kloepfer, K.M. Role of the Microbiome in Allergic Disease Development. Curr. Allergy Asthma Rep. 2020, 20, 44. [Google Scholar] [CrossRef]
- DeVore, S.B.; Gonzalez, T.; Sherenian, M.G.; Herr, A.B.; Khurana Hershey, G.K. On the surface: Skin microbial exposure contributes to allergic disease. Ann. Allergy Asthma Immunol. 2020, 125, 628–638. [Google Scholar] [CrossRef]
- Chen, P.; He, G.; Qian, J.; Zhan, Y.; Xiao, R. Potential role of the skin microbiota in Inflammatory skin diseases. J. Cosmet. Dermatol. 2021, 20, 400–409. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Sala-Cunill, A.; Lazaro, M.; Herraez, L.; Quinones, M.D.; Moro-Moro, M.; Sanchez, I. Skin Allergy Committee of Spanish Society of Allergy Committee of Spanish Society of Allergy and Clinical Immunology (SEAIC). Basic Skin Care and Topical Therapies for Atopic Dermatitis: Essential Approaches and Beyond. J. Investig. Allergol. Clin. Immunol. 2018, 28, 379–391. [Google Scholar] [CrossRef]
- Lynde, C.W.; Andriessen, A.; Bertucci, V.; McCuaig, C.; Skotnicki, S.; Weinstein, M.; Wiseman, M.; Zip, C. The Skin Microbiome in Atopic Dermatitis and Its Relationship to Emollients. J. Cutan. Med. Surg. 2016, 20, 21–28. [Google Scholar] [CrossRef]
- Glatz, M.; Jo, J.H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient use alters skin barrier and microbes in infants at risk for developing atopic dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.E.; Schaffer, J.V.; Orlow, S.J.; Gao, Z.; Li, H.; Alekseyenko, A.V.; Blaser, M.J. Cutaneous microbiome effects of fluticasone propionate cream and adjunctive bleach baths in childhood atopic dermatitis. J. Am. Acad. Dermatol. 2016, 75, 481–493.e8. [Google Scholar] [CrossRef] [Green Version]
- Smith Pease, C.K.; Basketter, D.A.; Patlewicz, G.Y. Contact allergy: The role of skin chemistry and metabolism. Clin. Exp. Dermatol. 2003, 28, 177–183. [Google Scholar] [CrossRef]
- Gerberick, G.F.; Ryan, C.A.; Kern, P.S.; Dearman, R.J.; Kimber, I.; Patlewicz, G.Y.; Basketter, D.A. A chemical dataset for evaluation of alternative approaches to skin-sensitization testing. Contact Dermatitis 2004, 50, 274–288. [Google Scholar] [CrossRef]
- Zesch, A. Adverse reactions of externally applied drugs and inert substances. Derm. Beruf Umwelt 1988, 36, 128–133. [Google Scholar]
- Zesch, A. Skin irritation by topical drugs. Derm. Beruf Umwelt 1983, 31, 74–78. [Google Scholar]
- Garg, T.; Rath, G.; Goyal, A.K. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. 2015, 22, 969–987. [Google Scholar] [CrossRef] [Green Version]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent Advances and Perspectives in Liposomes for Cutaneous Drug Delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Pham, Q.D.; Bjorklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum—Relation between molecular effects and barrier function. J. Control Release 2016, 232, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Chai, J.K.; Hu, Q.; Yu, Y.H.; Ma, L.; Liu, L.Y.; Zhang, X.L.; Li, B.L.; Zhang, D.H. Transdermal permeation of drugs with differing lipophilicity: Effect of penetration enhancer camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical Enhancer: A Simplistic Way to Modulate Barrier Function of the Stratum Corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.Y.; Yang, Y.Y.; Heng, P.W. Role of solvent in interactions between fatty acids-based formulations and lipids in porcine stratum corneum. J. Control Release 2004, 94, 207–216. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of microemulsion components into the stratum corneum and their molecular effects on skin barrier function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Lin, S.Y.; Duan, K.J.; Lin, T.C. Direct or indirect skin lipid-ordering effect of pyrrolidone carboxylate sodium after topical treatment with penetration enhancers. Biomed. Mater. Eng. 1995, 5, 9–20. [Google Scholar] [CrossRef]
- Guo, J.W.; Lin, T.K.; Wu, C.H.; Wei, K.C.; Lan, C.C.; Peng, A.C.; Tsai, J.C.; Sheu, H.M. Human sebum extract induces barrier disruption and cytokine expression in murine epidermis. J. Dermatol. Sci. 2015, 78, 34–43. [Google Scholar] [CrossRef]
- Shin, E.S.; Lee, J.Y.; Lee, S.J.; Nam, S.H. Non-invasive method to monitor molecular changes in human stratum corneum during acute barrier disruption using reflectance NIR spectroscopy. Annu. Int. Conf. IEEE. Eng. Med. Biol. Soc. 2018, 2018, 1542–1545. [Google Scholar] [CrossRef] [PubMed]
- Gloor, M. How do dermatological vehicles influence the horny layer? Skin Pharm. Physiol. 2004, 17, 267–273. [Google Scholar] [CrossRef]
- Gloor, M.; Gehring, W. Effects of emulsions on the stratum corneum barrier and hydration. Hautarzt 2003, 54, 324–330. [Google Scholar] [CrossRef]
- Witting, M.; Obst, K.; Friess, W.; Hedtrich, S. Recent advances in topical delivery of proteins and peptides mediated by soft matter nanocarriers. Biotechnol. Adv. 2015, 33, 1355–1369. [Google Scholar] [CrossRef]
- Pai, V.V.; Bhandari, P.; Shukla, P. Topical peptides as cosmeceuticals. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyanka, K.; Singh, S. A review on skin targeted delivery of bioactives as ultradeformable vesicles: Overcoming the penetration problem. Curr. Drug Targets 2014, 15, 184–198. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef]
- American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology. Contact dermatitis: A practice parameter. Ann. Allergy Asthma Immunol. 2006, 97, S1–S38. [Google Scholar] [CrossRef]
- Rocha, J.; Pereira, T.; Sousa-Basto, A.; Brito, C. Occupational protein contact dermatitis: Two case reports. Case Rep. Med. 2010, 2010, 489627. [Google Scholar] [CrossRef]
- Anliker, M.D.; Borelli, S.; Wuthrich, B. Occupational protein contact dermatitis from spices in a butcher: A new presentation of the mugwort-spice syndrome. Contact Dermatitis 2002, 46, 72–74. [Google Scholar] [CrossRef]
- Boehncke, W.H.; Pillekamp, H.; Gass, S.; Gall, H. Occupational protein contact dermatitis caused by meat and fish. Int. J. Dermatol. 1998, 37, 358–360. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Geneidi, A.S.; Mortada, N.D. Visualization, dermatopharmacokinetic analysis and monitoring the conformational effects of a microemulsion formulation in the skin stratum corneum. J. Colloid Interface Sci. 2011, 354, 124–130. [Google Scholar] [CrossRef]
- Man, M.-Q.; Fowler, A.J.; Schmuth, M.; Lau, P.; Chang, S.; Brown, B.E.; Moser, A.H.; Michalik, L.; Desvergne, B.; Wahli, W.; et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J. Investig. Dermatol. 2004, 123, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Flori, E.; Mastrofrancesco, A.; Kovacs, D.; Bellei, B.; Briganti, S.; Maresca, V.; Cardinali, G.; Picardo, M. The activation of PPARgamma by 2,4,6-Octatrienoic acid protects human keratinocytes from UVR-induced damages. Sci. Rep. 2017, 7, 9241. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.W.; Cheng, Y.P.; Liu, C.Y.; Thong, H.Y.; Lo, Y.; Wu, C.Y.; Jee, S.H. Magnolol may contribute to barrier function improvement on imiquimod-induced psoriasis-like dermatitis animal model via the downregulation of interleukin-23. Exp. Ther. Med. 2021, 21, 448. [Google Scholar] [CrossRef]
- Rush, A.K.; Nash, J.F.; Smith, E.D., III; Kasting, G.B. Formulation and Artificial Sebum Effects on the Percutaneous Absorption of Zinc Pyrithione through Excised Human Skin. Skin Pharm. Physiol. 2019, 32, 224–234. [Google Scholar] [CrossRef]
- Goldovsky, M.; Zhai, H.; Dika, E.; Maibach, H.I. Safety Assays in Skin Pharmacology. In Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays; Vogel, H.G., Hock, F.J., Maas, J., Mayer, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 365–383. [Google Scholar] [CrossRef]
- Guo, J.W.; Pu, C.M.; Liu, C.Y.; Lo, S.L.; Yen, Y.H. Curcumin-Loaded Self-Microemulsifying Gel for Enhancing Wound Closure. Skin Pharm. Physiol. 2020, 33, 300–308. [Google Scholar] [CrossRef]
- Guo, J.W.; Cheng, Y.P.; Liu, C.Y.; Thong, H.Y.; Huang, C.J.; Lo, Y.; Wu, C.Y.; Jee, S.H. Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Pharmaceutics 2020, 12, 457. [Google Scholar] [CrossRef]
- Try, C.; Moulari, B.; Beduneau, A.; Fantini, O.; Pin, D.; Pellequer, Y.; Lamprecht, A. Size dependent skin penetration of nanoparticles in murine and porcine dermatitis models. Eur. J. Pharm. Biopharm. 2016, 100, 101–108. [Google Scholar] [CrossRef]
- Riebeling, C.; Luch, A.; Tralau, T. Skin toxicology and 3Rs-Current challenges for public health protection. Exp. Dermatol. 2018, 27, 526–536. [Google Scholar] [CrossRef]
- Choksi, N.Y.; Truax, J.; Layton, A.; Matheson, J.; Mattie, D.; Varney, T.; Tao, J.; Yozzo, K.; McDougal, A.J.; Merrill, J.; et al. United States regulatory requirements for skin and eye irritation testing. Cutan. Ocul. Toxicol. 2019, 38, 141–155. [Google Scholar] [CrossRef]
- Tupker, R.A.; Pinnagoda, J.; Coenraads, P.J.; Nater, J.P. Susceptibility to irritants: Role of barrier function, skin dryness and history of atopic dermatitis. Br. J. Dermatol. 1990, 123, 199–205. [Google Scholar] [CrossRef]
- Piaserico, S.; Manfredini, S.; Borghi, A.; Gisondi, P.; Pazzaglia, M.; Stinco, G.; Venturini, M.; Conti, A. How to improve adherence to treatment in patients with mild-to-moderate psoriasis. G. Ital. Dermatol. Venereol. 2018, 153, 692–697. [Google Scholar] [CrossRef]
- Reich, K.; Bewley, A. What is new in topical therapy for psoriasis? J. Eur. Acad. Dermatol. Venereol. 2011, 25 (Suppl. 4), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, S.J. Skin acceptability of a cosmetic moisturizer formulation in female subjects with sensitive skin. Clin. Cosmet. Investig. Dermatol. 2018, 11, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snatchfold, J. Cutaneous acceptability of a moisturizing cream in subjects with sensitive skin. J. Cosmet. Dermatol. 2019, 18, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viennet, C.; Jeudy, A.; Fanian, F.; He, L.; Humbert, P. Assessment of the efficacy of a new complex antisensitive skin cream. J. Cosmet. Dermatol. 2018, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, L.; Elias, P.M.; Man, M.Q. Skin care products can aggravate epidermal function: Studies in a murine model suggest a pathogenic role in sensitive skin. Contact Dermatitis 2018, 78, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, P.M.; Wakefield, J.S.; Man, M.Q. Moisturizers versus Current and Next-Generation Barrier Repair Therapy for the Management of Atopic Dermatitis. Skin Pharm. Physiol. 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Elias, P.M.; Sugarman, J. Does moisturizing the skin equate with barrier repair therapy? Ann. Allergy Asthma Immunol. 2018, 121, 653–656.e2. [Google Scholar] [CrossRef]
- Zuber, R.; Anzenbacherova, E.; Anzenbacher, P. Cytochromes P450 and experimental models of drug metabolism. J. Cell. Mol. Med. 2002, 6, 189–198. [Google Scholar] [CrossRef]
- Smith, S.A.; Colley, H.E.; Sharma, P.; Slowik, K.M.; Sison-Young, R.; Sneddon, A.; Webb, S.D.; Murdoch, C. Expression and enzyme activity of cytochrome P450 enzymes CYP3A4 and CYP3A5 in human skin and tissue-engineered skin equivalents. Exp. Dermatol. 2018, 27, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Baron, J.M.; Holler, D.; Schiffer, R.; Frankenberg, S.; Neis, M.; Merk, H.F.; Jugert, F.K. Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J. Investig. Dermatol. 2001, 116, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Hotchkiss, S.A.; Boobis, A.R.; Edwards, R.J. Expression of P450 enzymes in rat whole skin and cultured epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2002, 297, 65–70. [Google Scholar] [CrossRef]
- Saeki, M.; Saito, Y.; Nagano, M.; Teshima, R.; Ozawa, S.; Sawada, J. mRNA expression of multiple cytochrome p450 isozymes in four types of cultured skin cells. Int. Arch. Allergy Immunol. 2002, 127, 333–336. [Google Scholar] [CrossRef]
- Vyas, P.M.; Roychowdhury, S.; Khan, F.D.; Prisinzano, T.E.; Lamba, J.; Schuetz, E.G.; Blaisdell, J.; Goldstein, J.A.; Munson, K.L.; Hines, R.N.; et al. Enzyme-mediated protein haptenation of dapsone and sulfamethoxazole in human keratinocytes: I. Expression and role of cytochromes P450. J. Pharm. Exp. Ther. 2006, 319, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Neis, M.M.; Ladd, P.A.; Keeney, D.S. Differentiation-specific factors modulate epidermal CYP1-4 gene expression in human skin in response to retinoic acid and classic aryl hydrocarbon receptor ligands. J. Pharm. Exp. Ther. 2006, 319, 1162–1171. [Google Scholar] [CrossRef] [Green Version]
- Janmohamed, A.; Dolphin, C.T.; Phillips, I.R.; Shephard, E.A. Quantification and cellular localization of expression in human skin of genes encoding flavin-containing monooxygenases and cytochromes P450. Biochem. Pharm. 2001, 62, 777–786. [Google Scholar] [CrossRef]
- Jugert, F.K.; Agarwal, R.; Kuhn, A.; Bickers, D.R.; Merk, H.F.; Mukhtar, H. Multiple cytochrome P450 isozymes in murine skin: Induction of P450 1A, 2B, 2E, and 3A by dexamethasone. J. Investig. Dermatol. 1994, 102, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Loden, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Guo, J.W.; Chien, C.C.; Chen, J.H. CYP3A Excipient-Based Microemulsion Prolongs the Effect of Magnolol on Ischemia Stroke Rats. Pharmaceutics 2020, 12, 737. [Google Scholar] [CrossRef]



| Ingredients | Benefits |
|---|---|
| Glycerol | Moisturizer |
| Linoleic acid | Agonists of peroxisome proliferator-activated receptor-γ, improved barrier recovery/homeostasis |
| Polyethylene glycol (PEG)-40 castor oil (RH-40) | Surfactant, increased viscosity and reduced the fluidity of the formulation |
| Silicon oil | Increased the occlusion effect on skin surface |
| Sorbitol | Moisturizer |
| Squalene | Component of human sebum, provided liquid lipid film on inflammatory skin surface which works as temporary barrier |
| Triglyceride | Component of human sebum, provided liquid lipid film on inflammatory skin surface, which works as temporary barrier |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.-W.; Jee, S.-H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. https://doi.org/10.3390/ijms22116078
Guo J-W, Jee S-H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. International Journal of Molecular Sciences. 2021; 22(11):6078. https://doi.org/10.3390/ijms22116078
Chicago/Turabian StyleGuo, Jiun-Wen, and Shiou-Hwa Jee. 2021. "Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment" International Journal of Molecular Sciences 22, no. 11: 6078. https://doi.org/10.3390/ijms22116078
APA StyleGuo, J. -W., & Jee, S. -H. (2021). Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. International Journal of Molecular Sciences, 22(11), 6078. https://doi.org/10.3390/ijms22116078