Abstract
Candida auris is a multidrug-resistant fungal pathogen that can cause disseminated bloodstream infections with up to 60% mortality in susceptible populations. Of the three major classes of antifungal drugs, most C. auris isolates show high resistance to azoles and polyenes, with some clinical isolates showing resistance to all three drug classes. We reported in this study a novel approach to treating C. auris disseminated infections through passive transfer of monoclonal antibodies (mAbs) targeting cell surface antigens with high homology in medically important Candida species. Using an established A/J mouse model of disseminated infection that mimics human candidiasis, we showed that C3.1, a mAb that targets β-1,2-mannotriose (β-Man3), significantly extended survival and reduced fungal burdens in target organs, compared to control mice. We also demonstrated that two peptide-specific mAbs, 6H1 and 9F2, which target hyphal wall protein 1 (Hwp1) and phosphoglycerate kinase 1 (Pgk1), respectively, also provided significantly enhanced survival and reduction of fungal burdens. Finally, we showed that passive transfer of a 6H1+9F2 cocktail induced significantly enhanced protection, compared to treatment with either mAb individually. Our data demonstrate the utility of β-Man3- and peptide-specific mAbs as an effective alternative to antifungals against medically important Candida species including multidrug-resistant C. auris.
1. Introduction
Candida auris is an emerging fungal pathogen first identified in Tokyo, Japan in 2009 [1]. It has since emerged throughout much of the world, with many countries reporting multiple clinical cases [2]. Unlike other pathogenic Candida species, C. auris has a propensity to colonize abiotic surfaces as well as the human skin [3]. This makes the nosocomial spread of the pathogen especially prevalent and contributes to a higher potential to disseminate into bloodstream infections compared to other Candida species. Consequently, ICU patients and nursing home residents are highly vulnerable to nosocomial infections with C. auris, and migration from the skin to a disseminated bloodstream infection is especially common in patients with underlying comorbidities, those under immunosuppressed conditions, or those who have undergone invasive surgical interventions [4,5]. This ease of spread has contributed to a slew of healthcare-associated outbreaks, with contamination of ICUs persisting for several weeks [2,6]. Furthermore, with the ongoing COVID-19 pandemic caused by the novel coronavirus, SARS-CoV-2, the rate of hospitalizations is currently extremely high. With many ICU units being filled to capacity, this creates the perfect environment for further C. auris ICU outbreaks [7,8,9]. Once systemic, C. auris infection is often fatal, having a case mortality rate of 33–60% [4,10,11,12], which is much higher than that of other pathogenic Candida species. Mortality is most often attributed to multiorgan failure, with the kidney and heart being most susceptible [13,14,15].
A defining feature of C. auris, among other Candida species, is its multidrug resistance. Although antifungal resistance has been reported in other Candida species, most notably with Candida glabrata, the degree of antifungal resistance observed in C. auris is unprecedented [2]. In a study that looked at antifungal resistance in 99 clinical isolates of C. auris from the United States, 89% of isolates were resistant to fluconazole, 30% were resistant to amphotericin B, and 6% were resistant to echinocandin drugs [16]. In another study of 1385 United States clinical isolates of C. glabrata, 9.6% of isolates were resistant to fluconazole and 6% were resistant to echinocandin drugs [17]. Similarly, C. auris clinical isolates from across the globe have consistently shown high resistance to antifungals within the azole and polyene drug classes [4,18]. Being so, the typical course of treatment for C. auris bloodstream infections is the daily administration of echinocandin drugs, such as micafungin, caspofungin, or anidulafungin. A major limitation of antifungals, however, is their associated drug toxicities. Immunocompromised patients, who are most susceptible to disseminated infection, are in a fragile state and often unable to tolerate additional organ toxicity caused by commonly prescribed antifungal drugs, therefore rending these drugs ineffective [19]. Furthermore, there have been several reports of C. auris isolates that are pan-resistant to all three major antifungal drug classes, which greatly limits treatment options [4]. Due to its high degree of antifungal resistance, the potential to spread throughout the hospital environment, and its associated high mortality rate, C. auris is the first fungal pathogen to be labeled a serious global public health threat, and new treatments are urgently needed [20].
To overcome the problems of C. auris antifungal resistance and drug toxicity, we sought to investigate if prophylactic treatment using Candida-specific monoclonal antibodies (mAbs) could induce protection against C. auris bloodstream infections in A/J mice, as an alternative to conventional antifungal drug treatment. Protective mAb therapy is an emerging, yet highly promising strategy for the treatment of microbial diseases [21,22]. Antibodies are known to confer protection to various pathogens via several mechanisms, including neutralization, opsonization, and complement activation [23]. As of today, the United States Food and Drug Administration (FDA) has approved five different synthetic mAb for the treatment of various viral and bacterial diseases, including human immunodeficiency virus (HIV) and Clostridioides difficile infections [24]. Additionally, two new synthetic mAb-based treatments, Eli Lilly’s Bamlanivimab and Regeneron’s REGN-COV2 cocktail, are currently in clinical trials and have shown promising efficacy against COVID-19 [25], and the FDA has approved both drugs for emergency use authorization (EUA) [26,27]. Presently, there are no mAb-derived drugs for the treatment of fungal diseases, even within clinical trials. Particularly with pathogens such as C. auris, which have developed high levels of drug resistance and cause high mortality in immunocompromised patients, mAb therapy is an attractive treatment option.
Since the cell wall is the first point of contact between Candida and the host’s immune system, we developed “universal mAbs” that target various Candida cell wall epitopes that share high homology among various Candida species. A major benefit of universal mAbs is that they could potentially be applied for the treatment of candidemia caused by multiple pathogenic species of Candida, such as Candida albicans, Candida glabrata, Candida tropicalis, Candida krusei, and C. auris. This is especially important because infected individuals often do not receive a timely diagnosis due to unspecific symptoms of invasive candidiasis.
Overall, we hypothesized that prophylactic treatment with universal mAbs would induce extended survival and enhanced fungal clearance within an A/J mouse model of C. auris disseminated infection. A/J mice are deficient in complement protein C5 and its cleaved product C5a, a pro-inflammatory chemoattractant important for anti-Candida protection [28,29,30]. This renders A/J mice highly susceptible to C. auris disseminated infection without the need for immunosuppressive drugs [31]. Using this model, we identified three mAbs that provided significant protection, as evidenced by extended survival and lower fungal burdens in the kidney, brain, and heart, compared to control mice. In addition, our results showed that two of our mAbs could be administered as a cocktail to further enhance their effectiveness. Overall, our results demonstrate the efficacy of passive transfer with universal mAbs as a novel treatment against multidrug-resistant C. auris.
2. Results
2.1. In Vitro and In Vivo Efficacy of Antifungals against Multidrug-Resistant C. auris
C. auris isolates can be grouped into five clades (I-V) originating from different geographic regions [4]. Within each clade, isolates may have differences in morphology, levels of virulence, growth rates, and antifungal-resistance profiles [4,32]. Being so, in preparation for our animal studies, we first investigated the antifungal susceptibility of two clinical isolates of C. auris belonging to distinct clades: AR-0386 (CAU-06) of Clade IV and AR-0389 (CAU-09) of Clade I. AR-0386 is a highly aggregative South American isolate that has been shown to be less virulent than C. albicans in mouse models [33], while AR-0389 is a nonaggregative South Asian isolate that is highly virulent in mouse models [31,34]. It has been reported that nonaggregating C. auris isolates such as AR-0389 are among the most virulent clinical isolates, with virulence comparable to that of C. albicans in the invertebrate Galleria mellonella model [35].
We first performed an in vitro minimum inhibitory concentration (MIC) assay using two commonly administered antifungal drugs, micafungin, and itraconazole. These two antifungals belong to the echinocandin and azole drug classes, respectively, and isolates AR-0386 and AR-0389 have been reported to be susceptible to both drugs in vitro [34,36]. As a comparison, we also tested the MICs for C. albicans reference strain SC5314. After 48-h of drug exposure, the micafungin MIC50 was determined to be 0.063 μg/mL for AR-0386, 0.125 μg/mL for AR-0389, and 0.031 μg/mL for C. albicans (Table 1). For itraconazole, the 48 h MIC50 was 2.0 μg/mL for AR-0386, 0.25 μg/mL for AR-0389, and 0.031 μg/mL for C. albicans. These results showed that AR-0386 and AR-0389 were susceptible to micafungin and itraconazole in vitro, although both isolates were much more resistant to itraconazole than was C. albicans.

Table 1.
Micafungin and itraconazole MIC50 for C. auris isolates AR-0386 and AR-0389 and C. albicans isolate SC5314 at 24 and 48 h.
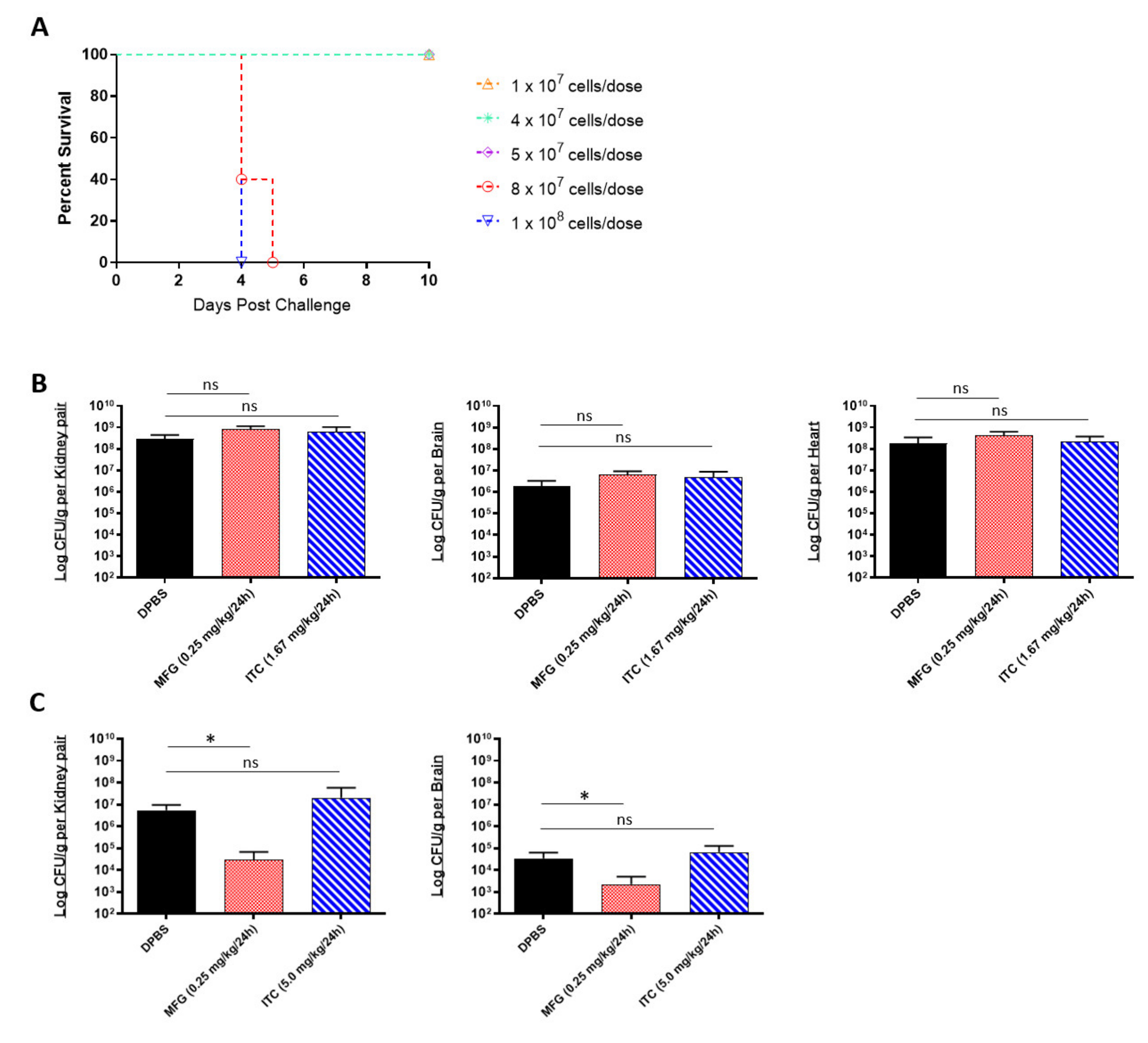
A limitation of in vitro assays is that they reflect the limited environment within the test tube, which is considerably different from the environmental conditions encountered in vivo. Being that MIC data cannot always reliably predict in vivo drug susceptibility [37], we next tested the micafungin and itraconazole susceptibility of C. auris using a complement C5-deficient A/J mouse model of disseminated infection [33]. To begin, we established an appropriate sublethal challenge dose that would result in 80–100% survival within 10 days post challenge using the highly virulent AR-0389 strain (Figure 1A). Our results showed that doses below 8 × 107 CFUs resulted in 100% survival by day 10. Accordingly, we decided to use a dose of 4 × 107 CFUs for our in vivo antifungal susceptibility study. After the challenge, mice were treated daily with a minimum protective dose of micafungin (0.25 mg/kg/day) or itraconazole (1.67 mg/kg/day), which were determined via an antifungal pilot study (data not shown). Upon termination on Day 6, micafungin-treated mice showed no reduction in fungal burdens in the kidney, brain, or heart compared to control mice (Figure 1B). Similarly, itraconazole provided no significant reduction in organ burdens using the minimum dose.
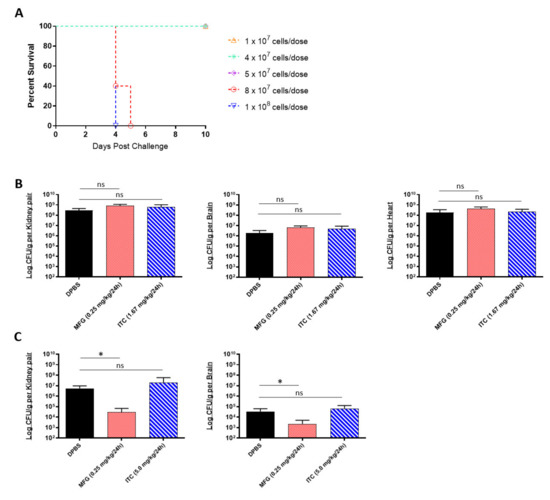
Figure 1.
In vivo efficacy of antifungals against multidrug-resistant C. auris: (A) the 10-day survival curve of 7-week-old female A/J mice challenged with C. auris AR-0389 doses ranging from 1 × 107 to 1 × 108 CFUs; (B) quantification of kidney, brain, and heart fungal burdens from 7-week-old female A/J treated daily for 5 days with a minimum protective dose of micafungin or itraconazole. Mice were challenged with a sub-lethal dose of 4 × 107 CFUs of C. auris isolate AR-0389. Starting 24 h later, mice received daily administration of 200 μL of DPBS, micafungin (0.25 mg/kg body weight), or itraconazole (1.67 mg/kg body weight). Mice were sacrificed on day 6 post challenge; (C) quantification of kidney and brain fungal burdens from 16- to 17-week-old male and female neutropenic C57BL/6 mice treated daily for 14 days with a minimum protective dose of micafungin or itraconazole. To induce neutropenia, mice were administered cyclophosphamide (200 mg/kg body weight) on day 3 and every 7 days after (150-mg/kg body weight). On day 0, mice were challenged with a sublethal dose of 4 × 107 CFUs of C. auris isolate AR-0389. Starting 24 h later, mice received daily administration of 200 μL of DPBS, micafungin (0.25 mg/kg body weight), or itraconazole (5.0 mg/kg body weight). Mice were sacrificed on day 15 post challenge. MFG = micafungin, ITC = itraconazole. Data are mean + SD (B,C). n = 4 (B) n = 5 (A,C). Log-rank (Mantel–Cox) test (A) or two-tailed t-test (B,C) were used to identify significant differences. * p < 0.05; ns = not significant.
Different inbred mouse strains have differences in MHC haplotypes, immunophenotype features, and isoforms of metabolic enzymes which can contribute to varying rates of drug metabolism [38,39]. Being so, we repeated our in vivo micafungin and itraconazole susceptibility assay using an immunosuppressed C57BL/6 mouse model of disseminated infection to compare C. auris susceptibility to that of A/J mice. C57BL/6 mice were immunosuppressed with cyclophosphamide prior to challenge with a sub-lethal dose of C. auris AR-0389. Beginning 18 h post challenge, mice were administered micafungin or itraconazole daily for 14 days and then sacrificed on day 15. As with our A/J mouse model, we saw no protective effect using itraconazole, even with a higher dose (5.0 mg/kg/day) (Figure 1C). Interestingly, with the minimum dose of micafungin, we observed a significant reduction in fungal burdens in the kidney and brain (2.9 × 104 and 2.2 × 103 CFUs/g, respectively) by day 15 as compared to DPBS mice kidney and brain burdens (5.5 × 106 and 3.4 × 104 CFUs/g, respectively). Comparing these results to our MIC data showed that although C. auris AR-0389 is susceptible to both micafungin and itraconazole in vitro, only low-dose micafungin was effective at significantly reducing fungal burdens in immunosuppressed C57BL/6 mice. The data showed that C. auris is susceptible to minimum doses of micafungin in vivo, but this could vary with mouse strain and cannot be predicted solely using in vitro MIC data.
2.2. Candida Cell Surface Binding of Universal Candida-Specific Monoclonal Antibodies
Considering that antifungals have limited efficacy against multidrug-resistant C. auris, we next investigated the protective efficacy of a panel of universal monoclonal antibodies (mAbs) that target different Candida cell surface epitopes, which share high homology among various Candida species (Table 2). First, we validated antibody binding to cell surface epitopes by flow cytometric analysis using C. auris AR-0386 and AR-0389 and C. albicans SC5314. We focused on three mAbs that were shown to be protective in our preliminary studies: C3.1, which targets β-1,2-mannotriose (β-Man3, IgG3), a mannose sugar that is abundantly expressed and distributed on the outer cell wall of most Candida species [40,41]; 6H1, which targets hyphal wall protein 1 (Hwp1, IgG2a), a cell wall mannoprotein that is involved in adhesion, biofilm formation, and hyphal development in several Candida species [42]; 9F2, which targets phosphoglycerate kinase 1 (Pgk1, IgG1), a metabolic enzyme that is primarily involved in glycolysis and gluconeogenesis within the cytoplasm [43,44].

Table 2.
Universal Candida monoclonal antibodies and their cell surface targets.
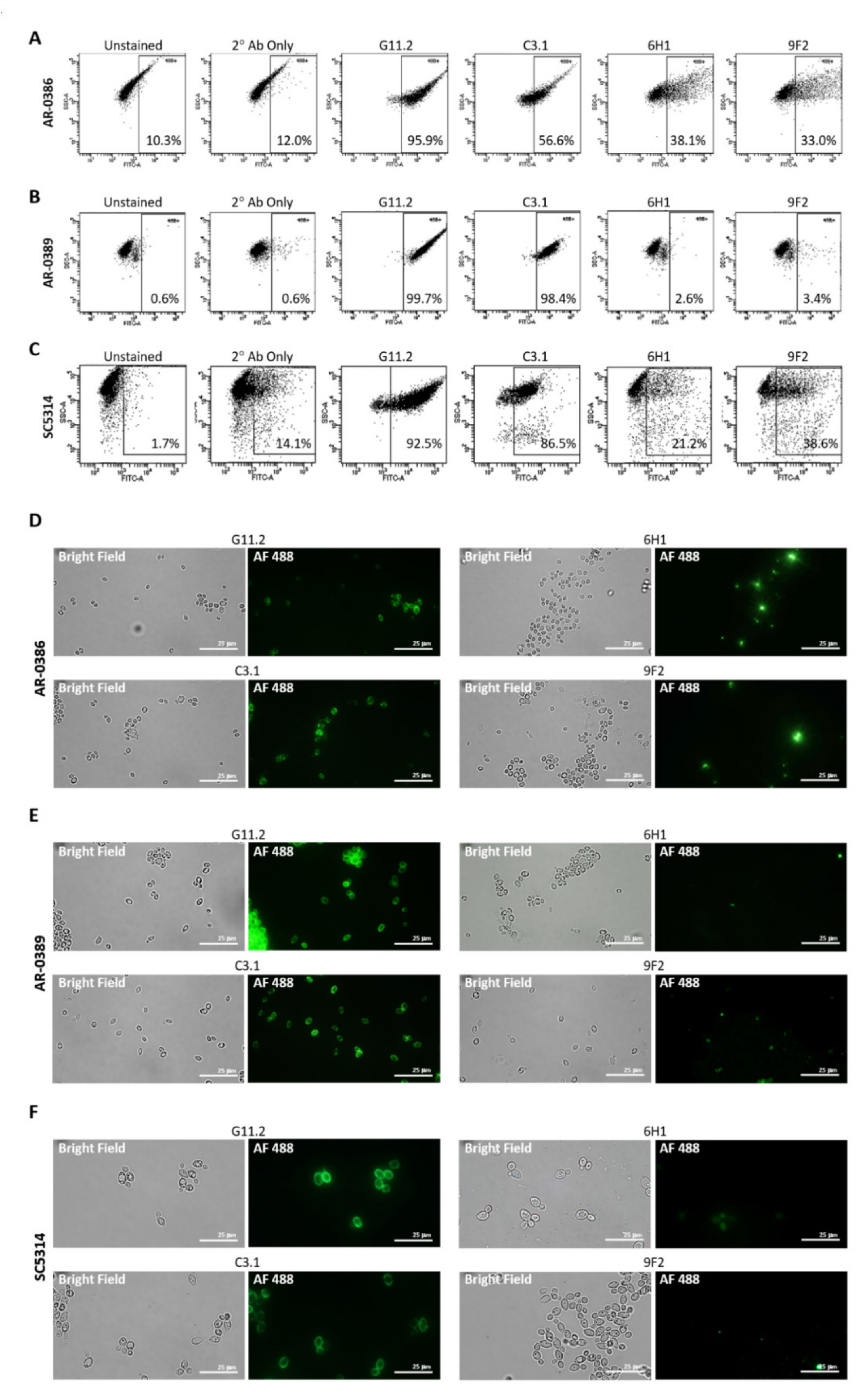
Isolates were incubated with each primary mAb, washed, and then incubated with goat anti-mouse secondary antibody conjugated to Alexa Fluor 488. Antibody binding was then detected using flow cytometry (Figure 2A–C). C3.1 (anti-β-Man3, IgG3) showed 56.6% binding to AR-0386, 98.4% binding to AR-0389, and 86.5% to C. albicans, while 6H1 (anti-Hwp1, IgG2b) showed binding of 38.1% to AR-0386, 2.6% to AR-0389, and 21.2% to C. albicans. Finally, binding of 9F2 (anti-Pgk 1, IgG2a) was at 33.0% for AR-0386, 3.4% for AR-0389, and 38.6% for C. albicans. Additionally, fluorescent microscopy imaging of cells stained with each antibody (Figure 2D–F) depicted levels of fluorescence that corresponded with our flow cytometry data. Since mannose sugars are abundantly expressed on the outer cell wall [40,41], a high level of C3.1 binding in all isolates was expected. Pgk1 and Hwp1, on the other hand, are not major components of the Candida cell wall and not as abundantly expressed as β-Man3, which was reflected in our mAb-binding data. It was, however, surprising to see modest Hwp1 binding, since C. auris has not been demonstrated to express Hwp1, and its genome has not been shown to contain an ortholog of the Hwp1 gene. This pointed to the possibility that our anti-Hwp1 mAb could be cross-reactive with another C. auris cell wall protein, in which further investigation is required to identify the target as well as confirm its sequence. It was also surprising to see such disparate levels of 6H1 or 9F2 binding between the two C. auris isolates. The lower levels of 9F2 and 6H1 binding to AR-0389 could be an indication of epitope masking, which could explain the higher level of virulence of this isolate, compared to AR-0386. Overall, the data showed that the universal mAbs bind to C. auris in an isolate-specific manner.
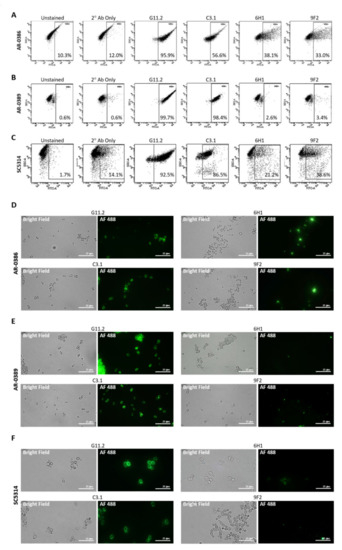
Figure 2.
Cell surface binding of universal Candida-specific monoclonal antibodies: (A–C) flow cytometry scatter plots depicting levels of cell surface binding of monoclonal antibodies as a measure of Alexa Fluor 488 expression in (A) C. auris isolate AR-0386, (B) C. auris isolate AR-0389, and (C) C. albicans isolate SC5314; (D–F) confocal microscopy analysis (1000X) showing antibody cell surface staining of (D) C. auris isolate AR-0386, (E) C. auris isolate AR-0389, and (F) C. albicans isolate SC5314 using mAbs C3.1, 6H1, and 9F2. G11.2 = β-1,2-mannotriose (IgG1), C3.1 = β-1,2-mannotriose (IgG3), Hwp1 = hyphal wall protein 1, Pgk1 = phosphoglycerate kinase 1. Bar = 25 μm.
2.3. In Vivo Protective Efficacy of Universal Candida β-1,2-Mannotriose- and Peptide-Specific Monoclonal Antibodies
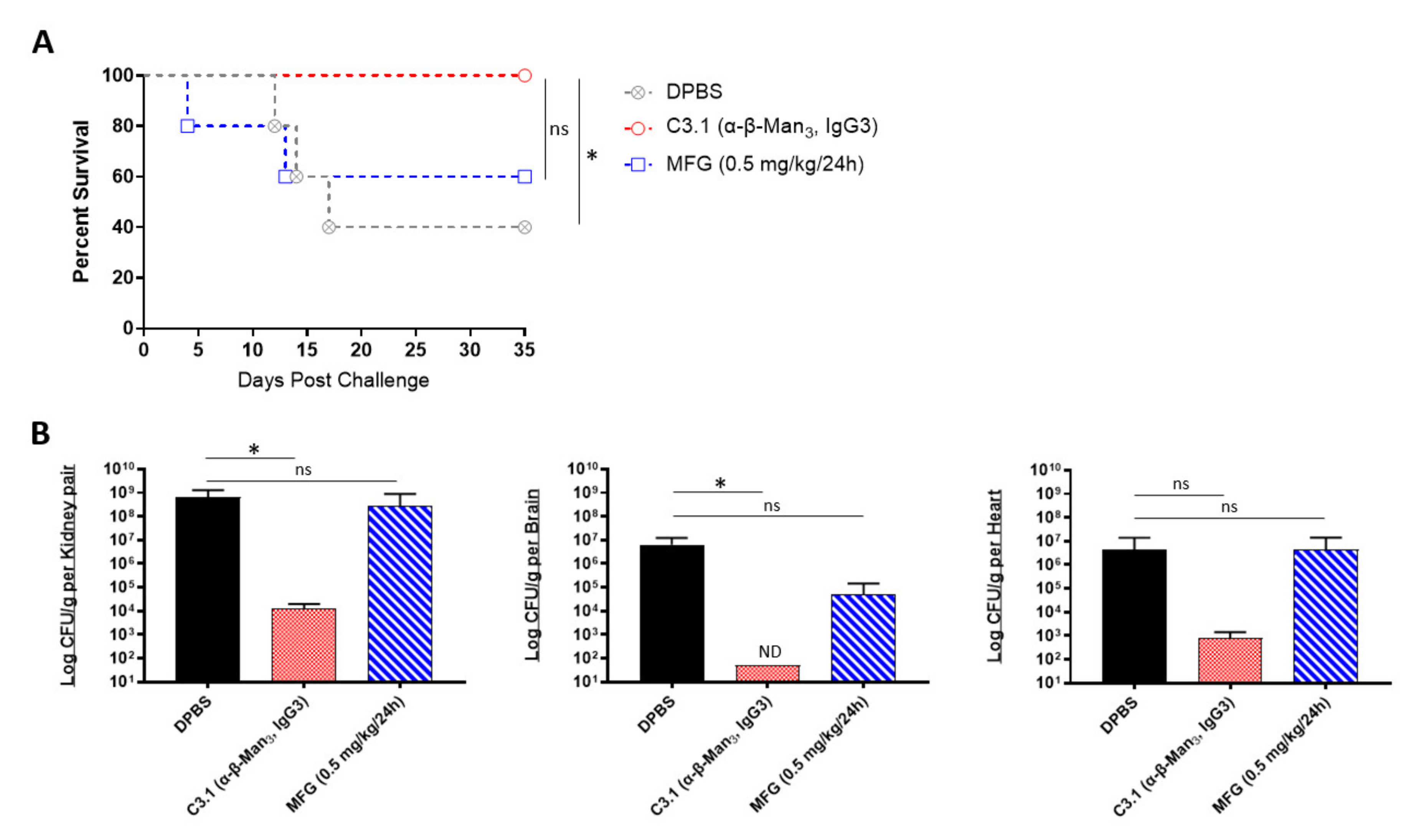
We next evaluated if the prophylactic passive transfer of our universal mAbs would protect mice against C. auris disseminated infection. We began with C3.1 (anti-β-Man3, IgG3), a mAb that has previously been shown to be highly protective against C. albicans disseminated bloodstream infections in mice [45]. Since the C. auris cell wall has a high composition of β-1-2-mannans—reportedly even higher than in C. albicans [46], we hypothesized that mAb C3.1 would also protect against disseminated infection caused by C. auris. To compare the efficacy of C3.1 to that of micafungin, A/J mice were treated one time with C3.1 or DPBS or treated daily for 7 days with micafungin. 24 h after C3.1 treatment, mice were challenged with a sublethal dose of C. auris AR-0386. By day 35 post infection, there was a significant increase in survival of C3.1-treated mice (100% survival), compared to DPBS control mice (40% survival) (Figure 3A). Micafungin-treated mice survival (60% survival) was not significantly extended, compared to the DPBS group. When quantifying fungal burdens, C3.1-treated mice had a significant reduction in kidney and brain burdens (1.2 × 104 and 5.0 × 101 CFUs/g, respectively), compared to control mice (6.6 × 108 and 6.2 × 106 CFUs/g, respectively) (Figure 3B). Remarkably, all C3.1-treated mice had undetectable brain burdens by day 35. While there was also a reduction in heart burdens in C3.1-treated mice (8.2 × 102 CFUs/g), compared to DPBS control (4.4 × 106 CFUs/g), this change was not statistically significant. Consistent with survival data, there was no significant reduction in the kidney, brain, or heart burdens in micafungin-treated mice (2.8 × 108, 4.9 × 104, and 4.4 × 106 CFUs/g, respectively), as compared to DPBS mice. The data showed that mAb C3.1 treatment outperformed low-dose micafungin, a gold-standard drug for the treatment of invasive C. auris infection in an A/J mouse model.
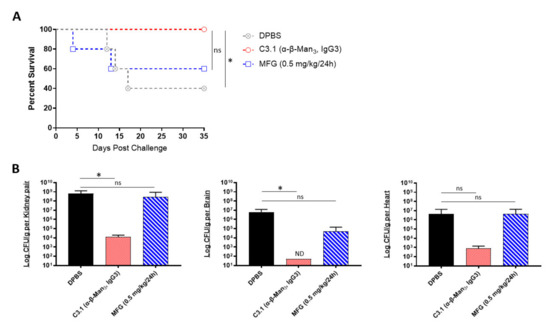
Figure 3.
In vivo protective efficacy of universal Candida β-1,2-mannotriose-specific monoclonal antibody: (A) the 35-day survival curve and (B) quantification of kidney, brain, and heart fungal burdens from 7-week-old female A/J mice treated with mAb C3.1 or micafungin. Mice were treated with 200 μL DPBS, mAb C3.1 (0.24 mg/200 μL DPBS), or micafungin (0.5 mg/kg body weight daily for 7 days) then challenged 18 h later with a sub-lethal dose of 4 × 107 CFUs of C. auris AR-0386. β-Man3 = β-1,2-mannotriose. Data are mean + SD (B). n = 5. Log-rank (Mantel–Cox) test (A) or two-tailed t-test (B) were used to identify significant differences. * p < 0.05; ns = not significant. ND = not detectable.
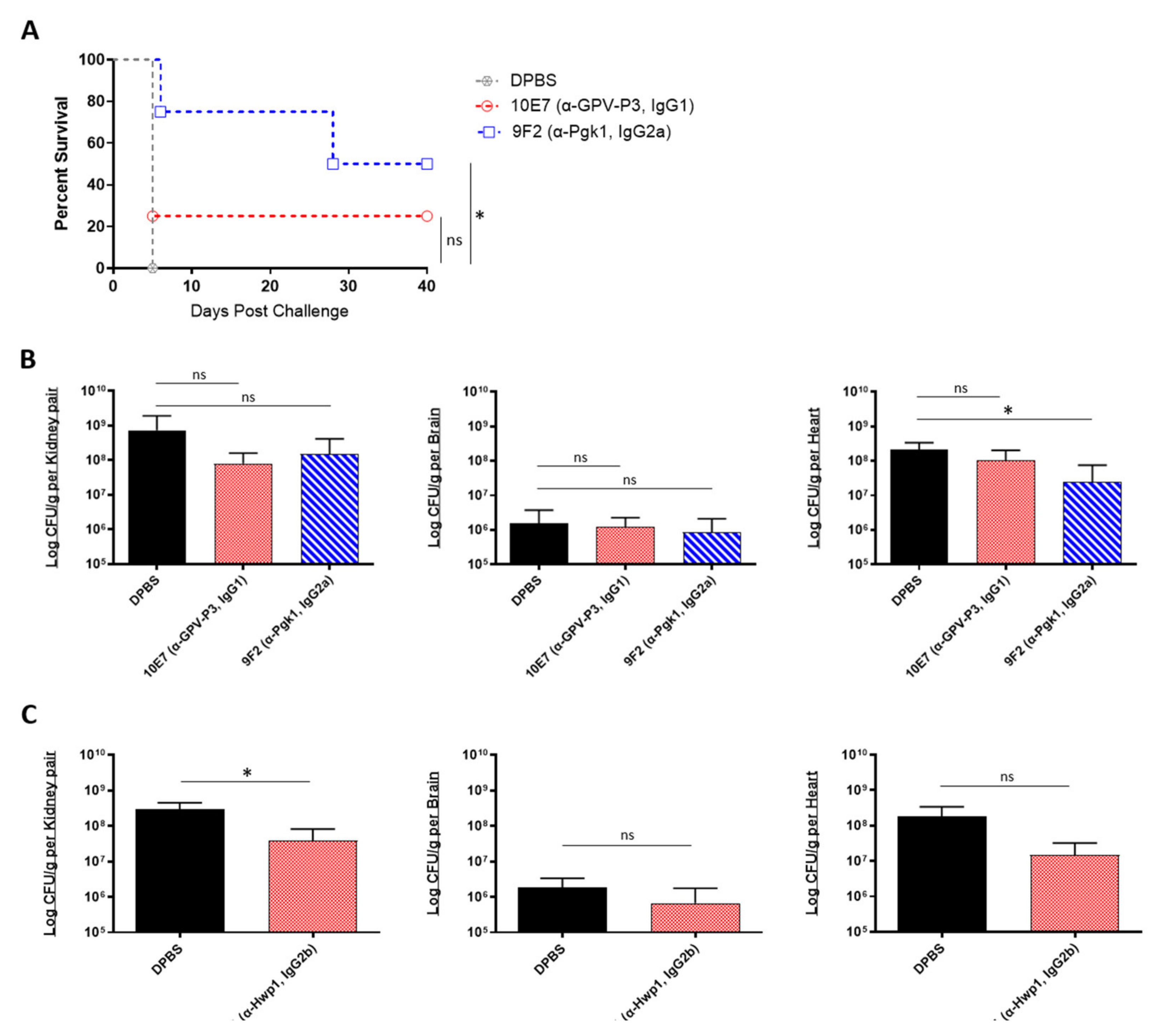
We then evaluated the prophylactic efficacy of our peptide-specific mAbs. In addition to the mAbs 6H1 and 9F2, we also screened one additional universal mAb, 10E7, which targets a different epitope on Pgk1 (GPV-P3, IgG1). A/J mice were treated with each mAb, followed by a lethal dose challenge with C. auris AR-0386 18 h later. Survival was observed for 40 days, and fungal burdens were quantified in the kidney, brain, and heart. With this lethal challenge dose, all control mice died on day 5 post challenge (Figure 4A). On the other hand, 9F2 and 10E7 mice had prolonged survival (50% and 25% survival, respectively) by day 40, with 9F2 inducing significantly higher survival, compared to control mice. Although not statistically significant, both mAb-treated groups had slightly reduced fungal burdens in the kidneys, with 9F2 also inducing significantly lower heart burdens (2.5 × 107 CFUs/g), compared to DPBS mice (2.1 × 108 CFUs/g) (Figure 4B). This reduction in heart burdens is host significant, because A/J mice ultimately succumb to cardiac failure, therefore making the heart the best indicator for evaluating disease progress and protection [33,47]. There was no difference in brain burdens among mAb-treated and control groups.
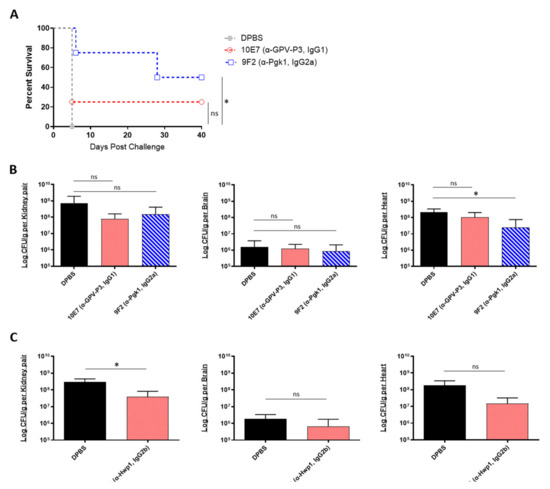
Figure 4.
In vivo protective efficacy of universal Candida peptide-specific monoclonal antibodies: (A) the 40-day survival curve and (B) quantification of kidney, brain, and heart fungal burdens from 7-week-old male A/J mice treated with two Pgk1-specific mAbs; (A,B) mice were treated with 200 μL DPBS or antibody (0.285 mg/200 μL DPBS), then challenged 18 h later with a lethal dose of 1 × 108 CFUs of C. auris AR-0386; (C) quantification of kidney, brain, and heart fungal burdens from 7-week-old female A/J mice treated with mAb 6H1 or micafungin. Mice were treated with 100 μL DPBS or mAb (0.135 mg/100 μL DPBS), then challenged 18 h later with a sublethal dose of 4 × 107 CFUs of C. auris AR-0389. GPV-P3 = phosphoglycerate kinase 1 (IgG1), Pgk1 = phosphoglycerate kinase 1 (IgG2a). Data are mean + SD (B,C). n = 4. Log-rank (Mantel–Cox) test (A) or two-tailed t-test (B,C) were used to identify significant differences. * p < 0.05; ns = not significant.
In a separate experiment, we also tested the prophylactic protective efficacy of 6H1 (anti-Hwp1, IgG2b) in A/J mice using a sublethal challenge dose of C. auris AR-0389. Mice were sacrificed on day 6, and fungal burdens were quantified as previously (Figure 4C). Based on the data, 6H1-treated mice had lower fungal burdens in the kidney, brain, and heart (3.8 × 107, 6.7 × 105, and 1.5 × 107 CFUs/g, respectively), compared to DPBS mice kidney, brain, and heart burdens (2.9 × 108, 1.8 × 106, and 1.8 × 108 CFUs/g, respectively). Of these, only the kidneys had a statistically significant reduction in fungal burdens, while the reduction in heart burdens was nearly significant (p = 0.0798). Collectively, the data showed that passive transfer of universal Candida peptide-specific mAbs, 9F2 and 6H1 provided significantly extended survival (9F2) and significantly reduced fungal burdens in the kidney (6H1) and heart (9F2), compared to control mice in an A/J mouse model of C. auris disseminated infection.
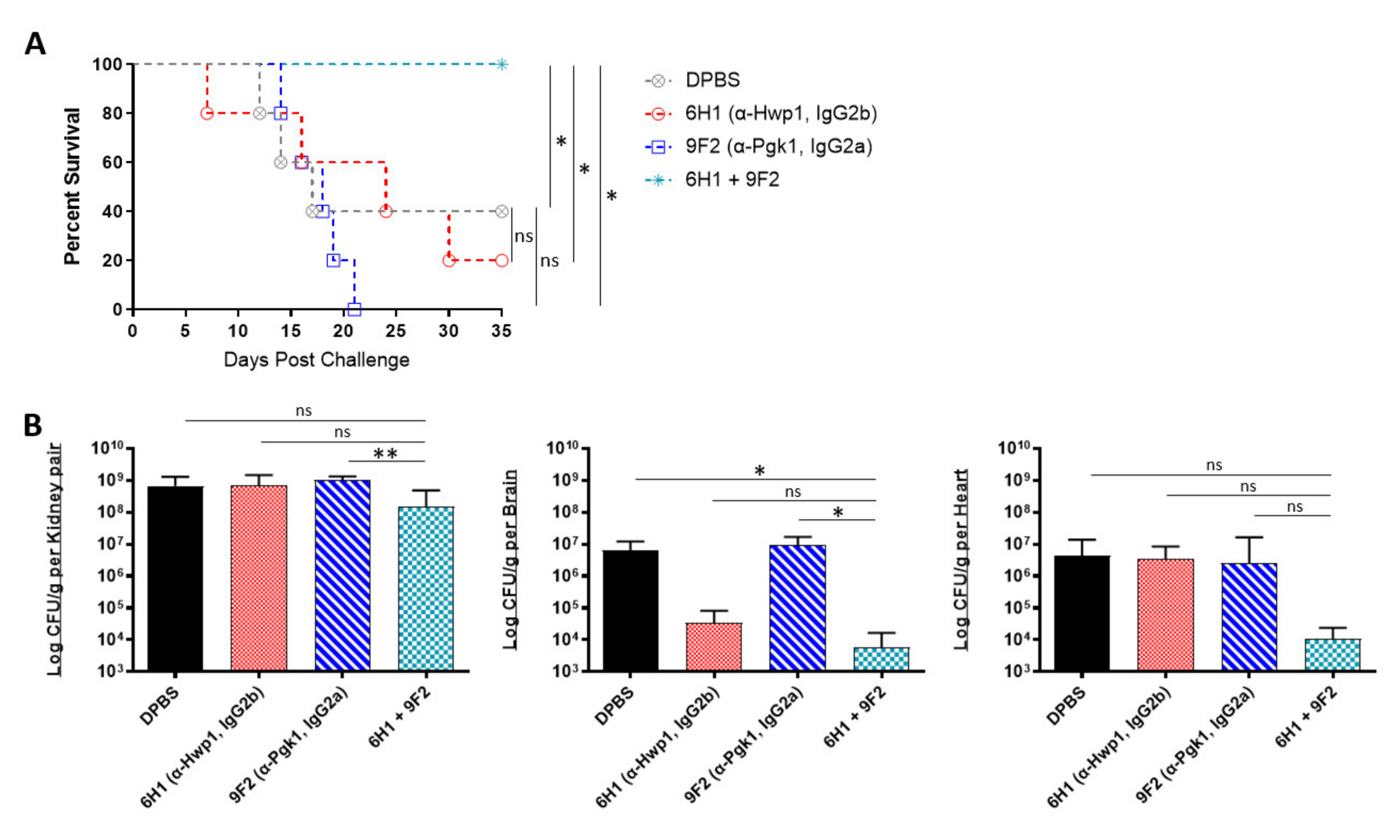
2.4. In Vivo Protective Efficacy of Monoclonal Antibody Cocktails
Being that several of our antibodies were able to induce protection in mice when administered individually, we further tested if combining two different mAbs would induce enhanced or even synergistic protection in mice. Since our mAbs are specific to different cell surface antigens, we hypothesized that using a cocktail of two mAbs could result in a more effective “double hit” protection against C. auris. The selected two-mAb cocktail consisted of 6H1, which performed best in the kidney (Figure 4C), and 9F2, which performed best in the heart (Figure 4B). A/J mice were treated with 6H1, 9F2, or both, followed by a sublethal dose challenge of AR-0386 24 h later. Survival was observed for 35 days. Mice that received the 6H1+9F2 cocktail had significantly higher survival (100% survival) by day 35, compared to mice that received only 6H1 (20% survival) or 9F2 (0% survival) (Figure 5A). Regarding fungal burdens (Figure 5B), mice that received the cocktail had significantly lower burdens in the kidney (1.5 × 108 CFUs/g), compared to mice that received only 9F2 (1.0 × 109 CFUs/g). Kidney burdens in the cocktail group were also lower than in the 6H1 group (6.7 × 108 CFUs/g), although this was not statistically significant. Similarly, fungal burdens in the brain were significantly lower in mice that received the cocktail (5.8 × 103 CFUs/g), compared to mice that received only 9F2 (9.3 × 106 CFUs/g). The cocktail group brain burdens were also lower than the 6H1 group (3.4 × 104 CFUs/g), although this was not statistically significant. We also observed a consistent trend of lower heart burdens for the mAb cocktail treated group (1.1 × 104 CFUs/g), compared to 6H1 mice (3.3 × 106 CFUs/g) or 9F2 (8.3 × 106 CFUs/g), although not statistically significant. Overall, the data showed that protective mAbs, such as 6H1 and 9F2, can be combined into cocktails to provide enhanced protection against C. auris disseminated infection compared to treatment with either mAb individually.
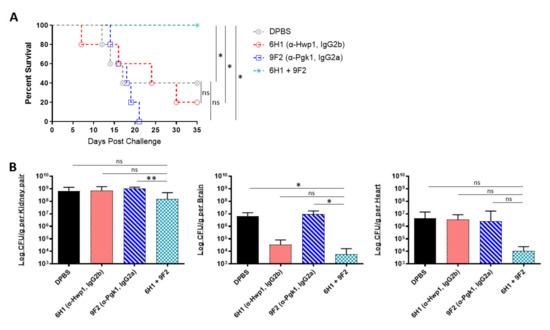
Figure 5.
In vivo protective efficacy of monoclonal antibody cocktails: (A) the 35-day survival curve and (B) quantification of kidney, brain, and heart fungal burdens from 7-week-old female A/J mice treated with mAbs 6H1, 9F2, or a cocktail of 6H1 + 9F2. Mice were treated with two 400-μL-doses of mAb 6H1 given 18 h apart, one 200-μL-dose of mAb 9F2, or a combination of both mAbs consisting of one dose of 9F2 and two doses of 6H1 given 18 h apart. Then, 18 h after first dose of mAb, mice were challenged with a sublethal dose of 4 × 107 CFUs of C. auris AR-0386. Hwp1 = hyphal wall protein 1, Pgk1 = phosphoglycerate kinase 1. Data are mean + SD (B). n = 5. Log-rank (Mantel–Cox) test (A) or two-tailed t-test (B) were used to identify significant differences. * p < 0.05; ** p < 0.01; ns = not significant.
3. Discussion
C. auris is the first fungal pathogen to cause a serious global public health threat [20]. In vitro and in vivo studies of drug efficacy against this MDR pathogen have shown that most isolates are highly resistant to azoles and polyenes, which severely limits effective drug treatments. As this pathogen easily spreads throughout hospital ICUs and assisted living facilities, C. auris seriously threatens the lives of patients living with comorbidities. In those who are most at risk, such as the immunocompromised, antifungal drugs are often not effective and cause additional organ toxicity, which can further exacerbate the conditions of these highly susceptible patients [19].
In this study, we demonstrated for the first time that passive transfer of Candida peptide- and glycan-specific universal mAbs is an effective means of immunotherapy for protecting against C. auris invasive infections in an established A/J mouse model. During systemic infection, Candida spp. disseminates through the bloodstream and enters target organs within hours of infection [48]. During early infection, Candida cells are rapidly eliminated from circulation, and the pathogen is often undetectable in the blood [49]. Consequently, our study evaluated antibody efficacy by quantifying fungal burdens in the kidney, brain, and heart, as well as overall survival. A key characteristic of our mAbs is that they target epitopes that share high homology among various clinically significant Candida species. This “universal” targeting allows for their potential application in preventing candidemia caused by different Candida species and isolates regardless of isolate-specific antifungal-resistance profiles.
The two C. auris isolates analyzed, AR-0386 (CAU-06) and AR-0389 (CAU-09), come from different clades and have unique genetic variations resulting in isolate-specific morphologies, rates of proliferation, virulence, and antifungal resistance [32]. These genetic differences may also affect cell wall composition. The lower level of antibody binding observed with isolate AR-0389 could be due to epitope masking by cell wall polysaccharides, which could account for evasion of immune responses and higher levels of virulence observed with isolate AR-0389, compared to AR-0386. This hypothesis is supported by evidence showing that the unmasking of cell wall components, such as β-(1,3)-glucan, can lead to attenuated C. albicans virulence in mouse models of systemic infection [50,51]. More work is required to determine why the same mAb has different binding patterns to the cell surface epitopes of C. auris isolates, and how this is related to isolate virulence, host–pathogen interaction, and immune escape.
Of our mAb panel, C3.1 (anti-β-Man3) had the highest level of cell surface binding, provided the best overall protection, and significantly outperformed micafungin as a treatment for invasive C. auris infection. β-mannose is a major glycan component of the outer cell wall of many Candida spp., and it is abundantly expressed on the surface of C. auris [46]. Our previous data with C. albicans has shown that C3.1-mediated protection depends on its ability to rapidly and efficiently fix complement to the fungal surface, which is associated with enhanced phagocytosis and killing of the fungus [40,45]. Being that A/J mice are C3-competent, C3.1 likely protects against C. auris via the same mechanism. Furthermore, it has been shown that immune complexes of IgG3 can bind to FcγRI receptors on phagocytic cells [52]. Thus, C3.1 opsonization of C. auris may lead to additional FcγRI binding, increased adherence and internalization, and enhanced phagocytosis; however, quantitation of these values will be the subject of a later study.
In contrast to C3.1, 6H1 (anti-Hwp1) and 9F2 (anti-Pgk1) mAbs target epitopes that are not abundantly expressed in the Candida cell wall, which was reflected in our flow cytometry data. Although Pgk1 is primarily a glycolytic enzyme localized in the cytoplasm, it is also exposed on the Candida surface and has been identified as a cell-wall-associated moonlight protein that is immunoreactive during invasive fungal infections in humans [43,44,53]. Hwp1, on the other hand, is expressed on the cell surface during hyphal morphology [54]; however, some evidence does suggest that it may also be expressed during pseudohyphal morphology [55], which has been induced in C. auris in vitro [56]. Interestingly, the C. auris genome does not appear to contain an ortholog of the Hwp1 gene [54,56], which points to the possibility of cross reaction of the anti-Hwp1 mAb with another cell wall protein. Nonetheless, both mAbs were able to induce significant protection in our mouse model.
Our animal model also showed the enhanced efficacy of two-mAb cocktails as a prophylactic treatment against C. auris disseminated infection. A benefit of mAb cocktails is that each antibody can individually induce a different effector mechanism, which can work in concert to inhibit the growth and dissemination of the pathogen [57]. This is a strategy that has been highly effective in treating other infectious diseases, such as HIV infection using highly active antiretroviral therapy (HAART), which uses a cocktail of three or more drugs that inhibit different steps of the virus’s replication cycle [58]. In the case of the 6H1+9F2 cocktail, the enhanced protection could be due to improved access of phagocytic cells to C. auris yeast, leading to increased oxidative damage. Research conducted by other groups has shown that Pgk1 confers protection to C. albicans against reactive oxygen species (ROS) [59] and that immunization with recombinant Pgk1 protein leads to a significant reduction in kidney burdens in mice infected with C. albicans or with C. glabrata [60]. Additionally, in an in vivo rat venous catheter model of infection, C. albicans Hwp1 mutants were shown to display severe biofilm defects with few hyphae [61]. This may indicate that 6H1 and 9F2 mAbs could function together to disrupt biofilm formation and increase susceptibility to respiratory burst by phagocytic cells, although this mechanism would need to be further investigated. Alternatively, the binding of one mAb could induce a conformational change that results in the unmasking of the second mAb’s target epitope, leading to functional cooperativity between mAbs targeting different epitopes. Further investigation may also show that these different mechanisms are not mutually exclusive and may both contribute to the observed enhanced efficacy.
In recent years, several novel antifungal compounds have been developed that have proven effective against MDR Candida species. One such compound, carvacrol, was shown to have antifungal activity against clinical isolates of C. auris while also inducing synergistic antifungal activity when combined with fluconazole, amphotericin B, caspofungin, and micafungin [62]. As with these compounds, mAbs have the potential to be combined with antifungals to induce synergistic protection while also significantly reducing drug MICs and associated toxicity. Future experiments will evaluate the enhanced protection of combining our mAbs with conventional antifungals as well as the therapeutic efficacy of these mAbs.
Finally, it is important to note that similar to most pathogenic Candida species, C. auris has the propensity to form biofilms within its host. It is well established that within biofilms, Candida spp. exhibit higher resistance to antifungal drugs. This is largely due to the upregulation of efflux pumps [63] and the production of extracellular polymeric substances (EPS), which can interfere with drug diffusion [64]. Although we observed that micafungin was effective at reducing fungal burdens within our immunosuppressed C57BL/6 model, this efficacy would likely be reduced against biofilm-derived C. auris cells. In one mouse study of disseminated candidiasis using biofilm-derived C. glabrata cells, micafungin treatment was ineffective at reducing liver and kidney burdens [65]. MAbs could be effective here as well. In one study, after incubating C. albicans and Candida dubliniensis with antibodies targeting the surface antigen, complement receptor 3-related protein (CR3-RP), both species had a reduction in surface adherence and in biofilm thickness in vitro [66]. Using a combination therapy, the binding of mAbs to the surface of the pathogen could interfere with biofilm formation, leading to increased diffusion of antifungals.
In summary, the potential for mAb therapy against microbial pathogens is vast since mAbs inherently have high specificity for their targets without selecting for resistance. The application of protective mAbs against C. auris disseminated infection represents a highly promising alternative to the often-ineffective use of antifungal drugs against this MDR pathogen. Not only can effective mAbs protect against severe infection more rapidly than antifungal drugs [67,68,69], but specific antibodies may also be synergistic with conventional antimicrobials. The data presented here have significant implications for both immunotherapy and vaccine development in the future, and the demonstration of preclinical efficacy of the immunoprotective mAbs in this study will provide compelling data that can be advanced into the clinical setting.
4. Materials and Methods
4.1. Candida Isolates and Culture Conditions
Two antifungal-resistant isolates of C. auris, AR-0386 (CAU-06) and AR-0389 (CAU-09), were supplied by the United States Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA). C. albicans reference strain SC5314 was supplied by the American Type Culture Collection (MYA-2876, ATCC, Manassas, VA, USA). For passive transfer of mAb experiments, the inoculum was serially passaged daily for three days in 25 mL glucose yeast peptide broth at 37 °C and then washed three times in Dulbecco’s phosphate-buffered saline (DPBS). Cell density was measured using a hemocytometer and adjusted to the desired density in DPBS. For MIC assays, culture was plated onto Sabouraud Dextrose Broth (SDB) agar plates and incubated at 35 °C for 24 h. Five colonies were selected and suspended in 1 mL of sterile deionized H2O, and cell density was measured using a hemocytometer. The density was then adjusted to desired concentration in RPMI 1640 + 0.165 M MOPS medium (with L-glutamine and phenol red, without bicarbonate).
4.2. Mice
Male and female A/J mice were purchased from the Jackson Laboratory (JAX, Bar Harbor, ME, USA) and Envigo (Indianapolis, IN, USA). Male and female C57BL/6 mice were purchased from the Jackson Laboratory (JAX, Bar Harbor, ME, USA). At the time of studies, A/J mice were 7 weeks old, and C57BL/6 mice were 16–17 weeks old. Mice were maintained in the Louisiana State University Health Sciences Center’s AAALAC-accredited animal facility (#000037, LSUHSC-NO, New Orleans, LA, USA), and all animal experiments were performed using a protocol approved by the Louisiana State University Health Sciences Center’s Institutional Animal Care and Use Committee (#3559, 1/18/2019, LSUHSC-NO IACUC, New Orleans, LA, USA).
4.3. Immunosuppression
16–17-week-old male and female C57BL/6 mice were immunosuppressed using cyclophosphamide monohydrate (#C0768, Sigma-Aldrich, St. Louis, MO, USA) three days prior to challenge by intraperitoneal (i.p.) injection using a dose of 200 mg/kg of body weight. Immunosuppression was maintained with additional i.p. injections of a 150 mg/kg dose of cyclophosphamide every 7 days.
4.4. Antifungals
Micafungin (≥97% HPLC) was purchased from Sigma-Aldrich (#SML2268, Sigma-Aldrich, St. Louis, MO, USA), and itraconazole (≥98% TLC) was purchased from Sigma-Aldrich (#I6657, Sigma-Aldrich, St. Louis, MO, USA). For MIC assay, micafungin and itraconazole were dissolved in RPMI 1640 + 0.165 M MOPS medium (with L-glutamine and phenol red, without bicarbonate) + 1% DMSO to the desired concentrations. For animal experiments, micafungin was dissolved in DPBS to the desired concentration, and itraconazole was dissolved in sterile deionized H2O + 10% DMSO to the desired concentration.
4.5. Antifungal Susceptibility
C. auris and C. albicans micafungin and itraconazole minimum inhibitory concentrations (MICs) were determined using the broth microdilution method (BMD) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard (CLSI M27-A3, 3rd Ed. 2008. Vol 28, No 14).
4.6. Antibodies
All monoclonal antibodies were isolated from hybridoma cells, purified, and sterile filtered in phosphate-buffered saline (PBS) by GenScript Biotech Corporation (GenScript, Piscataway, NJ, USA) and by Autoimmune Technologies (AiT, New Orleans, LA, USA). Antibody concentrations were determined using a Pierce BCA Protein Assay Kit (#23227, Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s directions. A standard curve was constructed using bovine gamma globulin standard (BGG) (#23212, Thermo Scientific, Waltham, MA, USA). Absorbance was read on a plate reader at 562 nm, and sample absorbances were compared to the BGG standard curve to determine antibody concentration.
4.7. Antibody Titers
Titers of mAbs were determined via enzyme-linked immunosorbent assay (ELISA). A 96-well polystyrene plate was coated with whole synthetic protein (Pgk1, Hwp1) (GenScript, Piscataway, NJ, USA) or mannan extract at 4 μg/mL in bicarbonate coating buffer and incubated overnight at 4 °C. The following day, the wells were blocked using 1% BSA blocking buffer for 1 h at room temperature. Monoclonal antibodies were then added in duplicate to respective wells using twofold serial dilutions from 1:500 to 1:256,000 and incubated for 2 h at room temperature. Horseradish peroxidase (HRP)-conjugated goat anti-mouse polyvalent IgG, IgA, IgM secondary antibody (#A0412, Sigma-Aldrich, St. Louis, MO, USA) was then added (1:3000) and incubated in the dark for 1 h at room temperature. Tetramethyl benzidine (TMB) (#34022, Thermo Scientific, Waltham, MA, USA) was then added to each well and incubated in the dark for 30 min at room temperature. HCl was added to stop the reaction, and the optical density was measured at 450 nm using a spectrophotometer. As a negative control, wells were incubated with secondary antibody alone. Titers were given as the dilution whose OD reading was greater than two times that of the negative control.
4.8. Antibody Cell Surface Staining
Overnight cultures of C. auris AR-0386, AR-0389, and C. albicans SC5314 were washed three times with DPBS. Pellets were resuspended in 100 μL of C3.1 (anti-β-Man3), 9F2 (anti-Pgk1), or 6H1 (anti-Hwp1) antibodies in 1X PBS + 1% BSA and incubated for 1 h at room temperature. Cells were washed three additional times, and pellets were resuspended in Alexa Fluor 488-conjugated goat anti-mouse IgG, IgM secondary antibody (#A10680, Invitrogen, Carlsbad, CA, USA) (1:100) in 1X PBS + 1% BSA and incubated for 1 h at room temperature. Cells were washed three additional times and resuspended in 500 μL DPBS and acquired by flow cytometry at 488 nm (FACSDiva 8.0.3, FACSCanto II, BD Biosciences, San Jose, CA, USA), As a positive control, an additional high-binding anti-β-Man3 mAb, G11.2 (IgG1), was used. As a negative control, cells were stained with secondary antibody alone. Gating was set on the secondary antibody (Alexa Fluor 488 only) control. A portion of the stained cells was spread on slides for fluorescent imaging.
4.9. In Vivo Model of Disseminated Infection
For this, 7-week-old A/J mice or 16–17-week-old immunosuppressed C57BL/6 mice were treated via intraperitoneal (i.p.) injection with 200 μL of monoclonal antibody or DPBS. Then, 18 h later, mice were challenged via intravenous (i.v.) injection in the tail vein with C. auris AR-0386 at a sublethal dose of 4 × 107 CFUs in 100 μL DPBS or a lethal dose of 1 × 108 CFUs in 100 μL DPBS or with C. auris AR-0389 at a sublethal dose of 4 × 107 in 100 μL DPBS, depending on the experiment. For experiments that measured antifungal efficacy, mice received daily i.p. administration of micafungin or itraconazole starting 24 h post challenge. All mice were monitored daily for death or the development of a moribund state, at which point they were sacrificed via CO2 inhalation. All surviving mice were sacrificed at the conclusion of each study.
4.10. Quantification of Fungal Burdens
Upon death, the kidney, brain, and heart were extracted from mice, and each organ was homogenized in DPBS. The homogenate was then serial diluted and plated onto GYEP agar plates containing chloramphenicol. The plates were incubated for 48 h at 37 °C at which time CFUs were quantified. The limit of detection was 50 CFUs/g for each organ.
4.11. Statistical Analysis
Plots and statistical comparisons were performed using Prism Software (Version 9, GraphPad Software, San Diego, CA, USA). Survival data was evaluated by Kaplan–Meier analysis, and statistical significance was calculated using a log-rank (Mantel–Cox) test. For fungal burden data, results were expressed as mean ± SD, and statistical significance was calculated using a two-tailed t-test to compare mAb-treated groups to the control group. Each study contained five mice per group unless otherwise stated. Significant p values were defined as follows: * p < 0.05; ** p < 0.01.
Author Contributions
Conceptualization and methodology, H.X.; investigation, J.R.-C., K.E., A.A., and E.C.; data curation, J.R.-C. and K.E.; writing—original draft preparation, J.R.-C.; writing—review and editing, H.X.; supervision, H.X.; funding acquisition, H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Defense CDMRP, Award PR171482.
Institutional Review Board Statement
The study was approved by and conducted according to the guidelines of the Louisiana State University Health Sciences Center’s Institutional Animal Care and Use Committee (#3559, 1/18/2019, LSUHSC-NO IACUC, New Orleans, LA, USA). Mice were maintained in the Louisiana State University Health Sciences Center’s AAALAC-accredited animal facility (#000037, LSUHSC-NO, New Orleans, LA, USA).
Data Availability Statement
Data supporting reported results are available upon request (jcolo1@lsuhsc.edu).
Acknowledgments
We thank Constance Porretta for technical assistance with flow cytometry acquisition and analysis, Louisiana State University Health Sciences Center (LSUHSC) for the support of our research, and the National Institutes of Health (NIH) for funding support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2018, 57, 1–12. [Google Scholar] [CrossRef]
- Schelenz, S.; Hagen, F.; Rhodes, J.L.; Abdolrasouli, A.; Chowdhary, A.; Hall, A.; Ryan, L.; Shackleton, J.; Trimlett, R.; Meis, J.F.; et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob. Resist. Infect. Control. 2016, 5, 35. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kumar, V.A.; Sharma, C.; Prakash, A.; Agarwal, K.; Babu, R.; Dinesh, K.R.; Karim, S.; Singh, S.K.; Hagen, F.; et al. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 919–926. [Google Scholar] [CrossRef]
- Zhu, Y.; O’Brien, B.; Leach, L.; Clarke, A.; Bates, M.; Adams, E.; Ostrowsky, B.; Quinn, M.; Dufort, E.; Southwick, K.; et al. Laboratory Analysis of an Outbreak of Candida auris in New York from 2016 to 2018: Impact and Lessons Learned. J. Clin. Microbiol. 2019, 58, e01503–e01519. [Google Scholar] [CrossRef] [PubMed]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; De Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, V.; Codda, G.; Ball, L.; Giacobbe, D.; Willison, E.; Mikulska, M.; Magnasco, L.; Crea, F.; Vena, A.; Pelosi, P.; et al. Molecular Epidemiological Investigation of a Nosocomial Cluster of C. auris: Evidence of Recent Emergence in Italy and Ease of Transmission during the COVID-19 Pandemic. J. Fungi 2021, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.; Francisco, E.; Hagen, F.; Brandão, I.; Pereira, F.; Dias, P.P.; Costa, M.D.M.; Jordão, R.D.S.; de Groot, T.; Colombo, A. Emergence of Candida auris in Brazil in a COVID-19 Intensive Care Unit. J. Fungi 2021, 7, 220. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New Clonal Strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef]
- Morales, S.; Parra-Giraldo, C.M.; Garzón, A.C.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodriguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef]
- Armstrong, P.A.; Rivera, S.M.; Escandon, P.; Caceres, D.H.; Chow, N.; Stuckey, M.J.; Díaz, J.; Gomez, A.; Vélez, N.; Espinosa-Bode, A.; et al. Hospital-Associated Multicenter Outbreak of Emerging Fungus Candida auris, Colombia, 2016. Emerg. Infect. Dis. 2019, 25, 1339–1346. [Google Scholar] [CrossRef]
- Parra-Giraldo, C.M.; Valderrama, S.L.; Cortes-Fraile, G.; Garzón, J.R.; Ariza, B.E.; Morio, F.; Linares-Linares, M.Y.; Ceballos-Garzón, A.; De la Hoz, A.; Hernandez, C.; et al. First report of sporadic cases of Candida auris in Colombia. Int. J. Infect. Dis. 2018, 69, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Alatoom, A.; Sartawi, M.; Lawlor, K.; AbdelWareth, L.; Thomsen, J.; Nusair, A.; Mirza, I. Persistent candidemia despite appropriate fungal therapy: First case of Candida auris from the United Arab Emirates. Int. J. Infect. Dis. 2018, 70, 36–37. [Google Scholar] [CrossRef]
- Chow, N.A.; Gade, L.; Tsay, S.V.; Forsberg, K.; Greenko, J.A.; Southwick, K.L.; Barrett, P.M.; Kerins, J.L.; Lockhart, S.R.; Chiller, T.M.; et al. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: A molecular epidemiological survey. Lancet Infect. Dis. 2018, 18, 1377–1384. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Beldavs, Z.G.; Derado, G.; Pham, C.D.; Lockhart, S.R.; Smith, R.M. Epidemiology and Risk Factors for Echinocandin Nonsusceptible Candida glabrata Bloodstream Infections: Data From a Large Multisite Population-Based Candidemia Surveillance Program, 2008–2014. Open Forum Infect. Dis. 2015, 2, ofv163. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Kean, R.; Ramage, G. Combined Antifungal Resistance and Biofilm Tolerance: The Global Threat of Candida auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef]
- Motley, M.P.; Banerjee, K.; Fries, B.C. Monoclonal antibody-based therapies for bacterial infections. Curr. Opin. Infect. Dis. 2019, 32, 210–216. [Google Scholar] [CrossRef]
- Salazar, G.; Zhang, N.; Fu, T.-M.; An, Z. Antibody therapies for the prevention and treatment of viral infections. npj Vaccines 2017, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Elluru, S.R.; Kaveri, S.; Bayry, J. The protective role of immunoglobulins in fungal infections and inflammation. Semin. Immunopathol. 2015, 37, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 neutralizing monoclonal antibodies: Clinical pipeline. mAbs 2020, 12, 1854149. [Google Scholar] [CrossRef] [PubMed]
- FDA Press Announcements. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 12 January 2021).
- FDA Press Announcements. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibodies for Treatment of COVID-19. 2020. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibodies-treatment-covid-19 (accessed on 12 January 2021).
- Clarke, E.V.; Tenner, A.J. Complement modulation of T cell immune responses during homeostasis and disease. J. Leukoc. Biol. 2014, 96, 745–756. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Sprong, T.; Joosten, L.A.; Van Der Meer, J.W.M.; Kullberg, B.-J.; Hube, B.; Schejbel, L.; Garred, P.; Van Deuren, M.; Netea, M.G. Complement plays a central role in Candida albicans-induced cytokine production by human PBMCs. Eur. J. Immunol. 2012, 42, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.; Elias, M.; Picard, S.; Bourget, L.; Jovcevski, O.; Gauthier, S.; Tuite, A.; Harakidas, P.; Bihun, C.; Massie, B.; et al. Dysregulated Inflammatory Response to Candida albicans in a C5-Deficient Mouse Strain. Infect. Immun. 2004, 72, 5868–5876. [Google Scholar] [CrossRef]
- Torres, S.R.; Pichowicz, A.; Torres-Velez, F.; Song, R.; Singh, N.; Lasek-Nesselquist, E.; De Jesus, M. Impact of Candida auris Infection in a Neutropenic Murine Model. Antimicrob. Agents Chemother. 2019, 64, e01625-19. [Google Scholar] [CrossRef]
- Wurster, S.; Bandi, A.; Beyda, N.D.; Albert, N.D.; Raman, N.M.; Raad, I.I.; Kontoyiannis, D.P. Drosophila melanogaster as a model to study virulence and azole treatment of the emerging pathogen Candida auris. J. Antimicrob. Chemother. 2019, 74, 1904–1910. [Google Scholar] [CrossRef]
- Xin, H.; Mohiuddin, F.; Tran, J.; Adams, A.; Eberle, K. Experimental Mouse Models of Disseminated Candida auris Infection. mSphere 2019, 4, e00339-19. [Google Scholar] [CrossRef]
- Singh, S.; Uppuluri, P.; Mamouei, Z.; Alqarihi, A.; Elhassan, H.; French, S.; Lockhart, S.R.; Chiller, T.; Edwards, J.E., Jr.; Ibra-him, A.S. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019, 15, e1007460. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef]
- AR Isolate Bank. Candida auris. 2021. Available online: https://wwwn.cdc.gov/ARIsolateBank/Panel/PanelDetail?ID=2 (accessed on 27 May 2021).
- Mouton, J.W.; Muller, A.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- Enríquez, J.A. Mind your mouse strain. Nat. Metab. 2019, 1, 5–7. [Google Scholar] [CrossRef]
- Barr, J.T.; Tran, T.B.; Rock, B.M.; Wahlstrom, J.L.; Dahal, U.P. Strain-Dependent Variability of Early Discovery Small Molecule Pharmacokinetics in Mice: Does Strain Matter? Drug Metab. Dispos. 2020, 48, 613–621. [Google Scholar] [CrossRef]
- Faille, C.; Michalski, J.C.; Strecker, G.; MacKenzie, D.W.; Camus, D.; Poulain, D. Immunoreactivity of neoglycolipids constructed from oligomannosidic residues of the Candida albicans cell wall. Infect. Immun. 1990, 58, 3537–3544. [Google Scholar] [CrossRef]
- Navarro-Arias, M.J.; Hernández-Chávez, M.J.; Garcia-Carnero, L.C.; Amezcua-Hernández, D.G.; Lozoya-Pérez, N.E.; Estrada-Mata, E.; Martínez-Duncker, I.; Franco, B.; Mora-Montes, H.M. Differential recognition of Candida tropicalis, Candida guilliermondii, Candida krusei, and Candida auris by human innate immune cells. Infect. Drug Resist. 2019, 12, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Modrzewska, B.; Kurnatowski, P. Adherence of Candida sp. to host tissues and cells as one of its pathogenicity features. Ann. Parasitol. 2015, 61, 3–9. [Google Scholar]
- Alloush, H.M.; Lopez-Ribot, J.; Masten, B.J.; Chaffin, W.L. 3-Phosphoglycerate kinase: A glycolytic enzyme protein present in the cell wall of Candida albicans. Microbiology 1997, 143, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Fujarte, I.; López-Romero, E.; Cuéllar-Cruz, M. Moonlight-like proteins of the cell wall protect sessile cells of Candida from oxidative stress. Microb. Pathog. 2016, 90, 22–33. [Google Scholar] [CrossRef]
- Han, Y.; Riesselman, M.H.; Cutler, J.E. Protection against Candidiasis by an Immunoglobulin G3 (IgG3) Monoclonal Antibody Specific for the Same Mannotriose as an IgM Protective Antibody. Infect. Immun. 2000, 68, 1649–1654. [Google Scholar] [CrossRef]
- Yan, L.; Xia, K.; Yu, Y.; Miliakos, A.; Chaturvedi, S.; Zhang, F.; Chen, S.; Chaturvedi, V.; Linhardt, R.J. Unique Cell Surface Mannan of Yeast Pathogen Candida auris with Selective Binding to IgG. ACS Infect. Dis. 2020, 6, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Mullick, A.; Leon, Z.; Min-Oo, G.; Berghout, J.; Lo, R.; Daniels, E.; Gros, P. Cardiac Failure in C5-Deficient A/J Mice after Candida albicans Infection. Infect. Immun. 2006, 74, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Conti, H.R.; Huppler, A.R.; Whibley, N.; Gaffen, S.L. Animal Models for Candidiasis. Curr. Protoc. Immunol. 2014, 105, 19.6.1–19.6.17. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Finding the “Missing 50%” of Invasive Candidiasis: How Nonculture Diagnostics Will Improve Understanding of Disease Spectrum and Transform Patient Care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef]
- Chen, T.; Wagner, A.S.; Tams, R.N.; Eyer, J.E.; Kauffman, S.J.; Gann, E.R.; Fernandez, E.J.; Reynolds, T.B. Lrg1 Regulates β (1,3)-Glucan Masking in Candida albicans through the Cek1 MAP Kinase Pathway. mBio 2019, 10, e01767-19. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.T.; Kombe, D.; Agarwala, S.D.; Fink, G.R. Dynamic, Morphotype-Specific Candida albicans β-Glucan Exposure during Infection and Drug Treatment. PLoS Pathog. 2008, 4, e1000227. [Google Scholar] [CrossRef] [PubMed]
- Gavin, A.L.; Barnes, N.; Dijstelbloem, H.M.; Hogarth, P.M. Identification of the mouse IgG3 receptor: Implications for antibody effector function at the interface between innate and adaptive immunity. J. Immunol. 1998, 160, 20–23. [Google Scholar] [PubMed]
- Satala, D.; Karkowska-Kuleta, J.; Zelazna, A.; Rapala-Kozik, M.; Kozik, A. Moonlighting Proteins at the Candidal Cell Surface. Microorganisms 2020, 8, 1046. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Gade, L.; Chow, N.A.; Loparev, V.N.; Juieng, P.; Berkow, E.L.; Farrer, R.A.; Litvintseva, A.P.; Cuomo, C.A. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat. Commun. 2018, 9, 5346. [Google Scholar] [CrossRef]
- Naglik, J.R.; Fostira, F.; Ruprai, J.; Staab, J.F.; Challacombe, S.J.; Sundstrom, P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J. Med. Microbiol. 2006, 55, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.B.; Ross, Z.K.; Gow, N.A.R.; Lorenz, A. Pseudohyphal Growth of the Emerging Pathogen Candida auris Is Triggered by Genotoxic Stress through the S Phase Checkpoint. mSphere 2020, 5, e00151-00120. [Google Scholar] [CrossRef]
- Logtenberg, T. Antibody cocktails: Next-generation biopharmaceuticals with improved potency. Trends Biotechnol. 2007, 25, 390–394. [Google Scholar] [CrossRef]
- Kumar, A.; Coquard, L.; Herbein, G. Targeting TNF-Alpha in HIV-1 Infection. Curr. Drug Targets 2015, 17, 15–22. [Google Scholar] [CrossRef]
- Ramírez-Quijas, M.D.; López-Romero, E.; Cuéllar-Cruz, M. Proteomic analysis of cell wall in four pathogenic species of Candida exposed to oxidative stress. Microb. Pathog. 2015, 87, 1–12. [Google Scholar] [CrossRef]
- Medrano-Díaz, C.L.; Vega-González, A.; Ruiz-Baca, E.; Moreno, A.; Cuéllar-Cruz, M. Moonlighting proteins induce protection in a mouse model against Candida species. Microb. Pathog. 2018, 124, 21–29. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans Adhesin Hwp1 in Biofilm Formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C. Candida auris: A Quick Review on Identification, Current Treatments, and Challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Correia, A.; Vilanova, M.; Henriques, M. Inflammatory Cell Recruitment in Candida glabrata Biofilm Cell-Infected Mice Receiving Antifungal Chemotherapy. J. Clin. Med. 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Chupáčová, J.; Borghi, E.; Morace, G.; Los, A.; Bujdáková, H. Anti-biofilm activity of antibody directed against surface antigen complement receptor 3-related protein—Comparison of Candida albicans and Candida dubliniensis. Pathog. Dis. 2018, 76, ftx127. [Google Scholar] [CrossRef]
- Saylor, C.; Dadachova, E.; Casadevall, A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 2009, 27, G38–G46. [Google Scholar] [CrossRef] [PubMed]
- Pachl, J.; Svoboda, P.; Jacobs, F.; Vandewoude, K.; Van Der Hoven, B.; Spronk, P.; Masterson, G.; Malbrain, M.; Aoun, M.; Garbino, J.; et al. A Randomized, Blinded, Multicenter Trial of Lipid-Associated Amphotericin B Alone versus in Combination with an Antibody-Based Inhibitor of Heat Shock Protein 90 in Patients with Invasive Candidiasis. Clin. Infect. Dis. 2006, 42, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Oishi, K.; Tao, M.; Matsumoto, K.; Pollack, M. Antibacterial Properties of Pseudomonas aeruginosa Immunotype 1 Lipopolysaccharide-Specific Monoclonal Antibody (MAb) in a Murine Thigh Infection Model: Combined Effects of MAb and Ceftazidime. Microbiol. Immunol. 2000, 44, 629–635. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).