The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Histological Types of Diagnosed Tumors
2.2. Tumor Markers
2.3. Serum Glutathione Levels
2.4. Correlation between GSH, GSSG, and GSSG/GSH%, Tumor Markers, and Biochemical Parameters
2.5. Glutathione Levels and Tumor Stages
2.6. Changes in Glutathione Levels after CRC Treatment
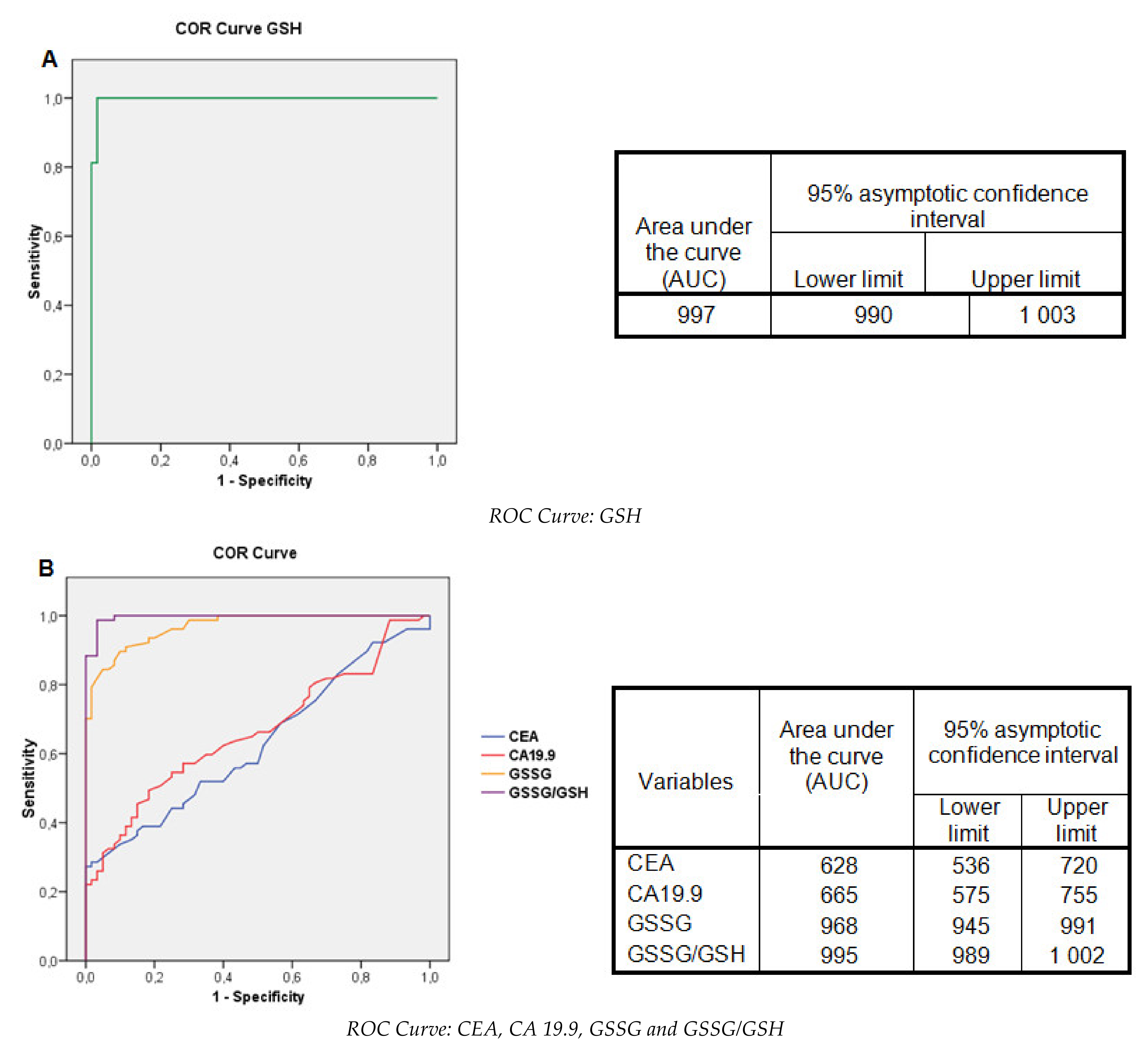
2.7. Evaluation of the Glutathione Levels: Contingency Tables and ROC Curves
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Analytical Assays
4.3.1. Biochemical and Hematological Studies in Serum Blood Samples
4.3.2. GSH, GSSG, and GSSG/GSH% Ratio
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Leonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology & Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide—production, fate and role in redox signaling of tumor cells. Cell. Commun. Signal. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landskron, G.; Marjorie De la Fuente, M.; Thuwajit, P.; Thuwajit, C.h.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva, M.R.; Ripoll, F.; Muñiz, P.; Iradi, A.; Trullenque, R.; Valls, V.; Drehmer, E.; Sáez, G.T. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol. Carcinog. 1997, 18, 232–243, Erratum in: Mol. Carcinog. 1997, 19, 280. [Google Scholar] [CrossRef]
- Ilangovan, G.; Li, H.; Jay, L.Z.; Kuppusamy, P. In vivo measurement of tumor redox environment using EPR spectroscopy. Mol. Cell. Biochem. 2002, 234, 393–398. [Google Scholar] [CrossRef] [PubMed]
- GLOBOCAN 2020: Colorectal Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 17 April 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiao, W.W.; Xie, W.H.; Cai, P.Q.; Wang, Q.X.; Chang, H.; Chen, B.Q.; Zhou, W.H.; Zeng, Z.F.; Wu, X.J.; et al. Neoadjuvant chemoradiotherapy for patients with unresectable radically locally advanced colon cancer: A potential improvement to overall survival and decrease to multivisceral resection. BMC Cancer 2021, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L.; Sarasqueta, C.; Hijona, E.; Hijona, L.; Cosme, A.; Gil, I.; Elorza, J.L.; Asensio, J.I.; Larburu, S.; Enríquez-Navascués, J.M.; et al. Colorectal cancer prognosis twenty years later. World J. Gastroenterol. 2010, 16, 862–867. [Google Scholar] [CrossRef]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C., Jr.; ASCO. ASCO: Update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Zafirellis, K.; Zachaki, A.; Agrogiannis, G.; Gravani, K. Inducible nitric oxide synthase expression and its prognostic significance in colorectal cancer. APMIS 2010, 118, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Perse, M. Oxidative stress in the pathogenesis of colorectal cancer: Cause or consequence? Biomed. Res. Int. 2013, 725710. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kahn, A.R.; Gupta, A.K. Role of glutathione in cancer pathophysiology and therapeutic interventions. J. Exp. Ther. Oncol. 2012, 9, 303–316, PMID: 22545423. [Google Scholar] [PubMed]
- Lo, H.W.; Ali-Osman, F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr. Opin. Pharmacol. 2007, 7, 367–374. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/deteccion-diagnosis-stating/survival-rates.html (accessed on 17 April 2021).
- WHO. Biomarkers in Risk Assessment: Validity and Validation. Available online: http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed on 17 April 2021).
- Biomarkers Definition Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Monzó-Beltrán, L.; Vázquez-Tarragón, A.; Cerdá, C.; García-Pérez, P.; Iradi, A.; Sánchez, C.; Climent, B.; Tormos, C.; Vázquez-Prado, A.; Girbés, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef]
- Mitjavila, M.T.; Fandos, M.; Salas-Salvadó, J.; Covas, M.I.; Borrego, S.; Estruch, R.; Lamuela-Raventós, R.; Corella, D.; Martínez-González, M.Á.; Sánchez, J.M.; et al. The mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin. Nutr. 2013, 32, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The isoprostanes-25 years later. Biochim. Biophys. Acta. 2015, 1851, 433–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biomarkers of oxidative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev. 2005, 24, 55–99. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J., 2nd; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, Y.; Saito, Y.; Nakano, Y.; Mochizuki, K.; Sakata, O.; Ito, R.; Saito, K.; Nakazawa, H.J. Chromatographic and mass spectrometric analysis of glutathione in biological samples. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2009, 877, 3309–3317. [Google Scholar] [CrossRef] [PubMed]
- Musaogullari, A.; Chai, Y.C. Redox regulation by protein S-Glutathionylation: From molecular mechanisms to implications in health and disease. W. Int. J. Mol. Sci. 2020, 30, 8113. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Nencioni, L.; Garaci, E.; Palamara, A.T.; Magnani, M. GSH and analogs in antiviral therapy. Mol. Aspects Med. 2009, 30, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of oxidative stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Swartz, H.M.; Kahn, N.; Khramtsov, V.V. Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid. Redox Signal. 2007, 9, 1757–1771. [Google Scholar] [CrossRef] [Green Version]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P., Jr.; Reed, R.L.; Jones, D.P. Glutathione in human plasma: Decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef]
- Jagust, P.; Alcalá, S.; Sainz, B., Jr.; Heeschen, C.; Sancho, P. Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells. World J. Stem Cells 2020, 26, 1410–1428. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hadi, N.; Reyes-Castellanos, G.; Carrier, A. Targeting Redox Metabolism in Pancreatic Cancer. Int. J. Mol. Sci. 2021, 3, 1534. [Google Scholar] [CrossRef]
- Scibior, D.; Skrzycki, M.; Podsiad, M.; Czeczot, H. Glutathione level and glutathione-dependent enzyme activities in blood serum of patients with gastrointestinal tract tumors. Clin. Biochem. 2008, 41, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Borrego, S.; Vazquez, A.; Dasí, F.; Cerdá, C.; Iradi, A.; Tormos, C.; Sánchez, J.M.; Bagán, L.; Boix, J.; Zaragoza, C.; et al. Oxidative stress and DNA damage in human gastric carcinoma: 8-oxo-7´8-dihydro-2´-deoxyguanosine (8-oxo-dG) as a possible tumor marker. Int. J. Mol. Sci. 2013, 14, 3467–3486. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell. Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veljković, A.; Stanojević, G.; Branković, B.; Pavlović, D.; Stojanović, I.; Cvetković, T.; Jevtović, T.; Sokolović, D.; Bašić, J.; Despotović, M.; et al. Parameters of oxidative stress in colon cancer tissue. Acta Med. Median. 2016, 55, 32–37. [Google Scholar] [CrossRef]
- Wu, R.; Feng, J.; Yang, Y.; Dai, C.; Lu, A.; Li, J.; Liao, Y.; Xiang, M.; Huang, Q.; Wang, D.; et al. Significance of Serum Total Oxidant/Antioxidant Status in Patients with Colorectal Cancer. PLoS ONE 2017, 19, e0170003. [Google Scholar] [CrossRef]
- Dusak, A.; Atasoy, N.; Demir, H.; Doğan, E.; Gürsoy, T.; Sarıkaya, E. Investigation of levels of oxidative stress and antioxidant enzymes in colon cancers. J. Clin. Anal. Med. 2017, 8, 469–473. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, M.; Zhu, J.; Wei, M.; Li, H.; Zhao, P.; Wang, J.; Li, W.; Tian, L.; Tao, Y.; et al. Tissue metabolic profiling reveals major metabolic alteration in colorectal cancer. J. Mol. Omics. 2021, 20. [Google Scholar] [CrossRef]
- Baltruskeviciene, E.; Kazbariene, B.; Badaras, R.; Bagdonaitė, L.; Krikštaponienė, A.; Zdanavičius, L.; Aleknavicius, E.; Didziapetrienė, J. Glutathione and glutathione S-transferase levels in patients with liver metastases of colorectal cancer and other hepatic disorders. Turk. J. Gastroenterol. 2016, 27, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasool, M.; Malik, A.; Ghuman, A.A.; Ashraf, M.A.B.; Arooj, M.; Waquar, S.; Zahid, S.; Shaheen, S.; Qazi, A.; Naseer, M.I.; et al. Implications of Isoprostanes and matrix metalloproteinase-7 having potential role in the development of colorectal cancer in males. Front. Oncol. 2018, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Baltruskeviciene, E.; Kazbariene, B.; Aleknavicius, E.; Krikstaponiene, A.; Venceviciene, L.; Suziedelis, K.; Stratilatovas, E.; Didziapetriene, J. Changes of reduced glutathione and glutathione S-transferase levels in colorectal cancer patients undergoing treatment. J. Tumori. 2018, 104, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Moghadamyeghaneh, Z.; Hanna, M.H.; Carmichael, J.C.; Mills, S.D.; Pigazzi, A.; Stamos, M.J. Preoperative leukocytosis in colorectal cancer patients. J. Am. Coll. Surg. 2015, 221, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Tang, Z.H.; Liu, S.; Guo, S.S. Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J. Gastroenterol. 2017, 23, 1780–1786. [Google Scholar] [CrossRef]
- Bujanda, L.; Cosme, A.; Gil, I.; Arenas-Mirave, J.I. Malignant colorectal polyps. World J. Gastroenterol. 2010, 16, 3103–3111. [Google Scholar] [CrossRef] [PubMed]




| Variable | Control (n = 60) | CRC (n = 80) | p-Value | * Adjusted p-Value |
|---|---|---|---|---|
| Age (years) | 64.0 ± 9.0 | 67.5 ± 11.8 | 0.052 | - |
| Male/Female (n; %) | 36/24; 60/40 | 52/28; 65/35 | 0.548 | - |
| Weight (kg) | 74.4 ± 16.3 | 77.3 ± 15 | <0.001 | - |
| Height (cm) | 168 ± 11 | 165.5 ± 9.8 | <0.001 | - |
| BMI (kg/m2) | 26.1 ± 3.0 | 28.1 ± 3.9 | 0.001 | - |
| Glucose (mg/dL) | 96.2 ± 14.4 | 116.6 ± 52.3 | <0.001 | 0.001 |
| Creatinine (mg/dL) | 0.9 ± 0.2 | 2.0 ± 8.6 | 0.319 | 0.269 |
| Urea (mg/dL) | 40.9 ± 7.2 | 38.8 ± 15.9 | 0.878 | 0.296 |
| EGF (mL/min) | 81.1 ± 8.7 | 78.9 ± 20.9 | 0.399 | 0.720 |
| Total cholesterol (mg/dL) | 195.7 ± 34.3 | 180.4 ± 39.1 | 0.018 | 0.026 |
| HDL cholesterol (mg/dL) | 50.7 ± 12.8 | 43.2 ± 10.8 | <0.001 | <0.001 |
| LDL cholesterol (mg/dL) | 144.9 ± 30.0 | 114.5 ± 34.7 | <0.001 | <0.001 |
| Triglycerides (mg/dL) | 112.0 (98;142.8) | 108.5 (83.3;141) | 0.954 | 0.777 |
| Uric acid (mg/dL) | 4.5 ± 1.6 | 5.3 ± 1.7 | 0.003 | 0.058 |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.2 ± 0.5 | 0.001 | <0.001 |
| Total proteins (g/dL) | 7.0 ± 0.5 | 6.9 ± 0.4 | 0.472 | 0.577 |
| Ferritin (µg/L) | 133.5 ± 75.4 | 67.5 ± 144.9 | 0.002 | 0.008 |
| Iron (µg/dL) | 79.7 ± 19.1 | 57.6 ± 41.0 | <0.001 | <0.001 |
| Transferrin (mg/dL) | 269.3 ± 46.5 | 291.1 ± 51.4 | 0.011 | 0.016 |
| TSI (%) | 30.5 ± 8.9 | 16.2 ± 11.1 | <0.001 | <0.001 |
| CRP (mg/L) | 6.2 ± 1.3 | 11.0 ± 23.6 | 0.072 | 0.076 |
| IL-6 (pg/mL) | 2.7 ± 1.4 | 19.7 ± 26.5 | <0.001 | <0.001 |
| Leukocytes (x103/mm3) | 7.0 ± 1.7 | 7.7 ± 1.8 | 0.016 | 0.018 |
| Neutrophils (%) | 56.0 ± 6.0 | 62.9 ± 8.4 | <0.001 | <0.001 |
| Lymphocytes (%) | 29.8 ± 10.2 | 27.7 ± 12.0 | 0.221 | 0.531 |
| N/L (-) | 2.1 ± 1.4 | 2.7 ± 1.6 | 0.005 | 0.013 |
| Platelets (×105/mm3) | 206.0 ± 60.0 | 253.5 ± 74.0 | <0.001 | <0.001 |
| Fibrinogen (mg/dL) | 352.3 ± 70.8 | 483.8 ± 101.7 | <0.001 | <0.001 |
| GSH (μmol/mL) | GSSG (μmol/mL) | GSSG/GSH (%) | |
|---|---|---|---|
| Tumor markers | |||
| CEA (ng/mL) | −0.270 ** | 0.292 ** | 0.276 ** |
| CA 19.9 (IU/mL) | −0.292 ** | 0.345 *** | 0.322 *** |
| Inflammatory markers | |||
| CRP (mg/L) | n.s. | n.s. | n.s. |
| IL-6 (pg/mL) | −0.328 *** | 0.419 *** | 0.385 *** |
| Leukocytes (×103/mm3) | n.s. | 0.186 * | 0.173 * |
| Neutrophils (%) | −0.318 *** | 0.362 *** | 0.330 *** |
| Lymphocytes (%) | 0.226 ** | −0.280 ** | −0.240 ** |
| N/L (-) | −0.175 * | 0.232 ** | 0.181 * |
| Platelets (×105/mm3) | −0.300 *** | 0.240 ** | 0.239 ** |
| Fibrinogen (mg/dL) | −0.462 *** | 0.521 *** | 0.552 *** |
| Marker | Cut off | S (%) | SP (%) | PPV ‡ (%) | NPV ‡ (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| CEA (ng/mL) | 4.95 | 26.3 | 100 | 100 | 50.4 | 57.8 |
| CA 19.9 (IU/mL) | 40.0 | 17.5 | 100 | 100 | 47.6 | 52.9 |
| GSH (μmol/mL) | 3.17 * | 78.8 | 100 | 100 | 77.9 | 87.9 |
| GSSG (μmol/mL) | 0.73 | 75.0 | 98.3 | 98.4 | 74.7 | 85.0 |
| GSSG/GSH (%) | 14.3 | 98.8 | 98.3 | 92.8 | 98.3 | 98.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-León, D.; Monzó-Beltrán, L.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Camarasa-Lillo, N.; Pérez-Ebri, M.L.; Escandón-Álvarez, J.; Alonso-Iglesias, E.; Santaolaria-Ayora, M.L.; Carbonell-Moncho, A.; et al. The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6183. https://doi.org/10.3390/ijms22126183
Acevedo-León D, Monzó-Beltrán L, Gómez-Abril SÁ, Estañ-Capell N, Camarasa-Lillo N, Pérez-Ebri ML, Escandón-Álvarez J, Alonso-Iglesias E, Santaolaria-Ayora ML, Carbonell-Moncho A, et al. The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(12):6183. https://doi.org/10.3390/ijms22126183
Chicago/Turabian StyleAcevedo-León, Delia, Lidia Monzó-Beltrán, Segundo Ángel Gómez-Abril, Nuria Estañ-Capell, Natalia Camarasa-Lillo, Marisa Luisa Pérez-Ebri, Jorge Escandón-Álvarez, Eulalia Alonso-Iglesias, Marisa Luisa Santaolaria-Ayora, Araceli Carbonell-Moncho, and et al. 2021. "The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer" International Journal of Molecular Sciences 22, no. 12: 6183. https://doi.org/10.3390/ijms22126183
APA StyleAcevedo-León, D., Monzó-Beltrán, L., Gómez-Abril, S. Á., Estañ-Capell, N., Camarasa-Lillo, N., Pérez-Ebri, M. L., Escandón-Álvarez, J., Alonso-Iglesias, E., Santaolaria-Ayora, M. L., Carbonell-Moncho, A., Ventura-Gayete, J., Pla, L., Martínez-Bisbal, M. C., Martínez-Máñez, R., Bagán-Debón, L., Viña-Almunia, A., Martínez-Santamaría, M. A., Ruiz-Luque, M., Alonso-Fernández, J., ... Sáez, G. (2021). The Effectiveness of Glutathione Redox Status as a Possible Tumor Marker in Colorectal Cancer. International Journal of Molecular Sciences, 22(12), 6183. https://doi.org/10.3390/ijms22126183








