Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain
Abstract
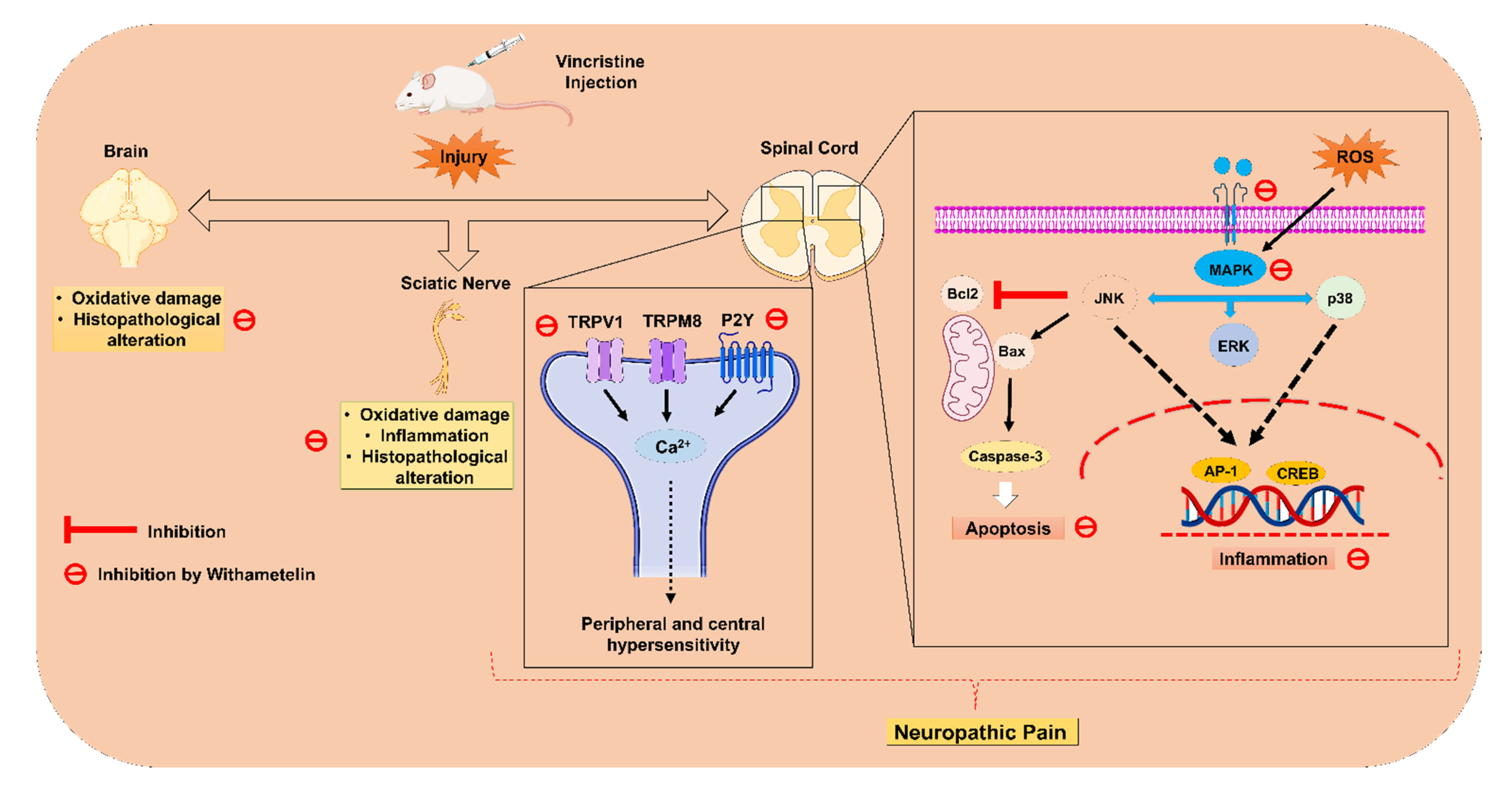
:1. Introduction
2. Results
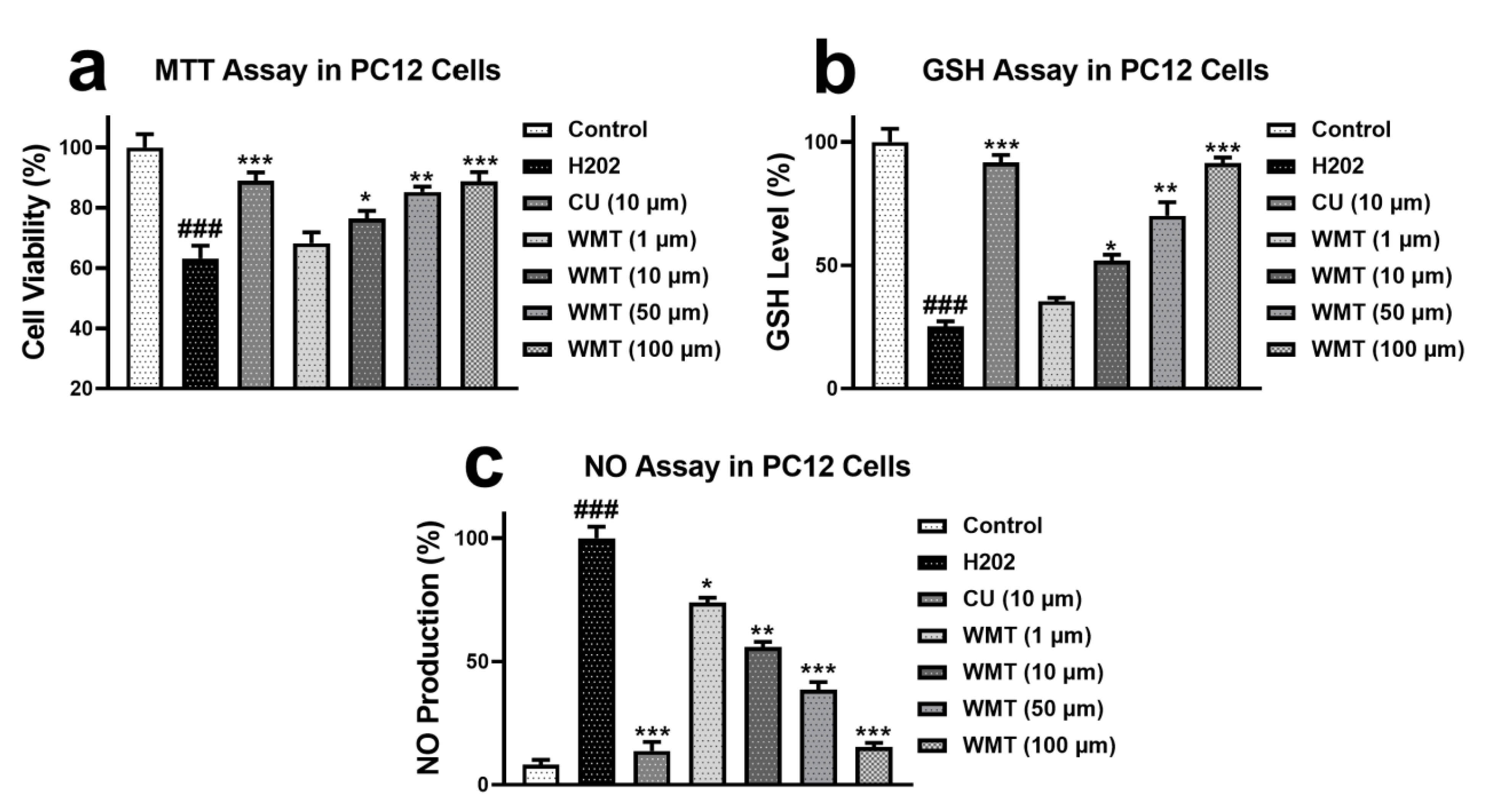
2.1. Effects of WMT on Cell Viability, GSH, and NO Level in H2O2-Induced PC12 Cells
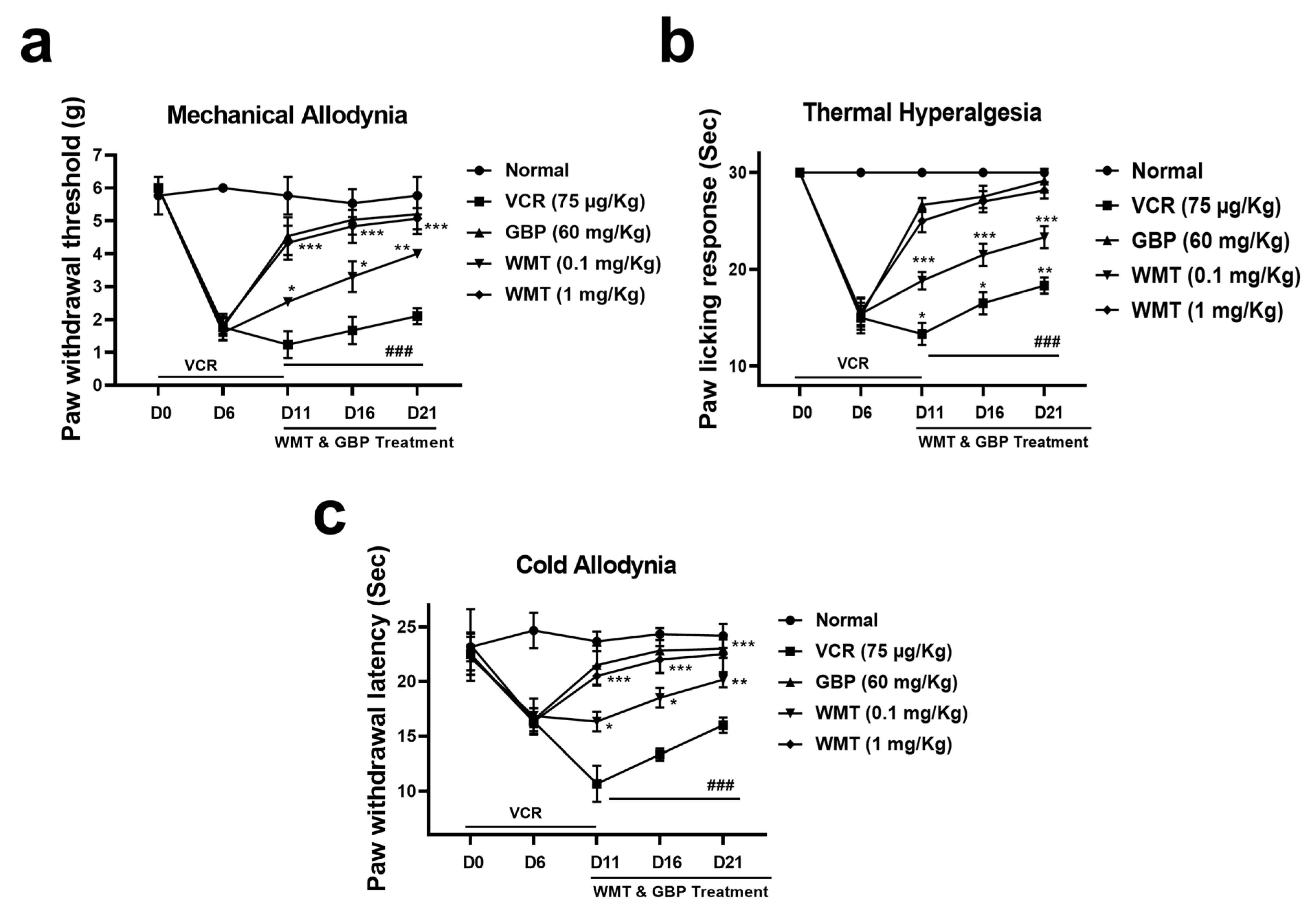
2.2. WMT Attenuated VCR-Induced Pain Hypersensitivity in Mice
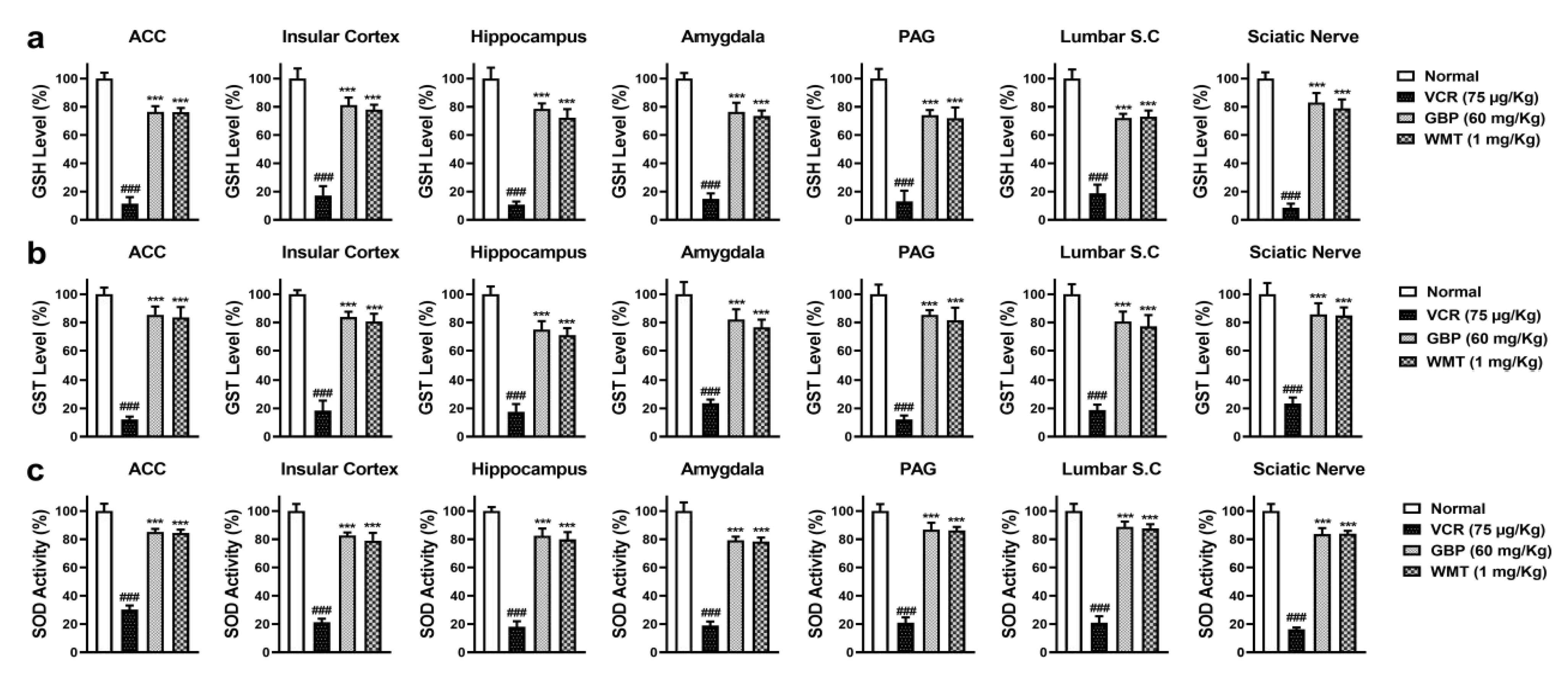
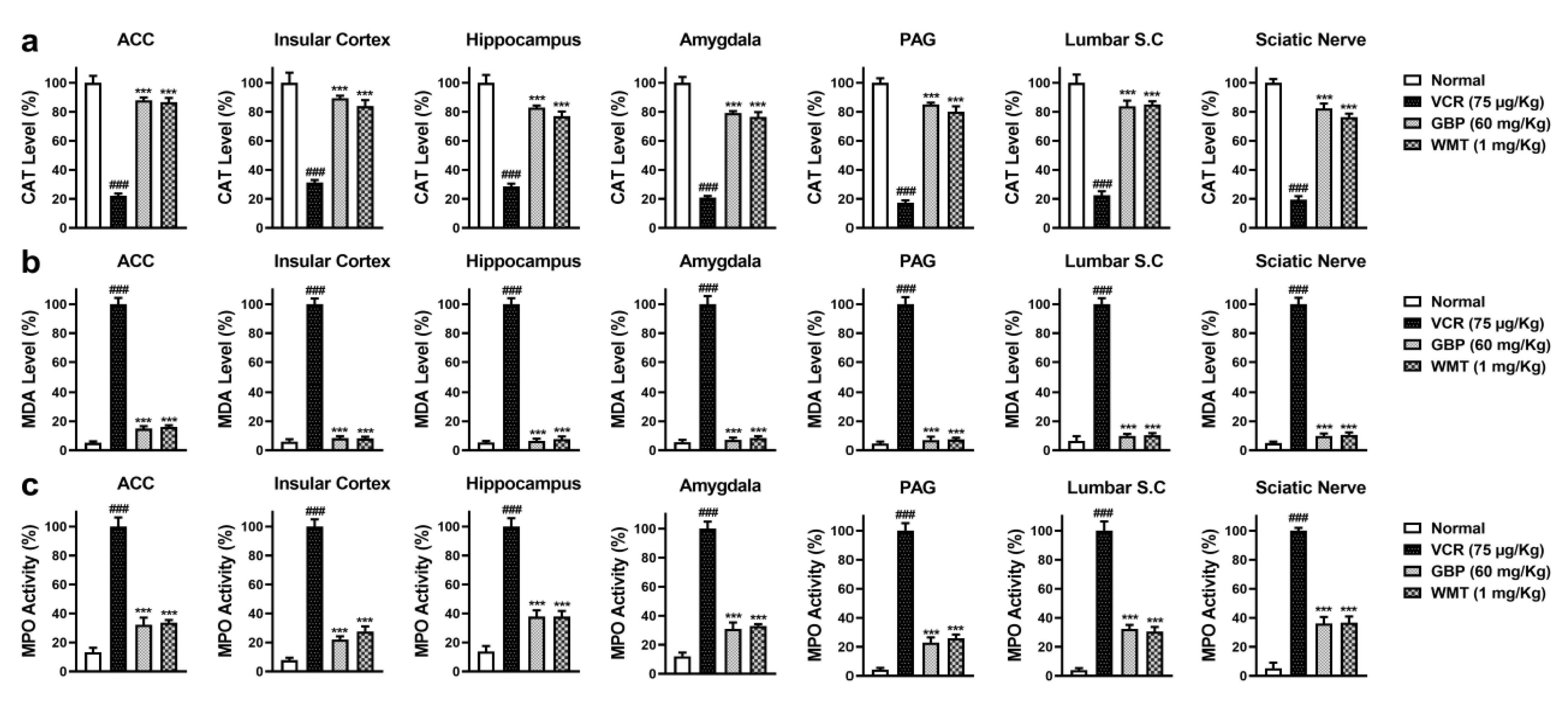
2.3. WMT Suppressed VCR-Induced Oxidative Stress in the Brain, Spinal Cord, and Sciatic Nerve
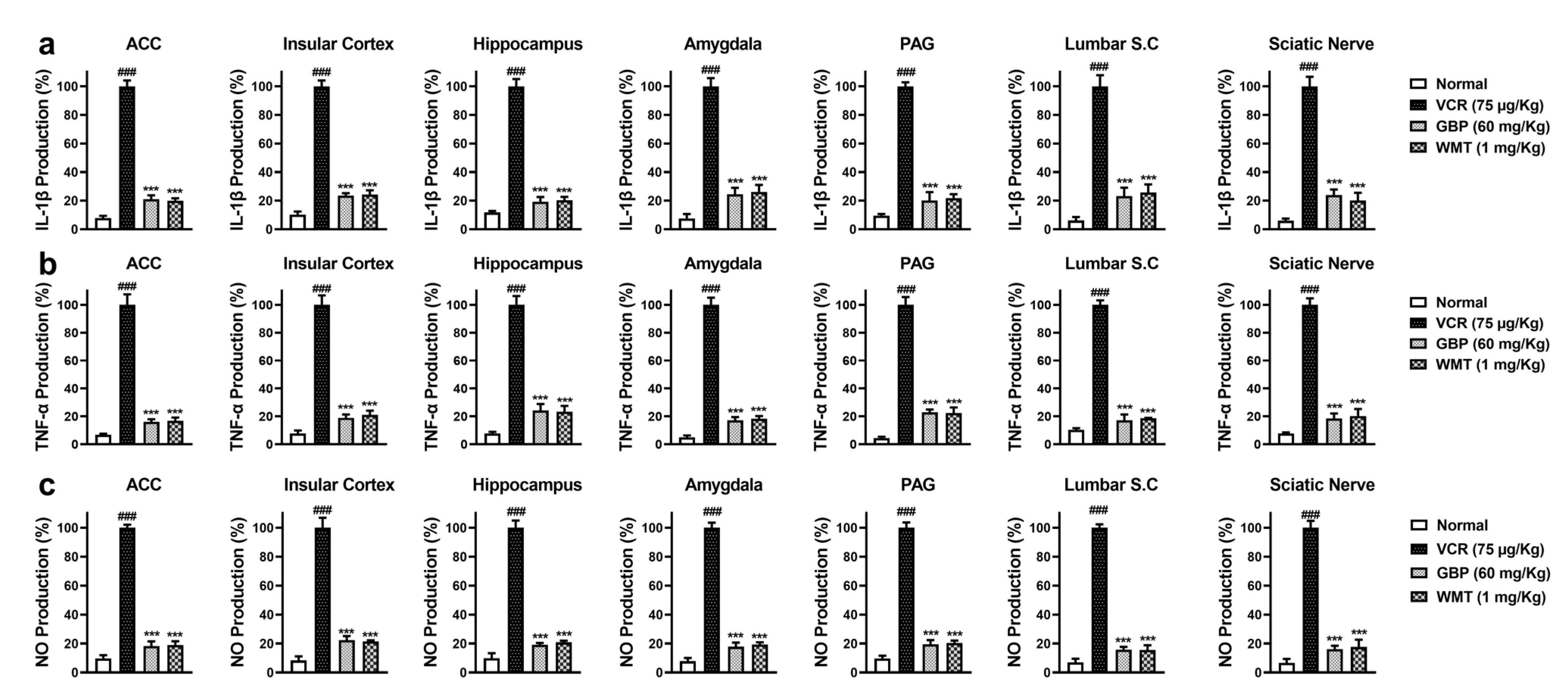
2.4. WMT Ameliorated VCR-Induced Inflammation in the Brain, Spinal Cord, and Sciatic Nerve
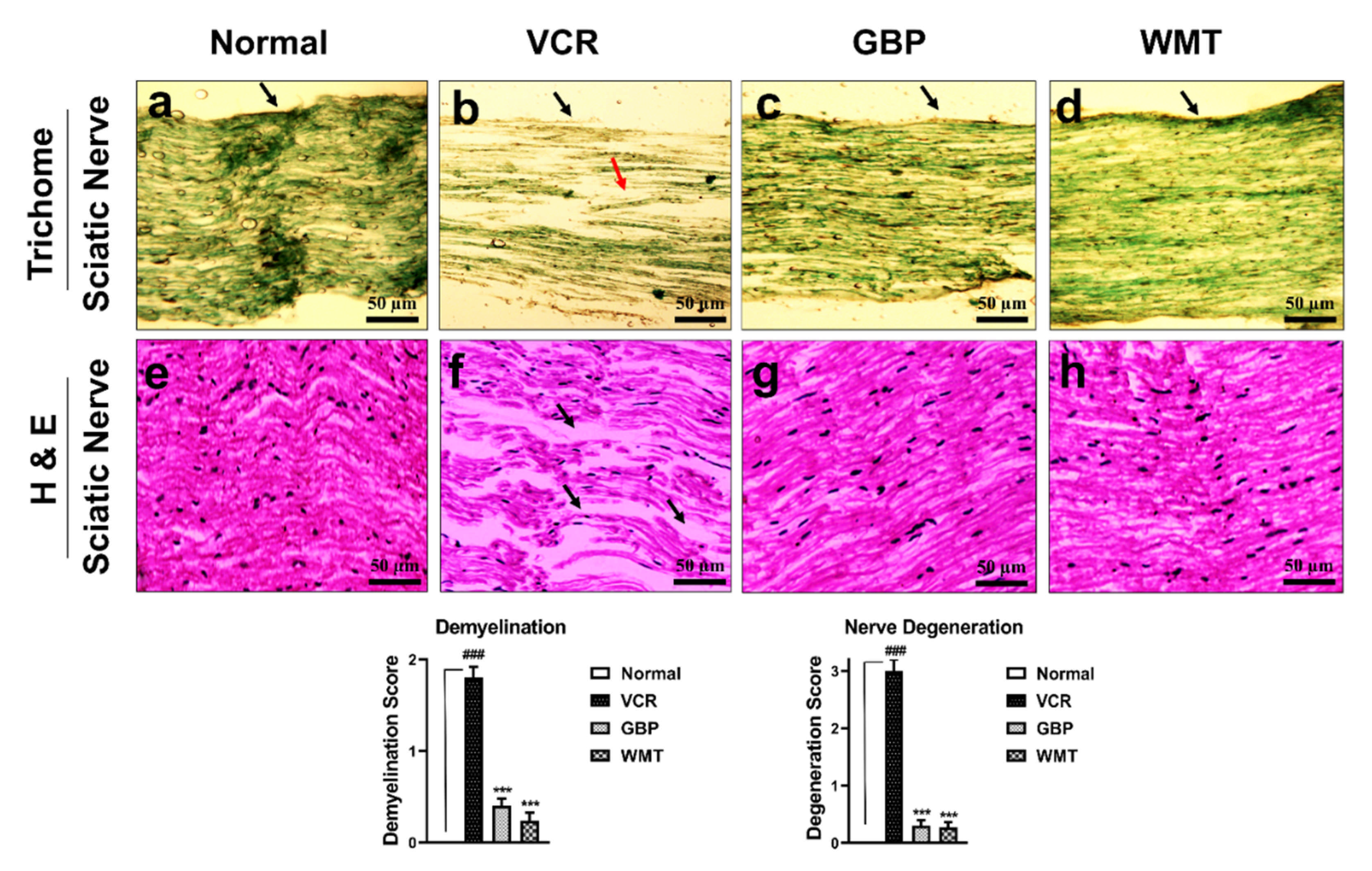
2.5. WMT Inhibited VCR-Induced Histopathological Changes in the Sciatic Nerve
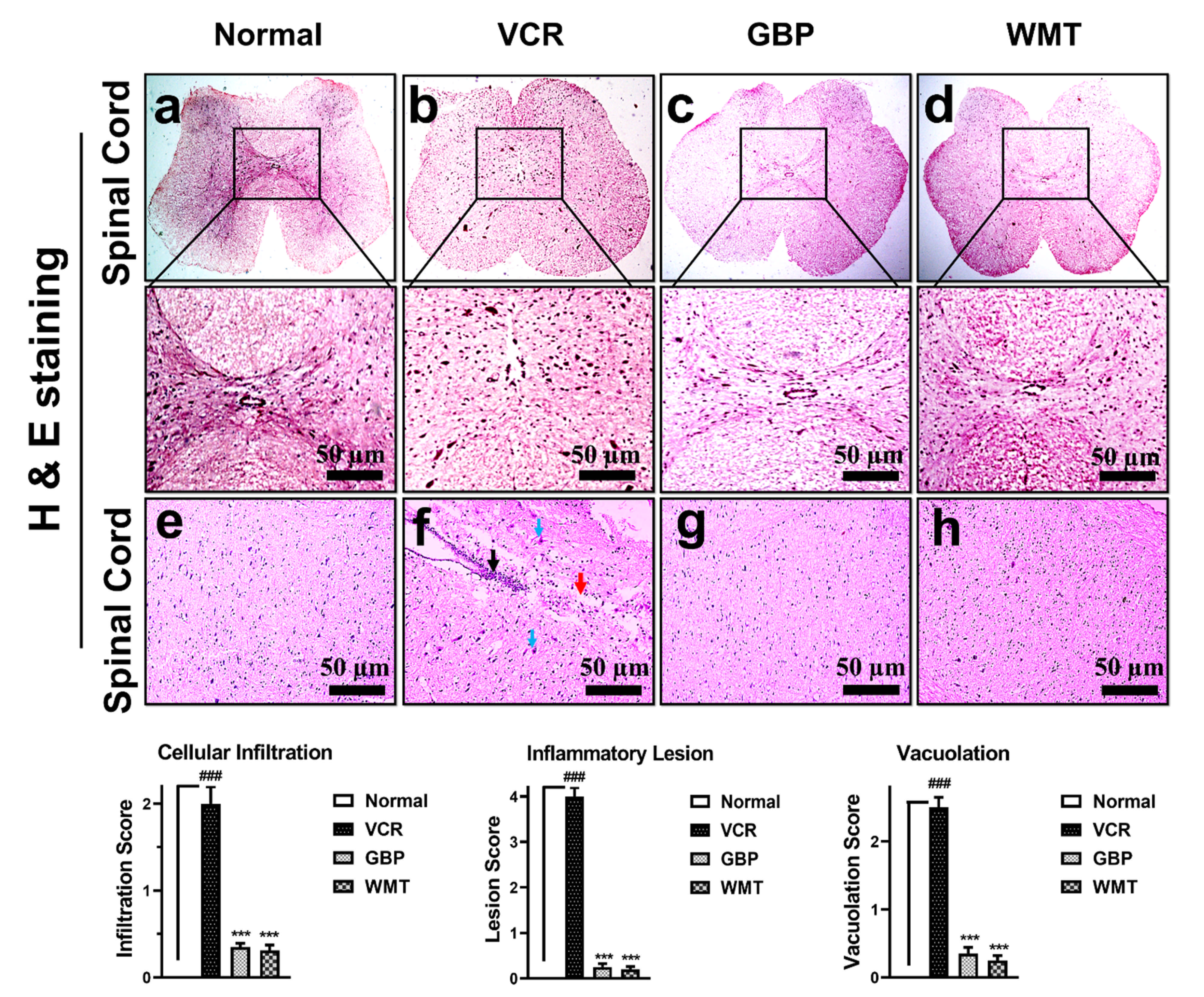
2.6. WMT Reversed VCR-Induced Histopathological Changes in the Spinal Cord
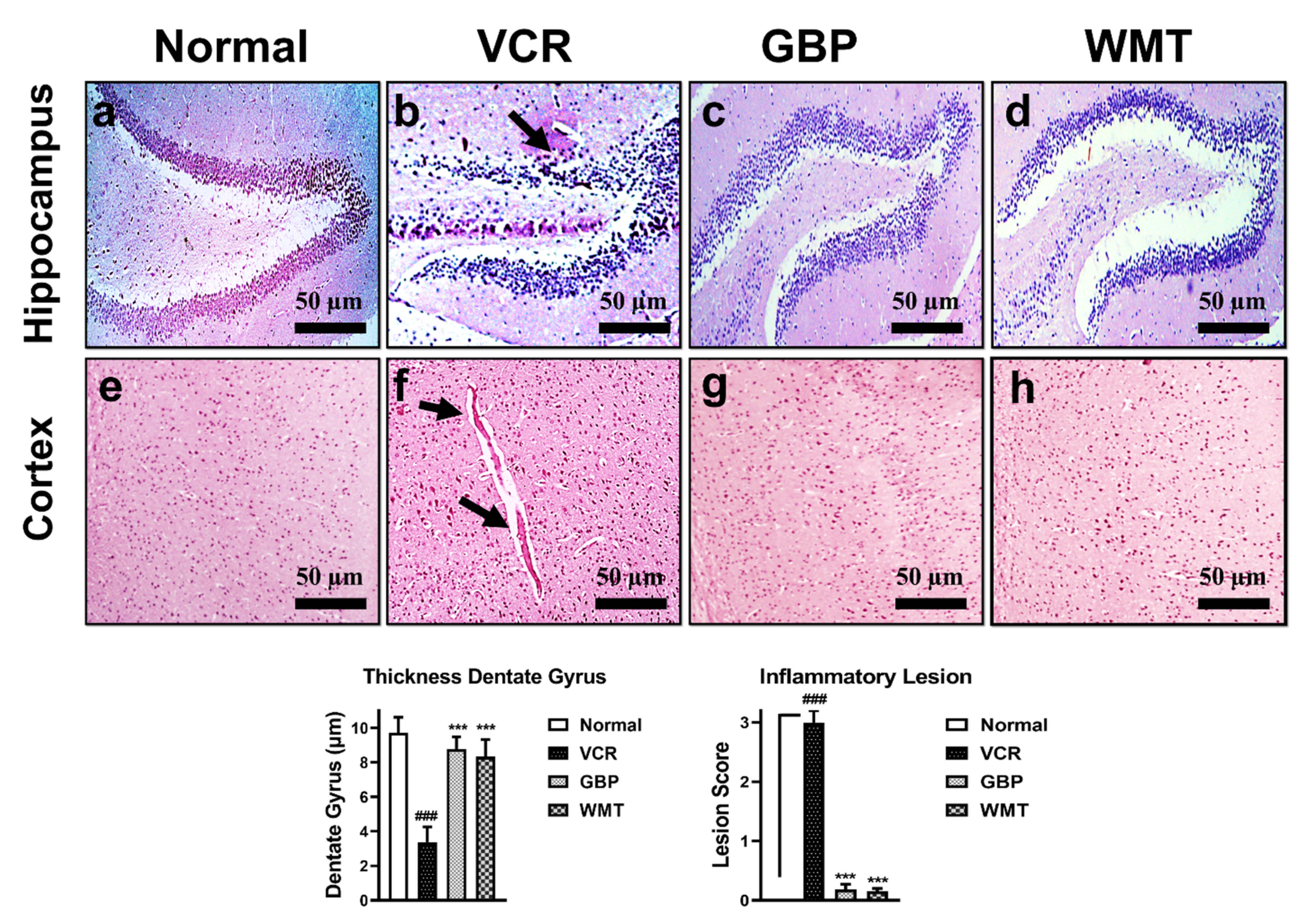
2.7. WMT Inhibited VCR-Induced Histopathological Changes in Brain
2.8. FTIR Spectroscopic and DSC Analysis of the Sciatic Nerve
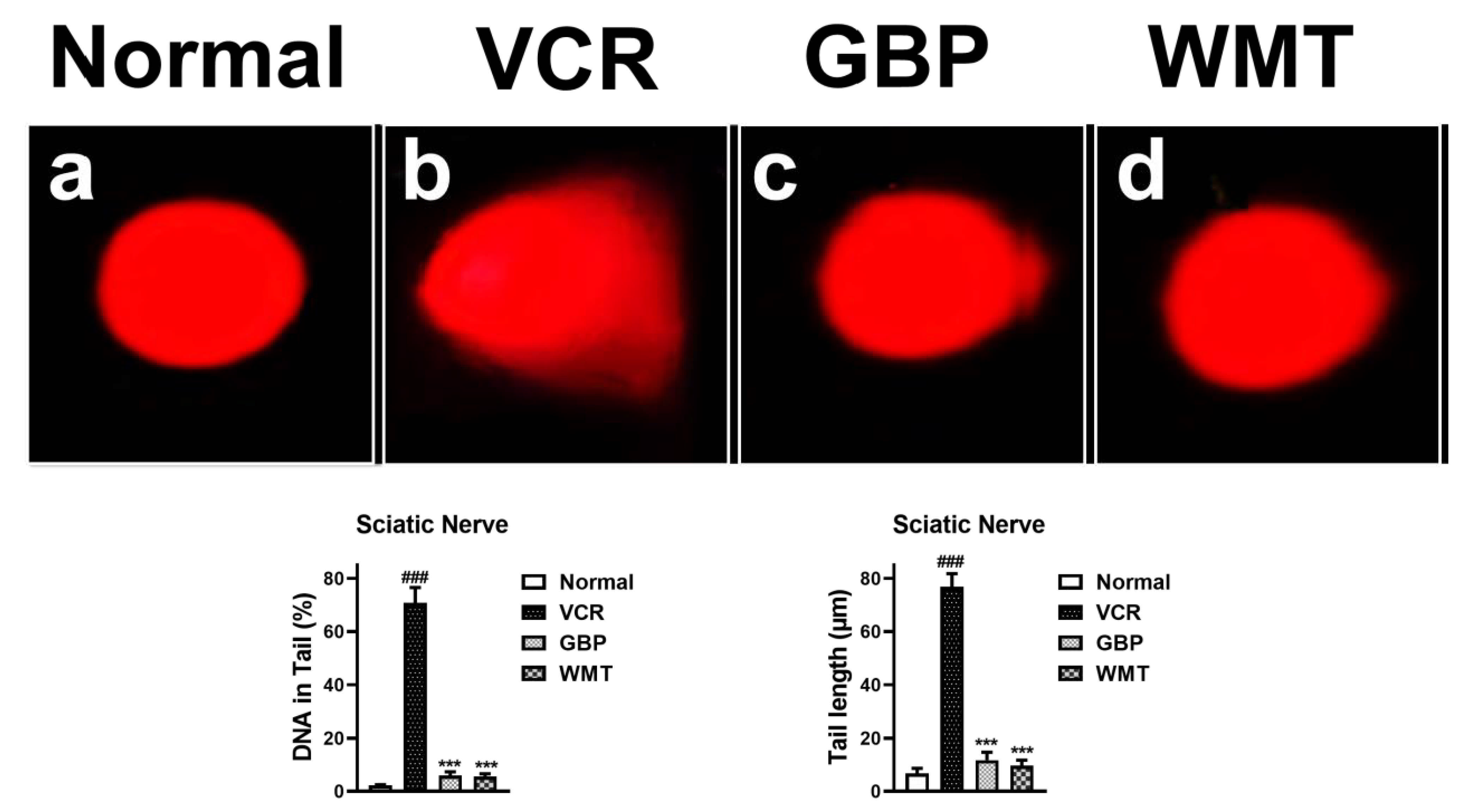
2.9. WMT Ameliorated VCR-Induced Genotoxic Effect in the Sciatic Nerve
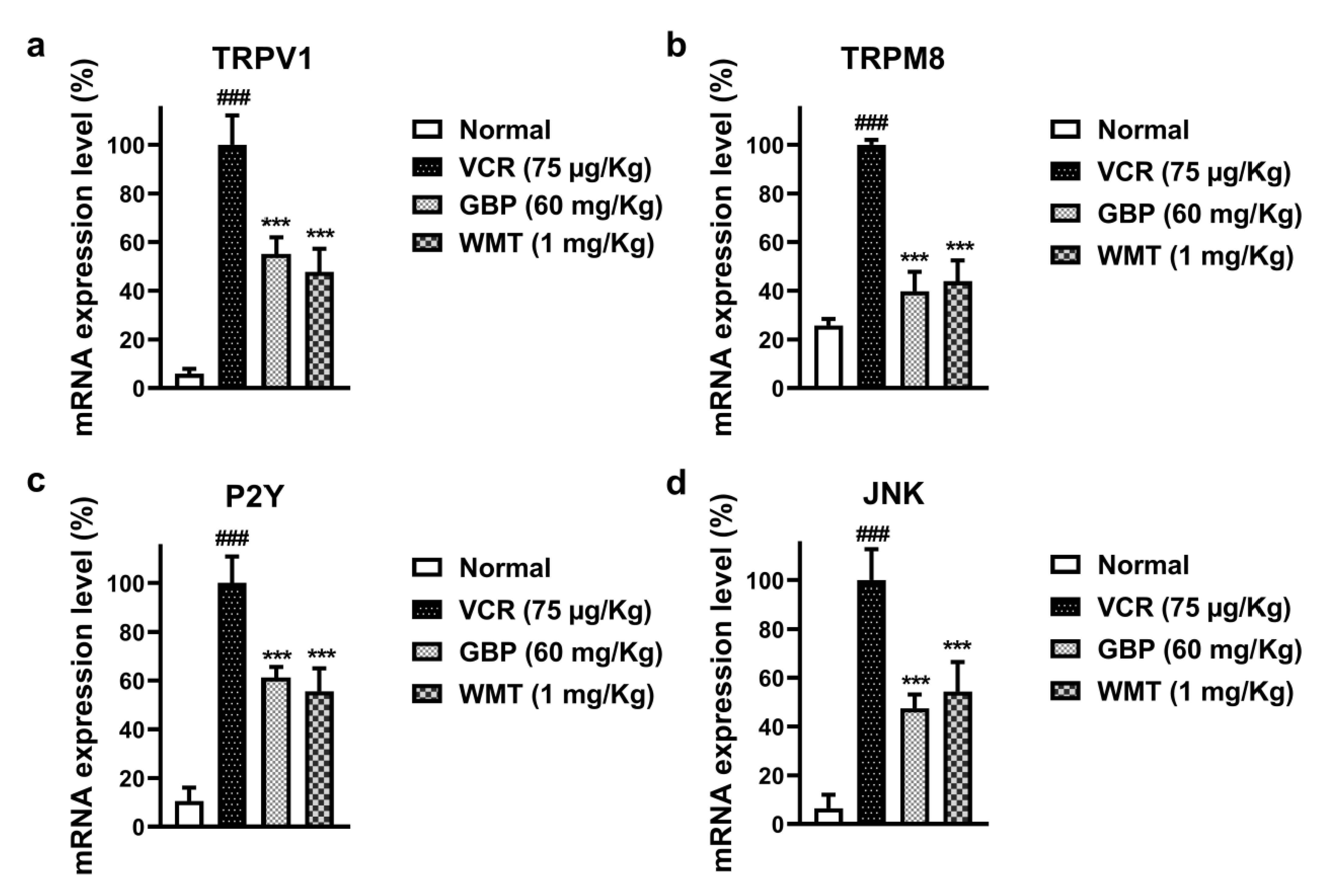
2.10. WMT Reduced the mRNA Expression Level of TRPV1/TRPM8/P2Y Nociceptors and JNK in the Spinal Cord after VCR Administration
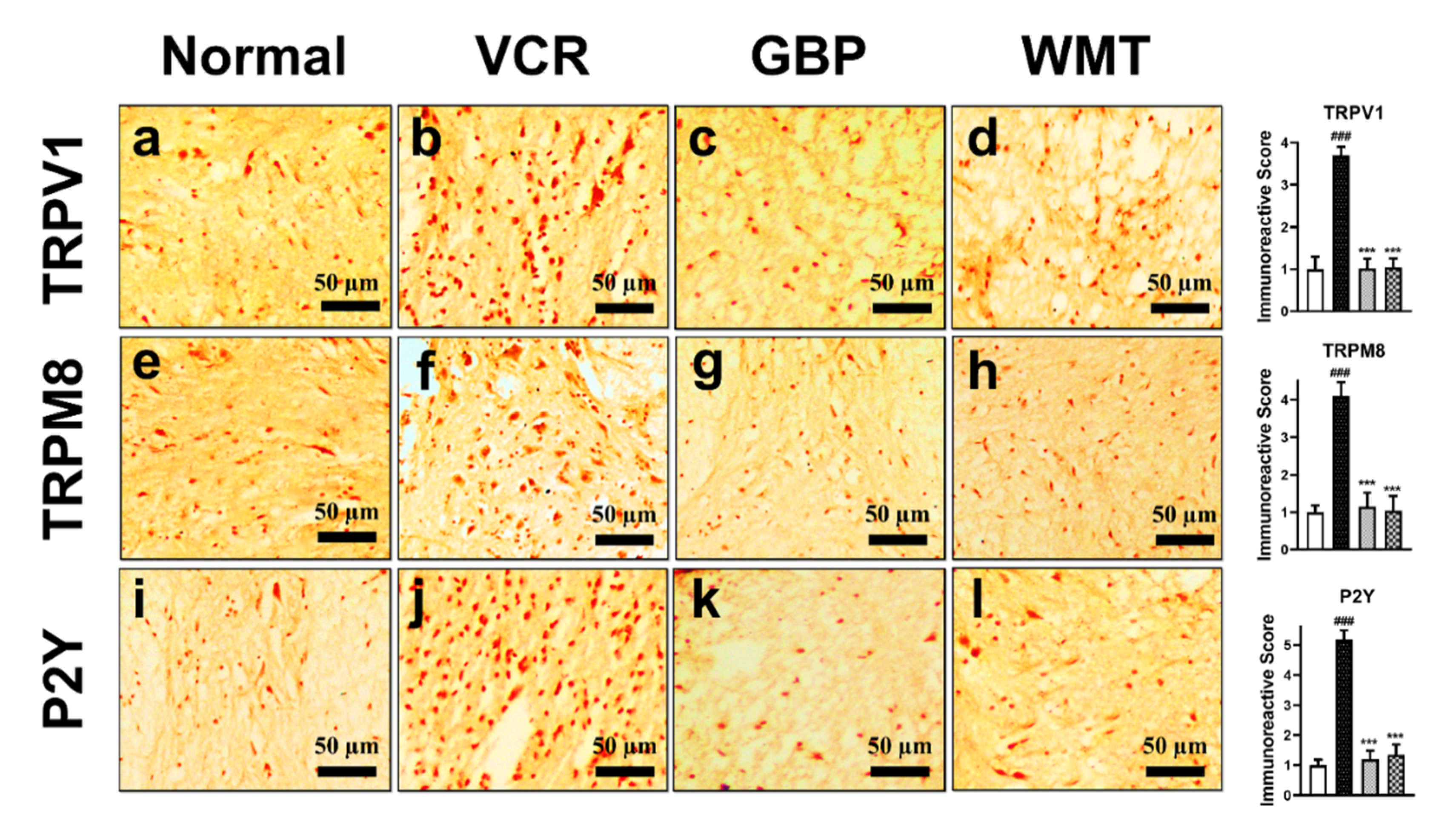
2.11. WMT downregulates TRPV1/TRPM8/P2Y Nociceptors in the Spinal Cord after VCR Administration
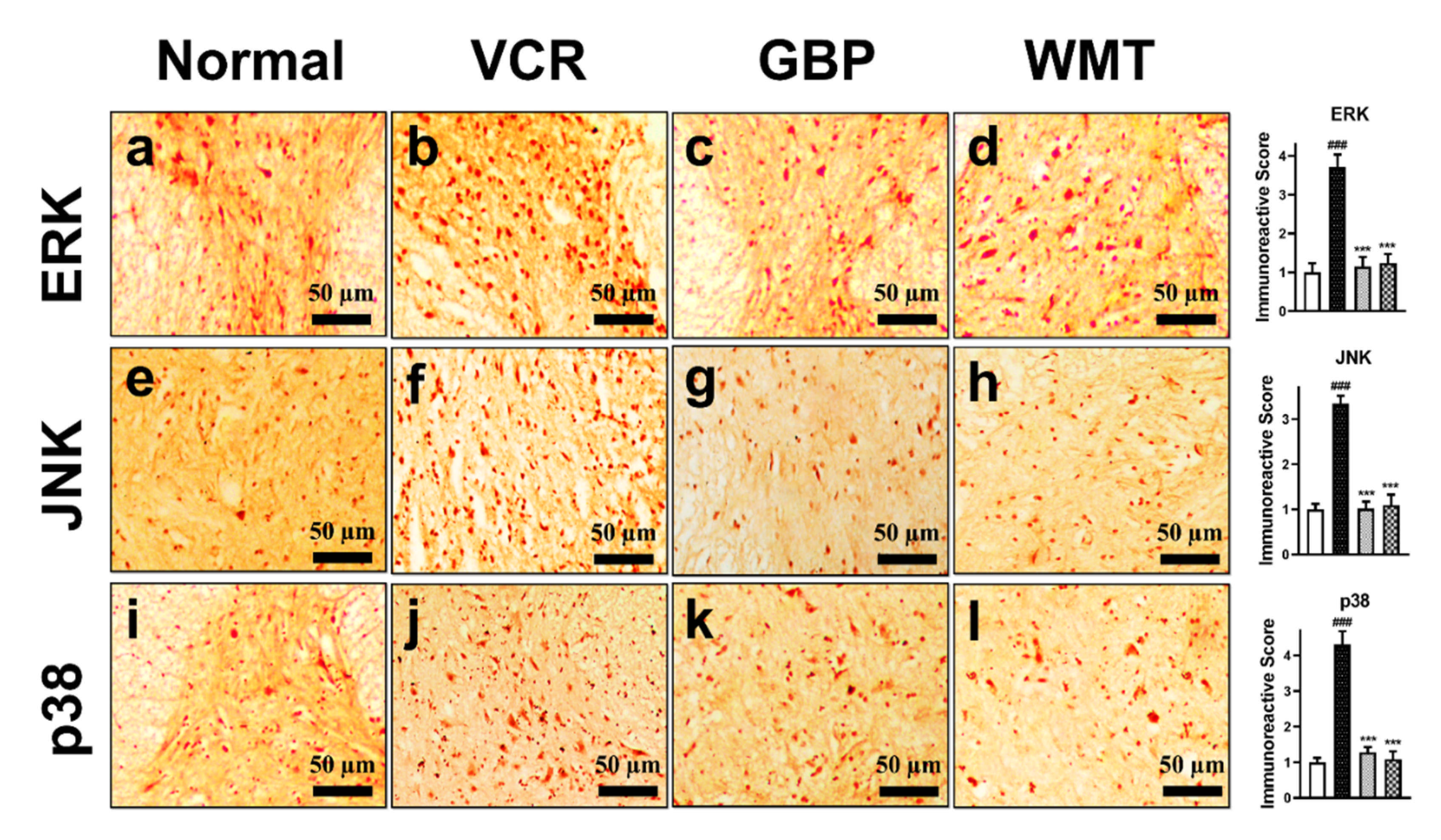
2.12. WMT Attenuates the ERK/JNK/p38 Expression in the Spinal Cord after VCR Administration
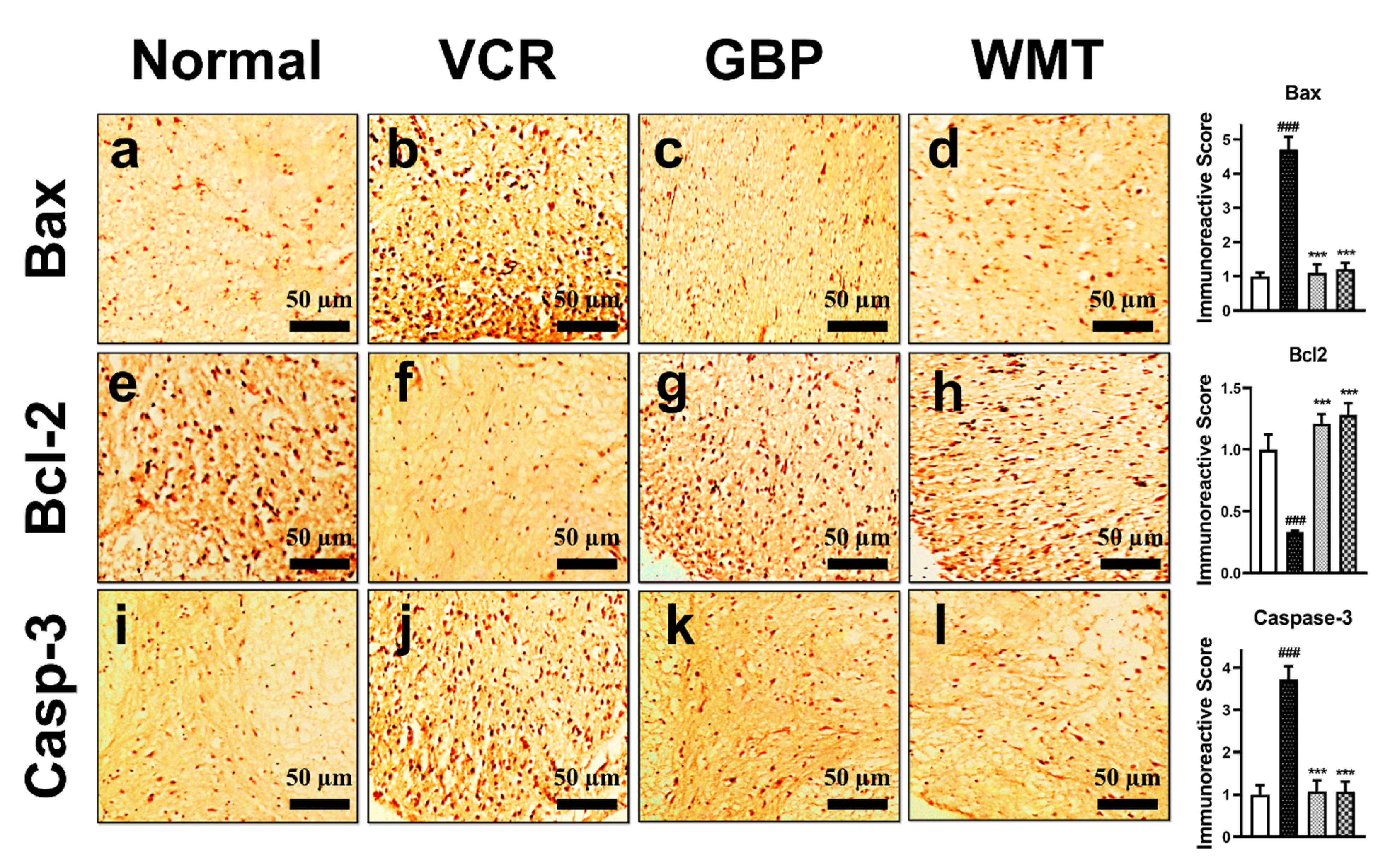
2.13. WMT Suppresses Apoptosis by Regulating the Bax/Bcl-2/Caspase-3 Expression in the Spinal Cord after VCR Administration
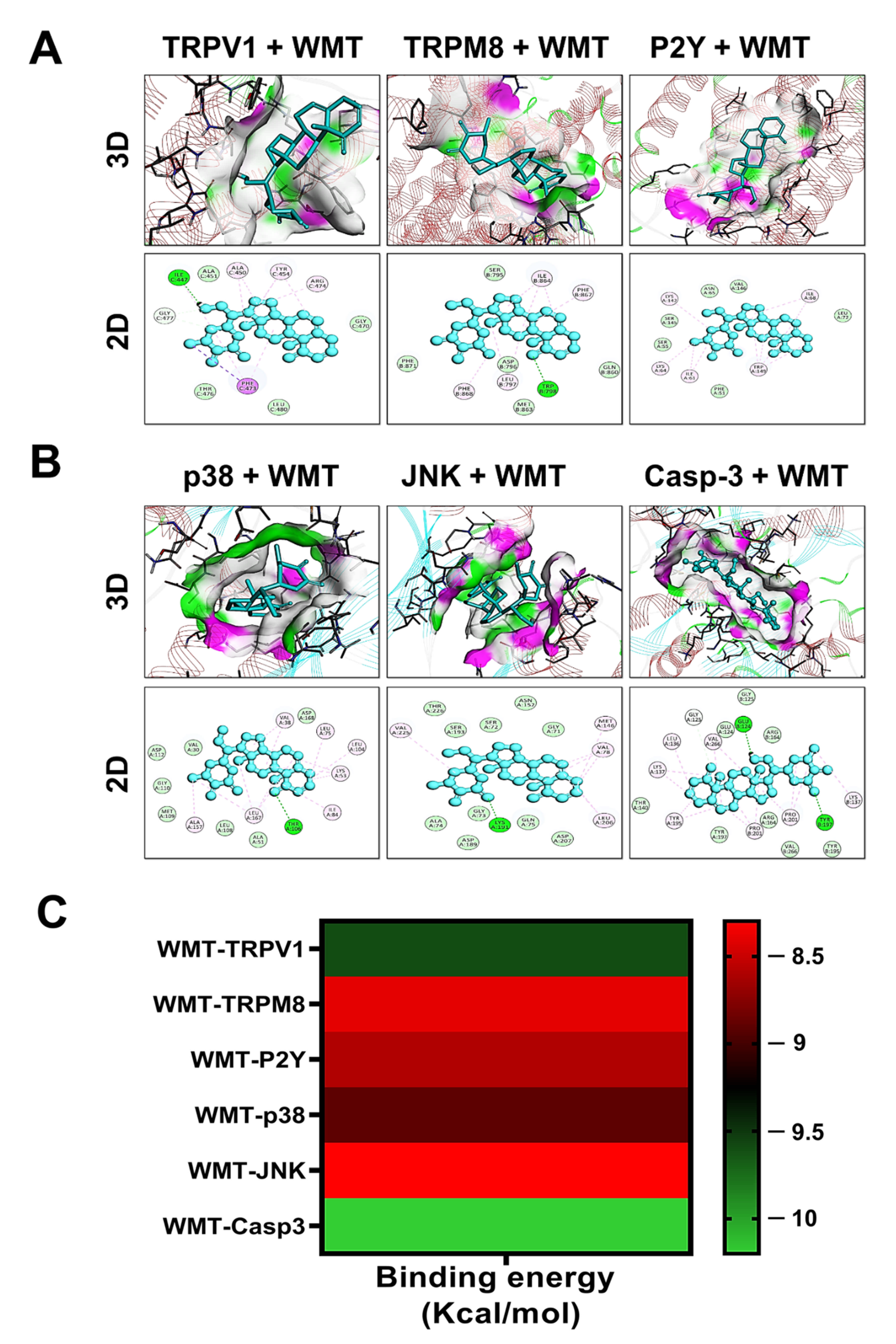
2.14. Docking Interaction with WMT
3. Discussion
4. Materials and Methods
4.1. Experimental Procedure
4.1.1. Reagent and Chemicals
4.1.2. Experimental Animals
4.1.3. Induction of VCR-Induced Peripheral Neuropathy
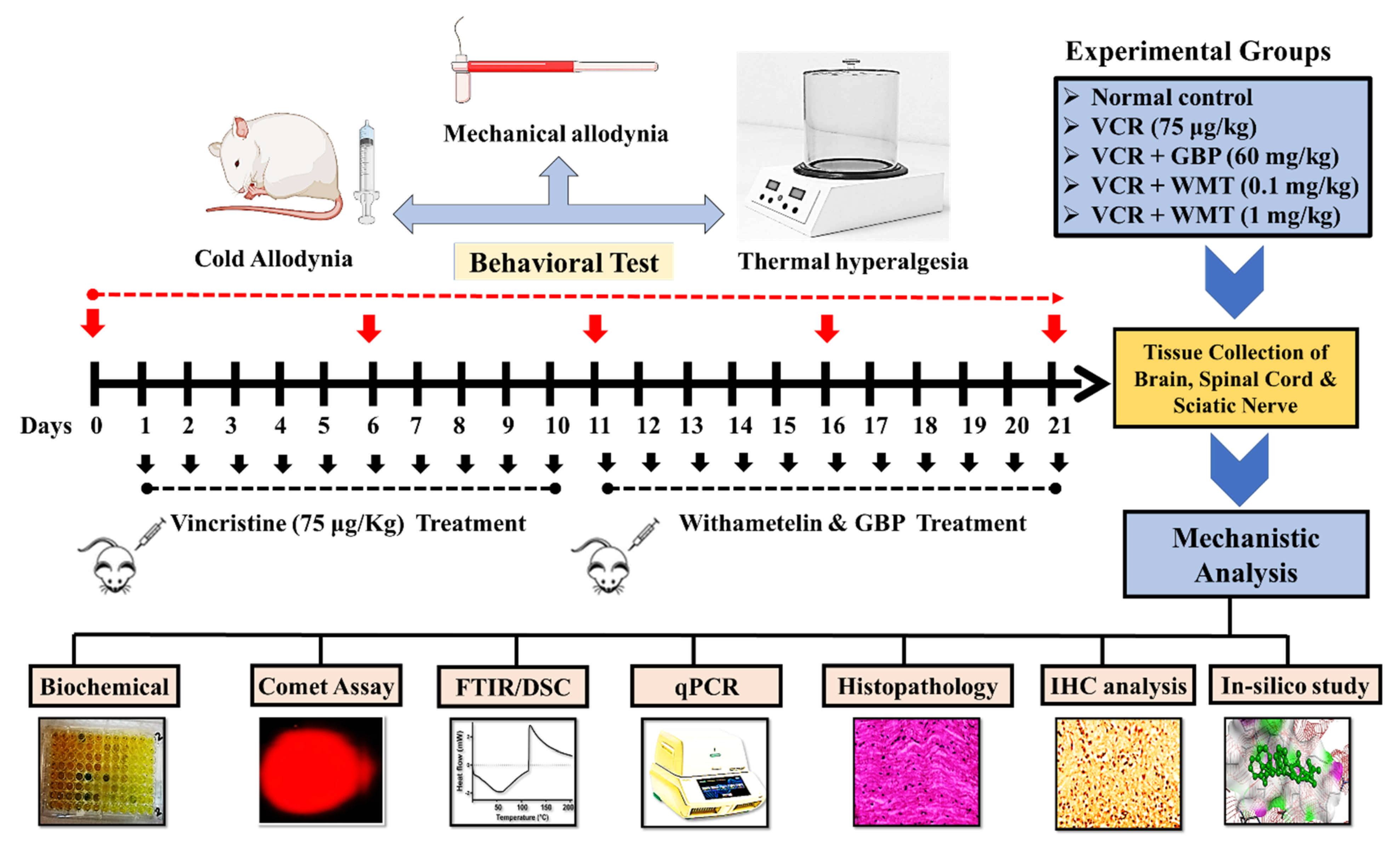
4.2. Experimental Protocol
4.3. Pain Behavioral Tests
4.3.1. Mechanical Allodynia
4.3.2. Thermal Hyperalgesia
4.3.3. Cold Allodynia
4.4. Cell Culture and Treatment
4.5. MTT Assay
4.6. Tissue Preparation
4.7. Biochemical Parameters
4.7.1. Reduced Glutathione
4.7.2. Glutathione S-transferase
4.7.3. Catalase Activity
4.7.4. Superoxide Dismutase Assay
4.7.5. Estimation of Lipid Peroxidation
4.7.6. Myeloperoxidase Activity
4.7.7. Nitric Oxide
4.7.8. Estimation of TNF-α and IL-1β
4.8. Histopathological Analysis
4.8.1. Hematoxylin and Eosin (H&E) Staining of the Brain, Spinal Cord, and Sciatic Nerve
4.8.2. Masson’s Trichrome Staining
4.9. Differential Scanning Calorimetry
4.10. Fourier-Transform Infrared Spectroscopy
4.11. qRT-PCR Analysis of the TRPV1/TRPM8 and JNK
4.12. Single-Cell Gel Electrophoresis
4.13. Immunohistochemical Analysis
4.14. Molecular Docking
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.; Singh, A.; Singh, P.; Bhatti, R. Bergapten ameliorates vincristine-induced peripheral neuropathy by inhibition of inflammatory cytokines and NFκB Signaling. ACS Chem. Neurosci. 2019, 10, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.-S.; Li, Y.-X.; Zhang, M.-T.; Du, J.; Ma, P.-S.; Yao, W.-X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.-Q. Neuroprotective effect of matrine in mouse model of vincristine-induced neuropathic pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef]
- Zhou, L.; Ao, L.; Yan, Y.; Li, C.; Li, W.; Ye, A.; Liu, J.; Hu, Y.; Fang, W.; Li, Y. Levo-corydalmine attenuates vincristine-induced neuropathic pain in mice by upregulating the Nrf2/HO-1/CO pathway to inhibit connexin 43 expression. Neurotherapeutics 2020, 17, 340–355. [Google Scholar] [CrossRef]
- Chen, X.-J.; Wang, L.; Song, X.-Y. Pharmacotherapy, Mitoquinone alleviates vincristine-induced neuropathic pain through inhibiting oxidative stress and apoptosis via the improvement of mitochondrial dysfunction. Biomed. Pharmacother. 2020, 125, 110003. [Google Scholar] [CrossRef]
- Diouf, B.; Evans, W. Pharmacogenomics of vincristine-induced peripheral neuropathy: Progress continues. Clin. Pharmacol. Ther. 2019, 105, 315. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Ali, G.; Khan, R.; Ullah, R.; Ullah, S. Attenuation of vincristine-induced neuropathy by synthetic cyclohexenone-functionalized derivative in mice model. Neurol. Sci. 2019, 40, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Li, Q.; Hou, J.; Li, Z.; Cao, X.; Liu, X.; Qin, B. Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-κB signaling in the dorsal root ganglion of rats with neuropathic pain. J. Pain Res. 2019, 12, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef] [Green Version]
- Rosasco, M.G.; Gordon, S.E.; Islas, L.D.; Katz, B.; Payne, R.; Minke, B.; Geffeney, S.; Moore, C.; Liedtke, W.B.; Yang, P. Neurobiology of TRP Channels; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Spicarova, D.; Palecek, J. The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level. J. Neurophysiol. 2009, 102, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Nazıroğlu, M.; Braidy, N. Thermo-sensitive TRP channels: Novel targets for treating chemotherapy-induced peripheral pain. Front. Physiol. 2017, 8, 1040. [Google Scholar] [CrossRef]
- Marwaha, L.; Bansal, Y.; Singh, R.; Saroj, P.; Bhandari, R.; Kuhad, A. TRP channels: Potential drug target for neuropathic pain. Inflammopharmacology 2016, 24, 305–317. [Google Scholar] [CrossRef]
- Raddatz, N.; Castillo, J.P.; Gonzalez, C.; Alvarez, O.; Latorre, R. Temperature and voltage coupling to channel opening in transient receptor potential melastatin 8 (TRPM8). J. Biol. Chem. 2014, 289, 35438–35454. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, G. P2Y receptors in neuropathic pain. Pharmacol. Biochem. Behav. 2019, 186, 172788. [Google Scholar] [CrossRef]
- Qu, Y.-J.; Jia, L.; Zhang, X.; Wei, H.; Yue, S.-W. MAPK pathways are involved in neuropathic pain in rats with chronic compression of the dorsal root ganglion. Evid. Based Complementary Altern. Med. 2016, 2016, 6153215. [Google Scholar] [CrossRef]
- Obata, K.; Noguchi, K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004, 74, 2643–2653. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.Y.; Jung, H.S.; Park, Y.J.; Kim, Y.S.; In, J.H.; Choi, J.W.; Kim, J.A.; Joo, J.D. Amitriptyline inhibits the MAPK/ERK and CREB pathways and proinflammatory cytokines through A3AR activation in rat neuropathic pain models. Korean J. Anesthesiol. 2019, 72, 60. [Google Scholar] [CrossRef]
- Liao, W.-T.; Tseng, C.-C.; Wu, C.-H.; Lin, C.-R. Early high-frequency spinal cord stimulation treatment inhibited the activation of spinal mitogen-activated protein kinases and ameliorated spared nerve injury-induced neuropathic pain in rats. Neurosci. Lett. 2020, 721, 134763. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Mika, J. Targeting the microglial signaling pathways: New insights in the modulation of neuropathic pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Yamanaka, H.; Yanamoto, F.; Okubo, M.; Noguchi, K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia 2012, 60, 1529–1539. [Google Scholar] [CrossRef]
- Chen, Y.; Willcockson, H.; Valtschanoff, J. Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthr. Cartil. 2009, 17, 244–251. [Google Scholar] [CrossRef] [Green Version]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory process involved in different preclinical models of chemotherapy-induced peripheral neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, M.; Zhang, J.; Xu, R. MAPK: A potential target of chronic pain. Curr. Med. Chem. 2014, 21, 4405–4418. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Hwang, S.-H.; Lee, S.-O.; Kim, S.H.; Abdi, S. Pentoxifylline ameliorates mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Pain Physician 2016, 19, E589–E600. [Google Scholar] [PubMed]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yu, J.; Zhai, D.; Zhang, D.; Shen, W.; Bai, L.; Cai, Z.; Yu, C. Role of JNK activation and mitochondrial Bax translocation in allicin-induced apoptosis in human ovarian cancer SKOV3 cells. Evid. Based Complementary Altern. Med. 2014, 2014, 378684. [Google Scholar] [CrossRef]
- Yardim, A.; Kandemir, F.M.; Ozdemir, S.; Kucukler, S.; Comakli, S.; Gur, C.; Celik, H. Quercetin provides protection against the peripheral nerve damage caused by vincristine in rats by suppressing caspase-3, NF-κB, ATF-6 pathways and activating Nrf2, Akt pathways. Neurotoxicology 2020, 81, 137–146. [Google Scholar] [CrossRef]
- Vashistha, B.; Sharma, A.; Jain, V. Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: An evidence of behavioral and biochemical examination. Nutr. Neurosci. 2017, 20, 60–70. [Google Scholar] [CrossRef]
- Chen, L.-X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef]
- Rao, P.C.; Begum, S.; Jahromi, M.A.F.; Jahromi, Z.H.; Sriram, S.; Sahai, M. Cytotoxicity of withasteroids: Withametelin induces cell cycle arrest at G2/M phase and mitochondria-mediated apoptosis in non-small cell lung cancer A549 cells. Tumor Biol. 2016, 37, 12579–12587. [Google Scholar] [CrossRef]
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.U. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complementary Altern. Med. 2015, 15, 376. [Google Scholar] [CrossRef] [Green Version]
- Baig, M.W.; Nasir, B.; Waseem, D.; Majid, M.; Khan, M.Z.I.; Haq, I.U. Withametelin: A biologically active withanolide in cancer, inflammation, pain and depression. Saudi Pharm. J. 2020, 28, 1526–1537. [Google Scholar] [CrossRef]
- Saki, K.; Bahmani, M.; Rafieian-Kopaei, M.; Hassanzadazar, H.; Dehghan, K.; Bahmani, F.; Asadzadeh, J. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac. J. Trop. Dis. 2014, 4, S895–S901. [Google Scholar] [CrossRef]
- Jiang, K.; Shi, J.; Shi, J. Morin alleviates vincristine-induced neuropathic pain via nerve protective effect and inhibition of NF-κB pathway in rats. Cell. Mol. Neurobiol. 2019, 39, 799–808. [Google Scholar] [CrossRef]
- Khalilzadeh, M.; Panahi, G.; Rashidian, A.; Hadian, M.R.; Abdollahi, A.; Afshari, K.; Shakiba, S.; Norouzi-Javidan, A.; Rahimi, N.; Momeny, M. The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology 2018, 67, 279–286. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Du, K.; Wu, W.; Feng, X. Puerarin alleviates vincristine-induced neuropathic pain and neuroinflammation via inhibition of nuclear factor-κB and activation of the TGF-β/Smad pathway in rats. Int. Immunopharmacol. 2020, 89, 107060. [Google Scholar] [CrossRef]
- Khan, A.; Khan, A.; Khalid, S.; Shal, B.; Kang, E.; Lee, H.; Laumet, G.; Seo, E.K.; Khan, S. 7β-(3-Ethyl-cis-crotonoyloxy)-1α-(2-methylbutyryloxy)-3, 14-dehydro-Z Notonipetranone Attenuates Neuropathic Pain by Suppressing Oxidative Stress, Inflammatory and Pro-Apoptotic Protein Expressions. Molecules 2021, 26, 181. [Google Scholar] [CrossRef]
- Valek, L.; Kanngießer, M.; Tegeder, I. Expression and regulation of redoxins at nociceptive signaling sites after sciatic nerve injury in mice. Data Brief 2015, 5, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Kazmi, Z.; Zeeshan, S.; Khan, A.; Malik, S.; Shehzad, A.; Seo, E.K.; Khan, S. Anti-epileptic activity of daidzin in PTZ-induced mice model by targeting oxidative stress and BDNF/VEGF signaling. NeuroToxicology 2020, 79, 150–163. [Google Scholar] [CrossRef]
- Tenchov, B.; Abarova, S.; Koynova, R.; Traikov, L.; Dragomanova, S.; Tancheva, L. A new approach for investigating neurodegenerative disorders in mice based on DSC. J. Therm. Anal. Calorim. 2017, 127, 483–486. [Google Scholar] [CrossRef]
- Sałat, K.; Filipek, B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B 2015, 16, 167–178. [Google Scholar] [CrossRef]
- Luo, J.; Bavencoffe, A.; Yang, P.; Feng, J.; Yin, S.; Qian, A.; Yu, W.; Liu, S.; Gong, X.; Cai, T.; et al. Zinc inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain. J. Neurosci. 2018, 38, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, T.; Liu, X.; Chen, Y.; Kong, D.; Tian, H.; Yue, M.; Huang, D.; Zeng, J. Activation of spinal dorsal horn P2Y13 receptors can promote the expression of IL-1β and IL-6 in rats with diabetic neuropathic pain. J. Pain Res. 2018, 11, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Samad, T.A.; Jin, S.-X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Horváth, G.; Gölöncsér, F.; Csölle, C.; Király, K.; Andó, R.D.; Baranyi, M.; Koványi, B.; Máté, Z.; Hoffmann, K.; Algaier, I.; et al. Central P2Y12 receptor blockade alleviates inflammatory and neuropathic pain and cytokine production in rodents. Neurobiol. Dis. 2014, 70, 162–178. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Wang, J.; Li, H.; Xia, L. IL-35 alleviates inflammation progression in a rat model of diabetic neuropathic pain via inhibition of JNK signaling. J. Inflamm. 2019, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. In Chemical Biology; Springer: Berlin, Germany, 2015; pp. 243–250. [Google Scholar]
- Khan, A.; Shal, B.; Naveed, M.; Nasir, B.; Irshad, N.; Ali, H.; Khan, S. Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology 2020, 237, 2327–2343. [Google Scholar] [CrossRef]
- Liaquat, I.; Khan, A.-U.; Khan, S. Pharmacotherapy, Pharmacological evaluation of continentalic acid for antidiabetic potential. Biomed. Pharmacother. 2021, 138, 111411. [Google Scholar] [CrossRef]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-κB and Nrf2 signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Khan, A.; Ullah, M.Z.; Afridi, R.; Rasheed, H.; Khalid, S.; Ullah, H.; Ali, H.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Antinociceptive properties of 25-methoxy hispidol A, a triterpinoid isolated from Poncirus trifoliata (Rutaceae) through inhibition of NF-κB signalling in mice. Phytother. Res. 2019, 33, 327–341. [Google Scholar] [CrossRef]
- Naveed, M.; Khan, S.Z.; Zeeshan, S.; Khan, A.; Shal, B.; Atiq, A.; Ali, H.; Ullah, R.; Khan, S. A new cationic palladium (II) dithiocarbamate exhibits anti-inflammatory, analgesic, and antipyretic activities through inhibition of inflammatory mediators in in vivo models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 961–977. [Google Scholar] [CrossRef]
- Khalid, S.; Khan, A.; Shal, B.; Ali, H.; Kim, Y.S.; Khan, S. Pharmacotherapy, Suppression of TRPV1 and P2Y nociceptors by honokiol isolated from Magnolia officinalis in 3rd degree burn mice by inhibiting inflammatory mediators. Biomed. Pharmacother. 2019, 114, 108777. [Google Scholar] [CrossRef]
- Ur Rehman, F.; Mazhar, K.; Malik, A.; Naz, S.S.; Shah, K.U.; Khan, A.; Khan, S.; Ahmed, R.; Qaisar, S. Surface modified multifaceted nanocarriers for oral non-conventional cancer therapy; synthesis and evaluation. Mater. Sci. Eng. 2021, 123, 111940. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Lu, C.; Wu, J.; Ding, M.; Wang, C.; Mao, N.; Shi, Z. Allicin protects against H2O2-induced apoptosis of PC12 cells via the mitochondrial pathway. Exp. Ther. Med. 2017, 14, 2053–2059. [Google Scholar] [CrossRef]
- Lou, H.; Jing, X.; Ren, D.; Wei, X.; Zhang, X. Eriodictyol protects against H2O2-induced neuron-like PC12 cell death through activation of Nrf2/ARE signaling pathway. Neurochem. Int. 2012, 61, 251–257. [Google Scholar] [CrossRef]
- Khan, A.; Shal, B.; Naveed, M.; Shah, F.A.; Atiq, A.; Khan, N.U.; Kim, Y.S.; Khan, S. Matrine ameliorates anxiety and depression-like behaviour by targeting hyperammonemia-induced neuroinflammation and oxidative stress in CCl4 model of liver injury. Neurotoxicology 2019, 72, 38–50. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Popov, G.; Shkondrov, A.; Manov, V.; Krasteva, I. Alcesefoliside protects against oxidative brain injury in rats. Rev. Bras. Farmacogn. 2019, 29, 221–227. [Google Scholar] [CrossRef]
- Ali, J.; Khan, A.U.; Shah, F.A.; Ali, H.; Islam, S.U.; Kim, Y.S.; Khan, S. Mucoprotective effects of Saikosaponin-A in 5-fluorouracil-induced intestinal mucositis in mice model. Life Sci. 2019, 239, 116888. [Google Scholar] [CrossRef] [PubMed]
- Sajad, M.; Zargan, J.; Chawla, R.; Umar, S.; Sadaqat, M.; Khan, H.A. Hippocampal neurodegeneration in experimental autoimmune encephalomyelitis (EAE): Potential role of inflammation activated myeloperoxidase. Mol. Cell. Biochem. 2009, 328, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, A.M.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Khan, S. The newly synthesized compounds (NCHDH and NTHDH) attenuates LPS-induced septicemia and multi-organ failure via Nrf2/HO1 and HSP/TRVP1 signaling in mice. Chem. Biol. Interact. 2020, 329, 109220. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, S.; Naveed, M.; Khan, A.; Atiq, A.; Arif, M.; Ahmed, M.N.; Kim, Y.S.; Khan, S. N-Pyrazoloyl and N-thiopheneacetyl hydrazone of isatin exhibited potent anti-inflammatory and anti-nociceptive properties through suppression of NF-κB, MAPK and oxidative stress signaling in animal models of inflammation. Inflamm. Res. 2019, 68, 613–632. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, A.; Ali, J.; Ullah, H.; Khan, A.; Ali, H.; Irshad, N.; Khan, S. Toxicology, Attenuation of LPS-induced acute lung injury by continentalic acid in rodents through inhibition of inflammatory mediators correlates with increased Nrf2 protein expression. BMC Pharmacol. Toxicol. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Khan, A.U.; Khan, A.; Khan, A.; Shal, B.; Aziz, A.; Ahmed, M.N.; Islam, S.U.; Ali, H.; Shehzad, A.; Khan, S. Inhibition of NF-κB signaling and HSP70/HSP90 proteins by newly synthesized hydrazide derivatives in arthritis model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 1–23. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Springer: Berlin, Germany, 2014; pp. 31–43. [Google Scholar]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef]
- Türedi, S.; Yuluğ, E.; Alver, A.; Bodur, A.; İnce, İ.J. A morphological and biochemical evaluation of the effects of quercetin on experimental sciatic nerve damage in rats. Exp. Ther. Med. 2018, 15, 3215–3224. [Google Scholar]
- Shal, B.; Khan, A.; Naveed, M.; Ali, H.; Seo, E.K.; Choi, H.; Khan, S. Neuroprotective effect of 25-Methoxyhispidol A against CCl4-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci. 2020, 253, 117684. [Google Scholar] [CrossRef]
- Shal, B.; Khan, A.; Naveed, M.; Khan, N.U.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Pharmacotherapy, Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. Biomed. Pharmacother. 2019, 111, 209–223. [Google Scholar] [CrossRef]
- Dhawan, A.; Bajpayee, M.; Parmar, D. Comet assay: A reliable tool for the assessment of DNA damage in different models. Cell Biol. Toxicol. 2009, 25, 5–32. [Google Scholar] [CrossRef]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
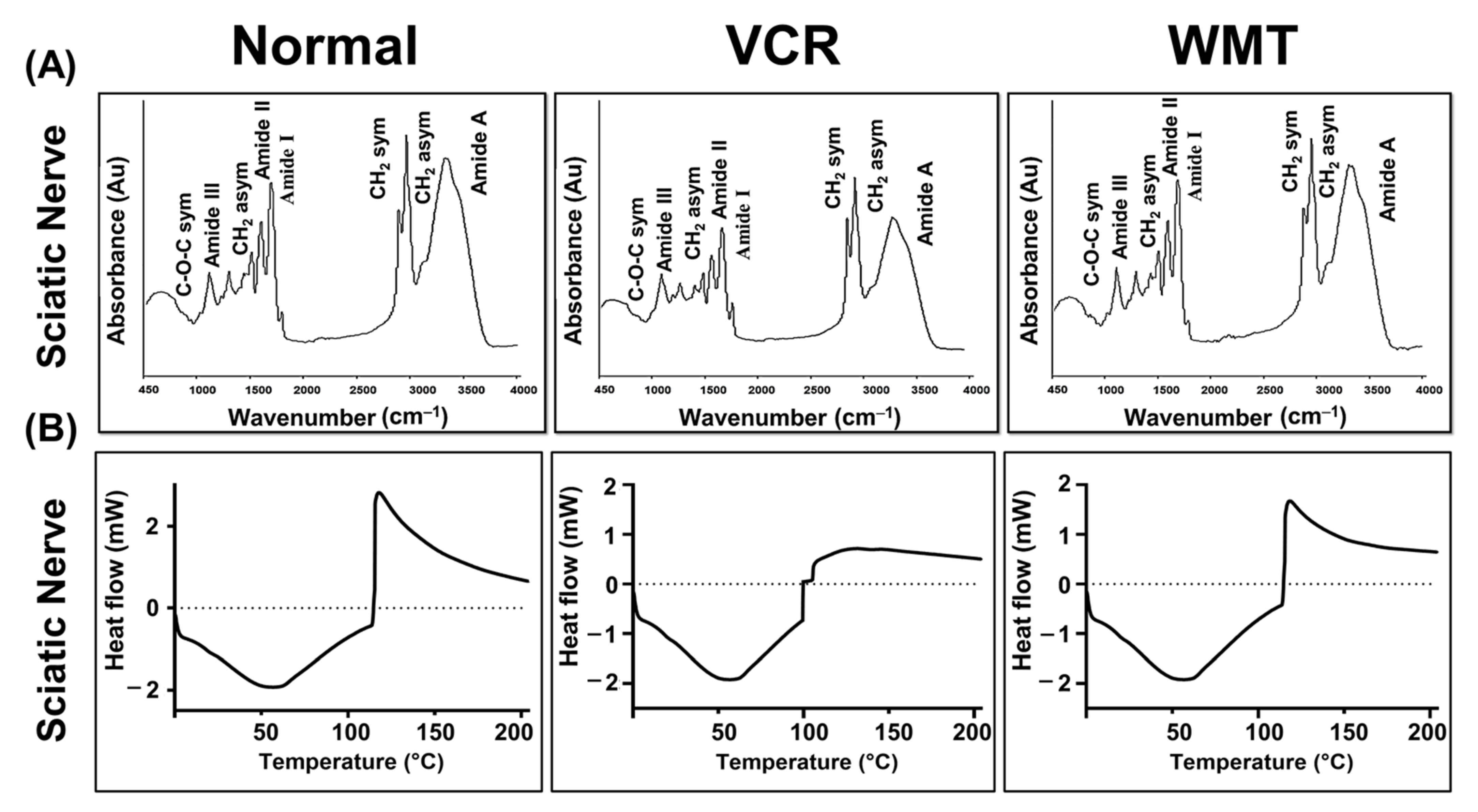
| Wavenumber (cm−1) | Spectral Assignment |
|---|---|
| 3285 | N-H str of amide A: Protein |
| 2926 | CH2 asym str: Lipids (fatty acid) |
| 2850 | CH2 sym str: Membrane lipids (membrane fatty acid) |
| 1650 | C=O sym str band of α-helical structure (amide I): Protein |
| 1545 | N-H bend, vib; C-N str of the amino acid (amide II): Protein |
| 1460 | CH2 sciss, vib; CH2 asym bend and vib of phospholipid: Membrane lipids |
| 1300 | C-N str, N-H bending; C=O str; O=C-N bend and vib (amide III): Protein |
| 1062 | Ester C-O-C sym str (phospholipids); ribose C-O str (Nucleic acids) |
| Genes | Forward Primers | Reverse Primers |
|---|---|---|
| TRPV1 | AAGGCTCTATGATCGCAGGA | CAGATTGAGCATGGCTTTGA |
| TRPM8 | ACATACCAAGGAGTTTCCAACAG | GCTGGGTCAGCAGTTCGTAG |
| P2Y | CGTGCTGGTGTGGCTCATT | GGACCCCGGTACCTGAGTAGA |
| JNK | AGCCTTGTCCTTCGTGTC | AAAGTGGTCAACAGAGCC |
| β-actin | CATCACCATCGGAATGAG | CACGGTGTTGGCATACAGG |
| WMT–Protein Interaction | Binding Energy (Kcal/mol) | Hydrogen Bond | Hydrophobic Interactions |
|---|---|---|---|
| WMT–TRPV1 | −9.6 | ILE C:447 | ALA C:451, TYR C 454, ARG C:474, GLY C:477 |
| WMT–TRPM8 | −8.4 | TRP B: 78 | ILE B:864, PHE B:867, ASP B: 796, PHE B:868 |
| WMT–P2Y | −8.6 | ---------- | ILE A:68, LEU A:72, TRP A: 149, LYS A: 142 |
| WMT–p38 | −8.9 | THR A:106 | VAL A:38, LEU A:75, LYS A:53, ALA A:157 |
| WMT–JNK | −8.3 | LYS A:191 | VAL A:78, LEU A: 206, VAL A:225, MET A:146 |
| WMT–Casp-3 | −10.2 | GLU B:124 TYR B:197 | VAL A:266, LYS A:137, TYR A:195, PRO B: 201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Shal, B.; Khan, A.U.; Ullah, R.; Baig, M.W.; ul Haq, I.; Seo, E.K.; Khan, S. Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain. Int. J. Mol. Sci. 2021, 22, 6084. https://doi.org/10.3390/ijms22116084
Khan A, Shal B, Khan AU, Ullah R, Baig MW, ul Haq I, Seo EK, Khan S. Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain. International Journal of Molecular Sciences. 2021; 22(11):6084. https://doi.org/10.3390/ijms22116084
Chicago/Turabian StyleKhan, Adnan, Bushra Shal, Ashraf Ullah Khan, Rahim Ullah, Muhammad Waleed Baig, Ihsan ul Haq, Eun Kyoung Seo, and Salman Khan. 2021. "Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain" International Journal of Molecular Sciences 22, no. 11: 6084. https://doi.org/10.3390/ijms22116084
APA StyleKhan, A., Shal, B., Khan, A. U., Ullah, R., Baig, M. W., ul Haq, I., Seo, E. K., & Khan, S. (2021). Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain. International Journal of Molecular Sciences, 22(11), 6084. https://doi.org/10.3390/ijms22116084







