In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein
Abstract
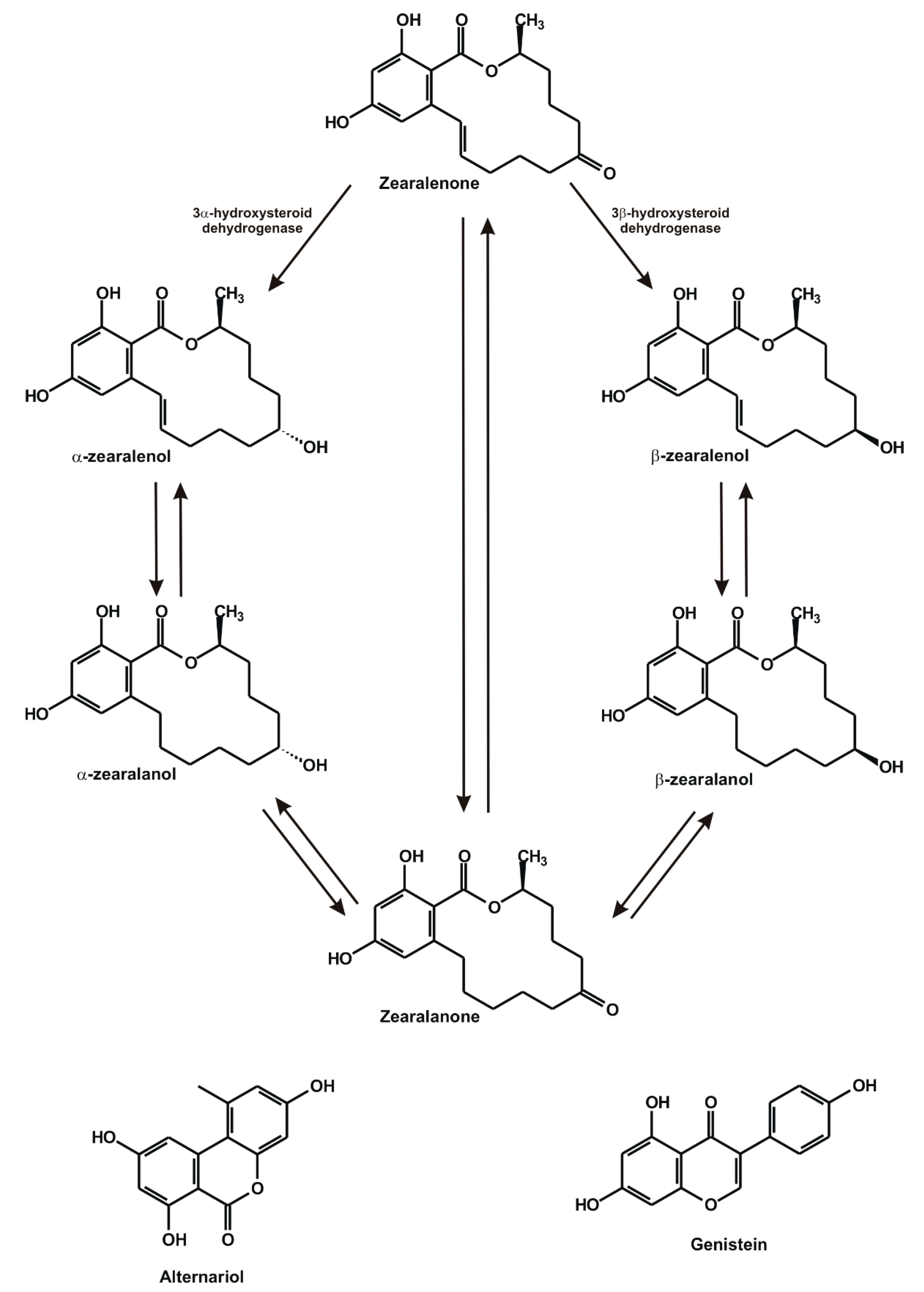
:1. Introduction
2. Results and Discussion
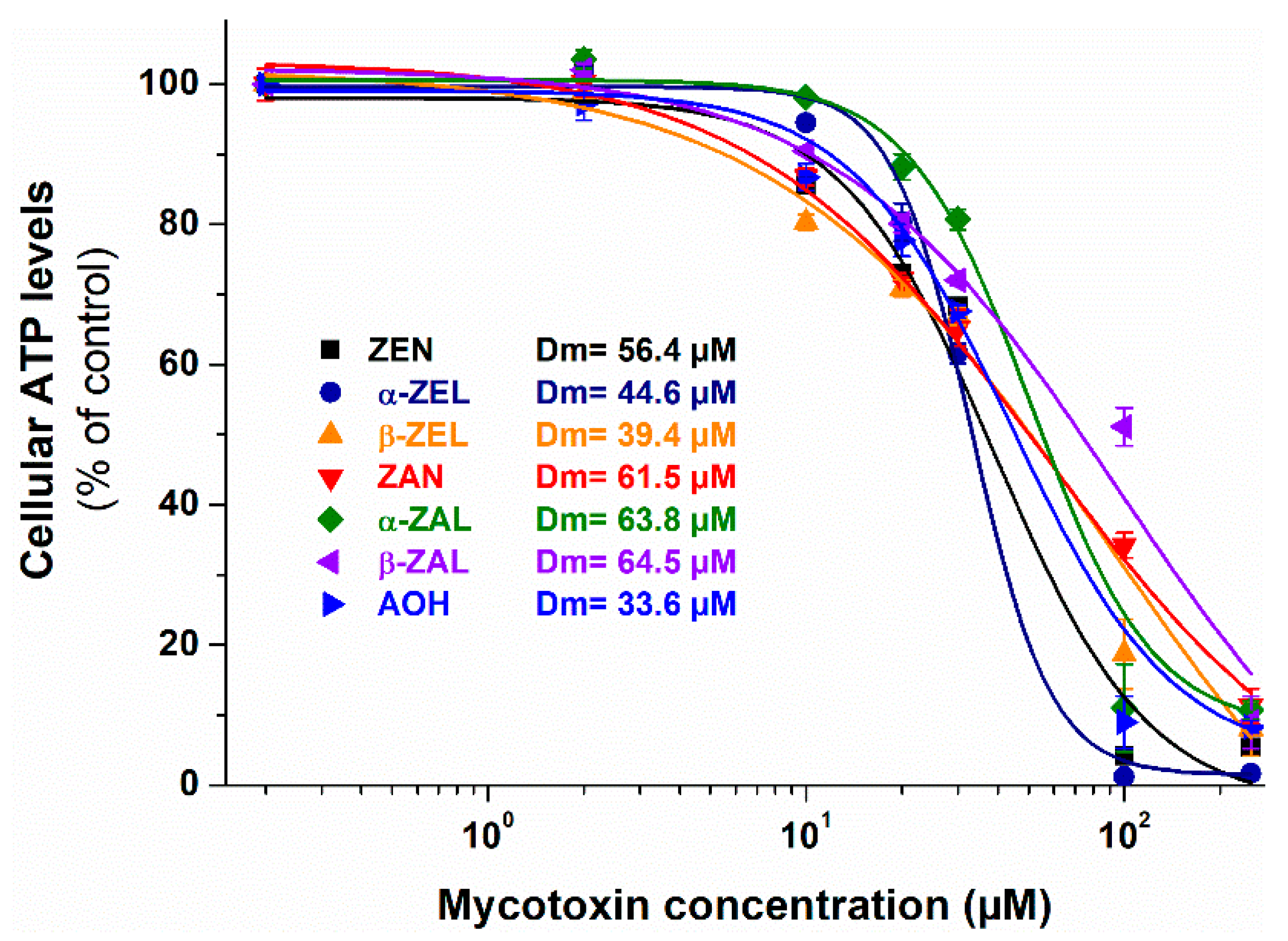
2.1. Individual Effects of ZEN, ZEN Metabolites, and AOH on Cell Viability
2.2. Combined Effects of Mycotoxins on Cell Viability
2.3. Individual Estrogenic Effects of ZEN, ZEN Metabolites, AOH, and GEN
2.4. Combined Estrogenic Effects of ZEN, ZEN Metabolites, AOH, and GEN
3. Materials and Methods
3.1. Reagents
3.2. Cell Culturing, Treatment, and Cell Viability Assay
3.3. BLYES Assay in Saccharomyces Cerevisiae
3.4. Assessment of Combined Effects
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.I. Mycotoxins. In Foodborne Infections and Intoxications, 4th ed.; Morris, J.G., Jr., Potter, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 30, pp. 409–418. [Google Scholar]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar] [CrossRef] [Green Version]
- Loi, M.; Fanelli, F.; Liuzzi, V.C.; Logrieco, A.F.; Mulè, G. Mycotoxin Biotransformation by Native and Commercial Enzymes: Present and Future Perspectives. Toxins 2017, 9, 111. [Google Scholar] [CrossRef]
- Shier, W.T.; Shier, A.C.; Xie, W.; Mirocha, C.J. Structure-activity relationships for human estrogenic activity in zearalenone mycotoxins. Toxicon 2001, 39, 1435–1438. [Google Scholar] [CrossRef]
- Filannino, A.; Stout, T.A.; Gadella, B.M.; Sostaric, E.; Pizzi, F.; Colenbrander, B.; Dell’Aquila, M.E.; Minervini, F. Dose-response effects of estrogenic mycotoxins (zearalenone, alpha- and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm. Reprod. Biol. Endocrinol. 2011, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R. Metabolism and pharmacokinetics of zearalenone following oral and intravenous administration in juvenile female pigs. Food Chem. Toxicol. 2017, 106, 193–201. [Google Scholar] [CrossRef]
- Frizzell, C.; Ndossi, D.; Verhaegen, S.; Dahl, E.; Eriksen, G.; Sørlie, M.; Ropstad, E.; Muller, M.; Elliott, C.T.; Connolly, L. Endocrine disrupting effects of zearalenone, alpha- and beta-zearalenol at the level of nuclear receptor binding and steroidogenesis. Toxicol. Lett. 2011, 206, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Royce, S.G.; Alexander, J.A.; Buckley, B.; Isukapalli, S.S.; Bandera, E.V.; Zarbl, H.; Georgopoulos, P.G. Physiologically-based toxicokinetic modeling of zearalenone and its metabolites: Application to the Jersey girl study. PLoS ONE 2014, 9, e113632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Demaegdt, H.; Daminet, B.; Evrard, A.; Scippo, M.-L.; Muller, M.; Pussemier, L.; Callebaut, A.; Vandermeiren, K. Endocrine activity of mycotoxins and mycotoxin mixtures. Food Chem. Toxicol. 2016, 96, 107–116. [Google Scholar] [CrossRef]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Stypuła-Trębas, S.; Minta, M.; Radko, L.; Jedziniak, P.; Posyniak, A. Nonsteroidal mycotoxin alternariol is a full androgen agonist in the yeast reporter androgen bioassay. Environ. Toxicol. Pharmacol. 2017, 55, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Camarena, E.; López-Rubalcava, C.; Valdés-Sustaita, B.; Azpilcueta-Morales, G.S.; González-Trujano, E.M. Use of phytoestrogens for the treatment of psychiatric symptoms associated with menopause transition. In A Multidisciplinary Look at Menopause; Rodriguez-Landa, J.F., Cueto-Escobedo, J., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 81–109. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.I.; Gross, M.; Gottschalk, C.; Usleber, E. Investigations on the occurrence of mycotoxins in beer. Food Control 2016, 63, 135–139. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Sulyok, M.; Warth, B.; Krska, R. Multi-microbial metabolites in fonio millet (acha) and sesame seeds in Plateau State, Nigeria. Eur. Food Res. Technol. 2012, 235, 285–293. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magistà, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-occurrence of toxigenic moulds, aflatoxins, ochratoxin A, Fusarium and Alternaria mycotoxins in fresh sweet peppers (Capsicum annuum) and their processed products. World Mycotoxin J. 2018, 11, 159–174. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulyok, M.; Krska, R.; Schuhmacher, R. Application of an LC–MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem. 2010, 119, 408–416. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a Changing Climate: Semi-Quantitative Multi-Mycotoxin Analysis of Grain Grown in Exceptional Climatic Conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Lee, J.J.; Kong, M.; Ayers, G.D.; Lotan, R. Interaction index and different methods for determining drug interaction in combination therapy. J. Biopharm. Stat. 2007, 17, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Meca, G.; Font, G.; Ruiz, M.-J. Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian CHO-K1 cells. Toxicol. In Vitro 2014, 28, 95–103. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Bulgaru, C.V.; Taranu, I. Cytotoxic and inflammatory effects of individual and combined exposure of HepG2 cells to zearalenone and its metabolites. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 937–947. [Google Scholar] [CrossRef]
- Zheng, N.; Gao, Y.N.; Liu, J.; Wang, H.W.; Wang, J.Q. Individual and combined cytotoxicity assessment of zearalenone with ochratoxin A or α-zearalenol by full factorial design. Food Sci. Biotechnol. 2018, 27, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Wang, J.Q.; Zheng, B.Q.; Li, S.L.; Zhang, Y.D.; Li, F.D.; Zheng, N. Cytotoxicity induced by ochratoxin A, zearalenone, and α-zearalenol: Effects of individual and combined treatment. Food Chem. Toxicol. 2014, 71, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Vejdovszky, K.; Hahn, K.; Braun, D.; Warth, B.; Marko, D. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch. Toxicol. 2017, 91, 1447–1460. [Google Scholar] [CrossRef] [Green Version]
- Vejdovszky, K.; Schmidt, V.; Warth, B.; Marko, D. Combinatory estrogenic effects between the isoflavone genistein and the mycotoxins zearalenone and alternariol in vitro. Mol. Nutr. Food Res. 2017, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, A.; Visconti, A.; Logrieco, A.; Solfrizzo, M.; Mirocha, C.J. Occurrence of Zearalenols (Diastereomeric Mixture) in Corn Stalk Rot and Their Production by Associated Fusarium Species. Appl. Environ. Microbiol. 1985, 49, 547–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boevre, M.; Di Mavungu, J.D.; Landschoot, S.; Audenaert, K.; Eeckhout, M.; Maene, P.; Haesaert, G.; De Saeger, S. Natural occurrence of mycotoxins and their masked forms in food and feed products. World Mycotoxin J. 2012, 5, 207–219. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural occurrence of 16 fusarium toxins in grains and feedstuffs of plant origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- De Nijs, M.; Rombouts, F.; Notermans, S. Fusarium molds and their mycotoxins. J. Food Saf. 1996, 16, 15–58. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 2nd ed.; Aspen Publ. Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Drochner, W. Natural occurrence of 16 Fusarium toxins in edible oil marketed in Germany. Food Control 2008, 19, 475–482. [Google Scholar] [CrossRef]
- Vepriková, Z.; Zachariasova, M.; Dzuman, Z.; Zachariasova, A.; Fenclova, M.; Slavikova, P.; Vaclavikova, M.; Mastovska, K.; Hengst, D.; Hajslova, J. Mycotoxins in plant-based dietary supplements: Hidden health risk for consumers. J. Agric. Food Chem. 2015, 63, 6633–6643. [Google Scholar] [CrossRef]
- Faisal, Z.; Lemli, B.; Szerencsés, D.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Kuzma, M.; Mayer, M.; Poór, M. Interactions of zearalenone and its reduced metabolites α-zearalenol and β-zearalenol with serum albumins: Species differences, binding sites, and thermodynamics. Mycotoxin Res. 2018, 34, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Faisal, Z.; Vörös, V.; Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Poór, M. Interactions of zearalanone, α-zearalanol, β-zearalanol, zearalenone-14-sulfate, and zearalenone-14-glucoside with serum albumin. Mycotoxin Res. 2020, 36, 389–397. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Dellafiora, L.; Dall’Asta, C.; Cruciani, G.; Pethő, G.; Poór, M. Interaction of Mycotoxin Alternariol with Serum Albumin. Int. J. Mol. Sci. 2019, 20, 2352. [Google Scholar] [CrossRef] [Green Version]
- Poór, M.; Kunsági-Máté, S.; Bálint, M.; Hetényi, C.; Gerner, Z.; Lemli, B. Interaction of mycotoxin zearalenone with human serum albumin. J. Photochem. Photobiol. B 2017, 170, 16–24. [Google Scholar] [CrossRef]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef] [Green Version]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Csepregi, R.; Temesfői, V.; Poór, M.; Faust, Z.; Kőszegi, T. Green Fluorescent Protein-Based Viability Assay in a Multiparametric Configuration. Molecules 2018, 23, 1575. [Google Scholar] [CrossRef] [Green Version]
- Ayed, Y.; Ayed-Boussema, I.; Ouanes, Z.; Bacha, H. In vitro and in vivo induction of chromosome aberrations by alpha- and beta-zearalenols: Comparison with zearalenone. Mut. Res. 2011, 726, 42–46. [Google Scholar] [CrossRef]
- Marin, D.E.; Taranu, I.; Burlacu, R.; Tudor, D.S. Effects of zearalenone and its derivatives on the innate immune response of swine. Toxicon 2010, 56, 956–963. [Google Scholar] [CrossRef]
- Tadpetch, K.; Kaewmee, B.; Chantakaew, K.; Kantee, K.; Rukachaisirikul, V.; Phongpaichit, S. Synthesis and cytotoxic activities of semisynthetic zearalenone analogues. Bioorg. Med. Chem. Lett. 2016, 26, 3612–3616. [Google Scholar] [CrossRef]
- Abid-Essefi, S.; Bouaziz, C.; Golli-Bennour, E.E.; Ouanes, Z.; Bacha, H. Comparative study of toxic effects of zearalenone and its two major metabolites alpha-zearalenol and beta-zearalenol on cultured human Caco-2 cells. J. Biochem. Mol. Toxicol. 2009, 23, 233–243. [Google Scholar] [CrossRef]
- Ayers, S.; Graf, T.N.; Adcock, A.F.; Kroll, D.J.; Matthew, S.; de Blanco Carcache, E.J.; Shen, Q.; Swanson, S.M.; Wani, M.C.; Pearce, C.J.; et al. Resorcylic acid lactones with cytotoxic and NF-κB inhibitory activities and their structure-activity relationships. J. Nat. Prod. 2011, 74, 1126–1131. [Google Scholar] [CrossRef] [Green Version]
- Ouanes-Ben Othmen, Z.O.-B.; El Golli, E.; Abid-Essefi, S.; Bacha, H. Cytotoxicity effects induced by zearalenone metabolites, α-zearalenol and β-zearalenol, on cultured Vero cells. Toxicology 2008, 252, 72–77. [Google Scholar] [CrossRef]
- Lu, J.; Yu, J.Y.; Lim, S.S.; Son, Y.O.; Kim, D.H.; Lee, S.A.; Shi, X.; Lee, J.C. Cellular mechanisms of the cytotoxic effects of the zearalenone metabolites α-zearalenol and β-zearalenol on RAW264.7 macrophages. Toxicol. In Vitro 2013, 27, 1007–1017. [Google Scholar] [CrossRef]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A. Individual and Combined Effect of Zearalenone Derivates and Beauvericin Mycotoxins on SH-SY5Y Cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Aichinger, G.; Pantazi, F.; Marko, D. Combinatory estrogenic effects of bisphenol A in mixtures with alternariol and zearalenone in human endometrial cells. Toxicol Lett. 2020, 319, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Pero, R.W.; Posner, H.; Blois, M.; Harvan, D.; Spalding, J.W. Toxicity of metabolites produced by the “Alternaria”. Environ. Health Perspect. 1973, 4, 87–94. [Google Scholar] [CrossRef]
- Juan-García, A.; Juan, C.; König, S.; Ruiz, M.J. Cytotoxic effects and degradation products of three mycotoxins: Alternariol, 3-acetyl-deoxynivalenol and 15-acetyl-deoxynivalenol in liver hepatocellular carcinoma cells. Toxicol. Lett. 2015, 235, 8–16. [Google Scholar] [CrossRef]
- Ueno, Y.; Tashiro, F. α-Zearalenol, a Major Hepatic Metabolite in Rats of Zearalenone, an Estrogenic Mycotoxin of Fusarium Species. J. Biochem. 1981, 89, 563–571. [Google Scholar] [CrossRef]
- Drzymala, S.S.; Binder, J.; Brodehl, A.; Penkert, M.; Rosowski, M.; Garbe, L.-A.; Koch, M. Estrogenicity of novel phase I and phase II metabolites of zearalenone and cis-zearalenone. Toxicon 2015, 105, 10–12. [Google Scholar] [CrossRef]
- Le Guevel, R.; Pakdel, F. Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum. Reprod. 2001, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; Sumpter, J.P. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ. Toxicol. Chem. 1996, 15, 241–248. [Google Scholar] [CrossRef]
- Makela, S.; Davis, V.L.; Tally, W.C.; Korkman, J.; Salo, L.; Vihko, R.; Santti, R.; Korach, K.S. Dietary estrogens act through estrogen receptor-mediated processes and show no antiestrogenicity in cultured breast cancer cells. Environ. Health Perspect. 1994, 102, 572–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miksicek, R.J. Estrogenic Flavonoids: Structural Requirements for Biological Activity. Exp. Biol. Med. 1995, 208, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Sanseverino, J.; Gupta, R.K.; Layton, A.C.; Patterson, S.S.; Ripp, S.A.; Saidak, L.; Simpson, L.; Schultz, T.W.; Sayler, G.S.; Simpson, M.L. Use of Saccharomyces cerevisiae BLYES Expressing Bacterial Bioluminescence for Rapid, Sensitive Detection of Estrogenic Compounds. Appl. Environ. Microbiol. 2005, 71, 4455–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frizzell, C.; Ndossi, D.; Kalayou, S.; Eriksen, G.S.; Verhaegen, S.; Sørlie, M.; Elliott, C.T.; Ropstad, E.; Connolly, L. An in vitro investigation of endocrine disrupting effects of the mycotoxin alternariol. Toxicol. Appl. Pharmacol. 2013, 271, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Maleki-Dizaji, M.; Styles, J.A.; White, I.N. Ishikawa cells exhibit differential gene expression profiles in response to oestradiol or 4-hydroxytamoxifen. Endocr. Relat. Cancer 2007, 14, 337–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.-C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984, 22 (Suppl. C), 27–55. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Appropriateness to set a group health-based guidance value for ZEN and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar] [CrossRef]
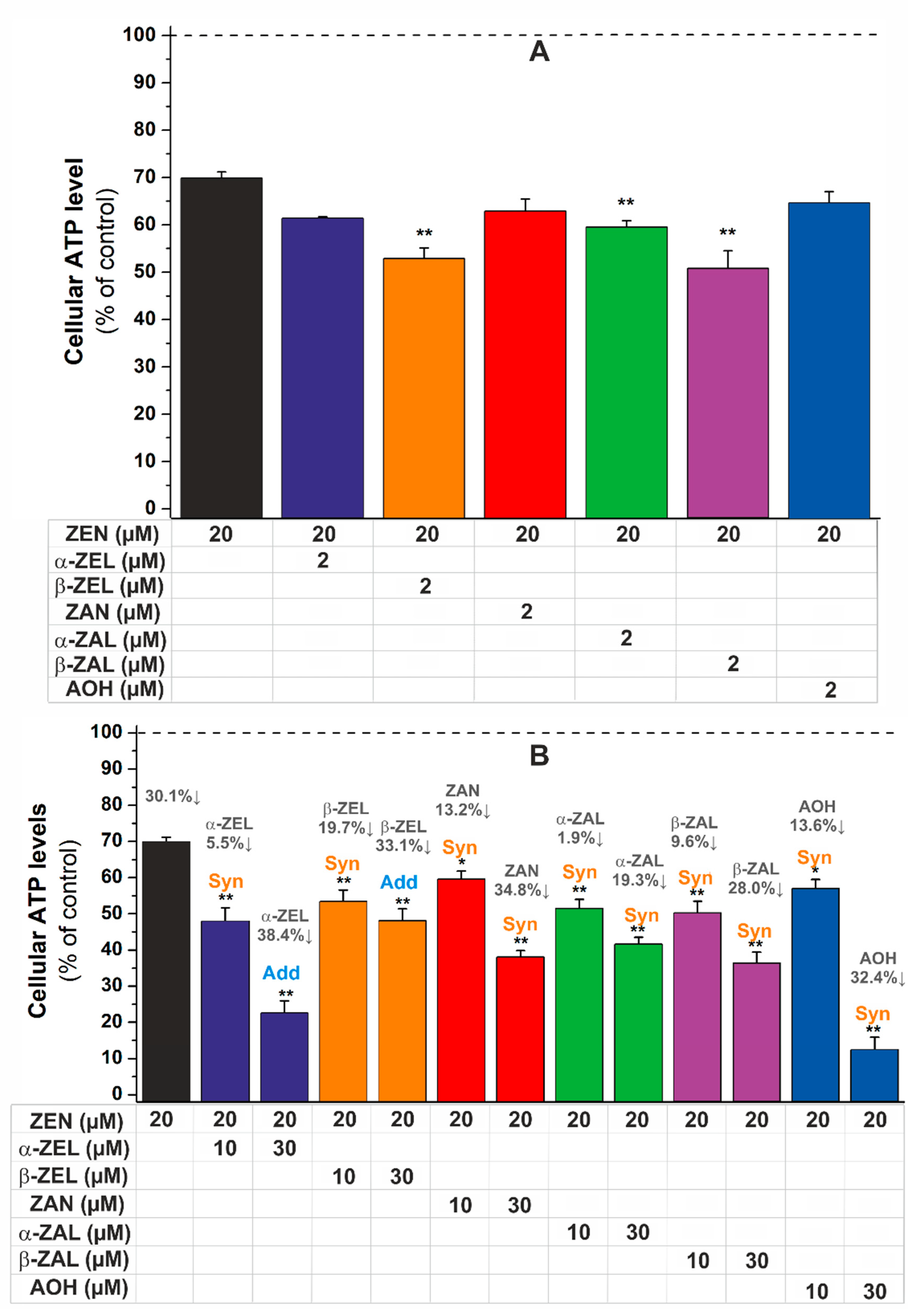


| 20 µM ZEN + | CI Value | Combined Cytotoxic Effect | |
|---|---|---|---|
| 10 µM α-ZEL | 0.57 | Synergism | ++ |
| 30 µM α-ZEL | 0.81 | Additive effect | ± |
| 10 µM β-ZEL | 0.64 | Synergism | + |
| 30 µM β-ZEL | 1.07 | Additive effect | ± |
| 10 µM ZAN | 0.55 | Synergism | ++ |
| 30 µM ZAN | 0.77 | Synergism | + |
| 10 µM α-ZAL | 0.52 | Synergism | ++ |
| 30 µM α-ZAL | 0.78 | Synergism | + |
| 10 µM β-ZAL | 0.51 | Synergism | ++ |
| 30 µM β-ZAL | 0.72 | Synergism | + |
| 10 µM AOH | 0.73 | Synergism | + |
| 30 µM AOH | 0.50 | Synergism | ++ |
| 6.28 × 10−2 µM ZEN + | CI Value | Combined Estrogenic Effect | |
|---|---|---|---|
| 6.24 × 10−3 µM α-ZEL | 0.37 | Synergism | ++ |
| 1.25 × 10−2 µM α-ZEL | 0.41 | Synergism | ++ |
| 1.22 µM β-ZEL | 0.77 | Synergism | + |
| 2.43 µM β-ZEL | 0.95 | Additive effect | ± |
| 2.50 × 10−2 µM ZAN | 0.58 | Synergism | ++ |
| 4.99 × 10−2 µM ZAN | 0.62 | Synergism | + |
| 1.24 × 10−2 µM α-ZAL | 0.97 | Additive effect | ± |
| 2.48 × 10−2 µM α-ZAL | 1.16 | Additive effect | ± |
| 1.24 × 10−1 µM β-ZAL | 1.19 | Additive effect | ± |
| 2.48 × 10−1 µM β-ZAL | 1.56 | Antagonism | -- |
| 7.4 × 10−1 µM GEN | 0.58 | Synergism | ++ |
| 1.48 µM GEN | 0.77 | Synergism | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balázs, A.; Faisal, Z.; Csepregi, R.; Kőszegi, T.; Kriszt, B.; Szabó, I.; Poór, M. In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein. Int. J. Mol. Sci. 2021, 22, 6281. https://doi.org/10.3390/ijms22126281
Balázs A, Faisal Z, Csepregi R, Kőszegi T, Kriszt B, Szabó I, Poór M. In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein. International Journal of Molecular Sciences. 2021; 22(12):6281. https://doi.org/10.3390/ijms22126281
Chicago/Turabian StyleBalázs, Adrienn, Zelma Faisal, Rita Csepregi, Tamás Kőszegi, Balázs Kriszt, István Szabó, and Miklós Poór. 2021. "In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein" International Journal of Molecular Sciences 22, no. 12: 6281. https://doi.org/10.3390/ijms22126281
APA StyleBalázs, A., Faisal, Z., Csepregi, R., Kőszegi, T., Kriszt, B., Szabó, I., & Poór, M. (2021). In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein. International Journal of Molecular Sciences, 22(12), 6281. https://doi.org/10.3390/ijms22126281







