Modulation of Glutamate Transporter EAAT1 and Inward-Rectifier Potassium Channel Kir4.1 Expression in Cultured Spinal Cord Astrocytes by Platinum-Based Chemotherapeutics
Abstract
:1. Introduction
2. Results
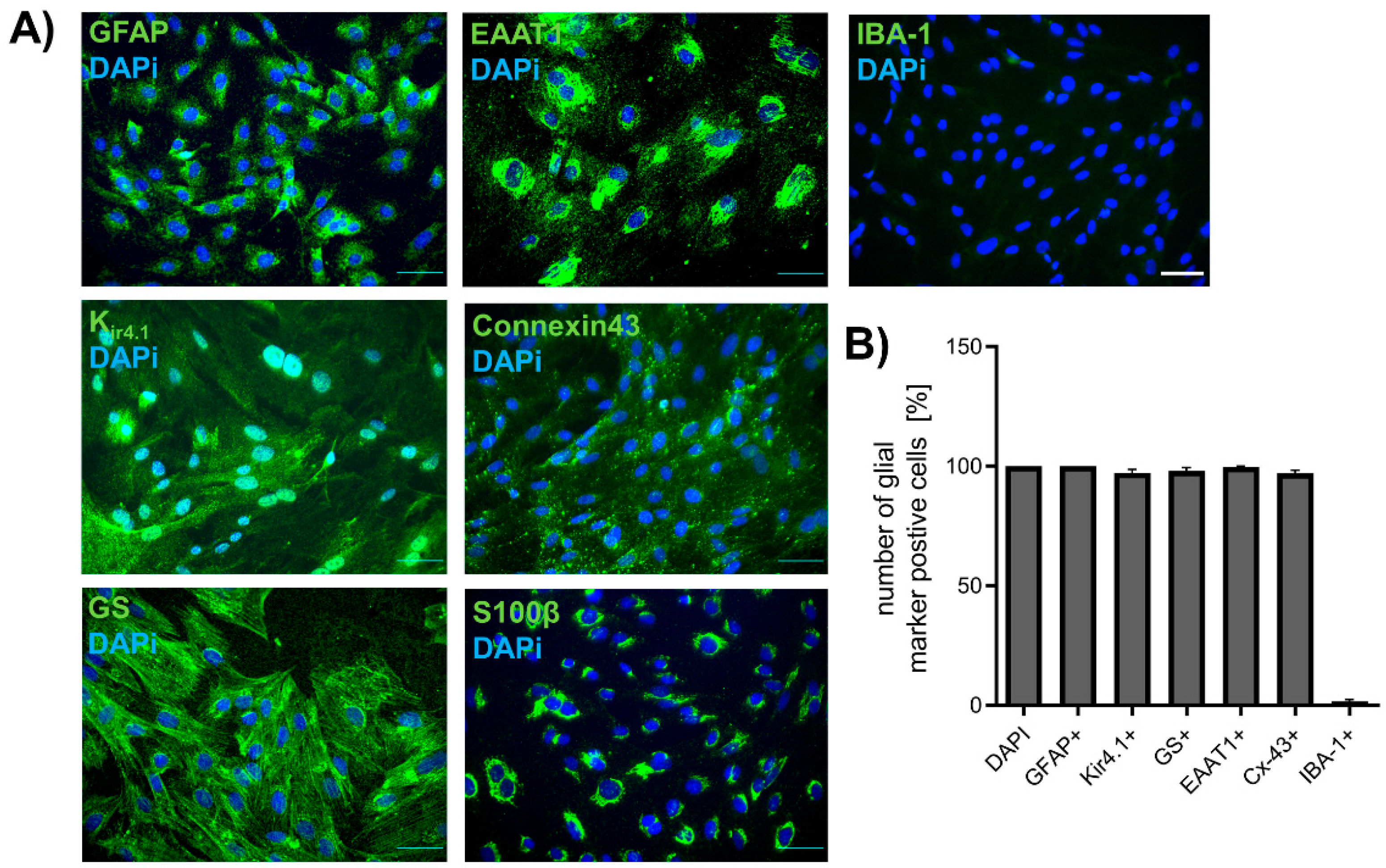
2.1. Isolation of Spinal Astrocytes from Juvenile Rats
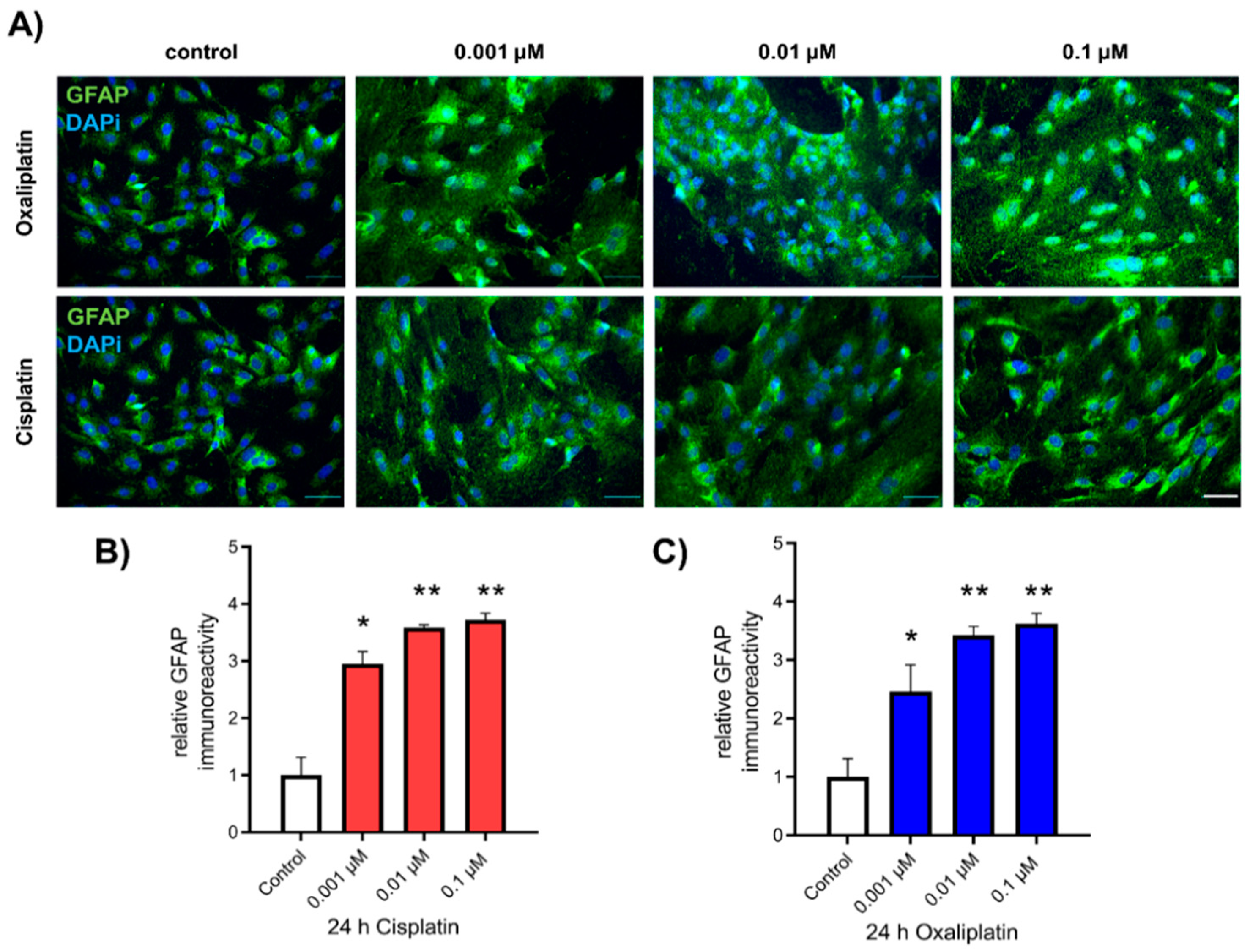
2.2. Activation of Spinal Astrocytes after Exposure to Chemotherapeutics
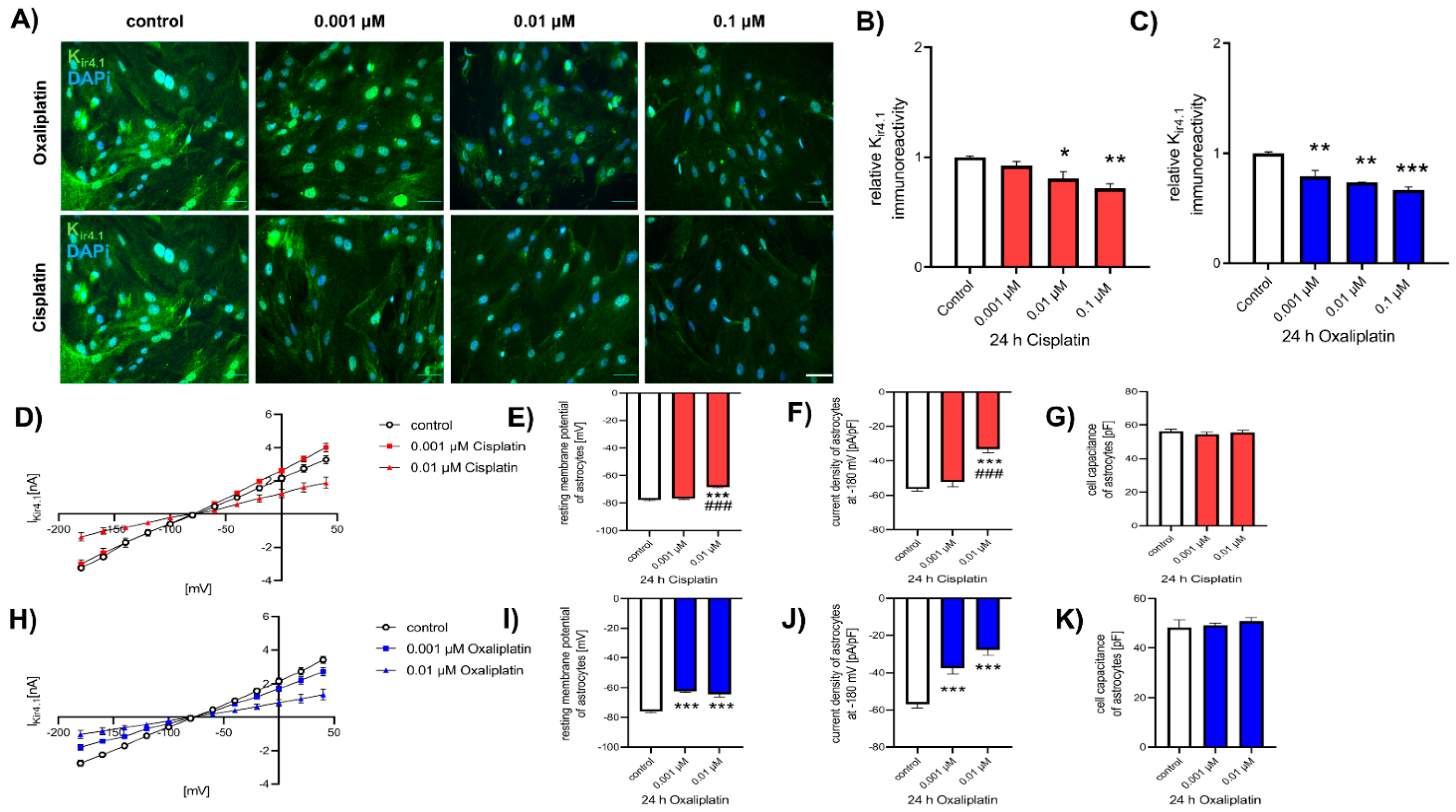
2.3. Reduction of Kir4.1 Protein Expression after Exposure to Chemotherapeutics
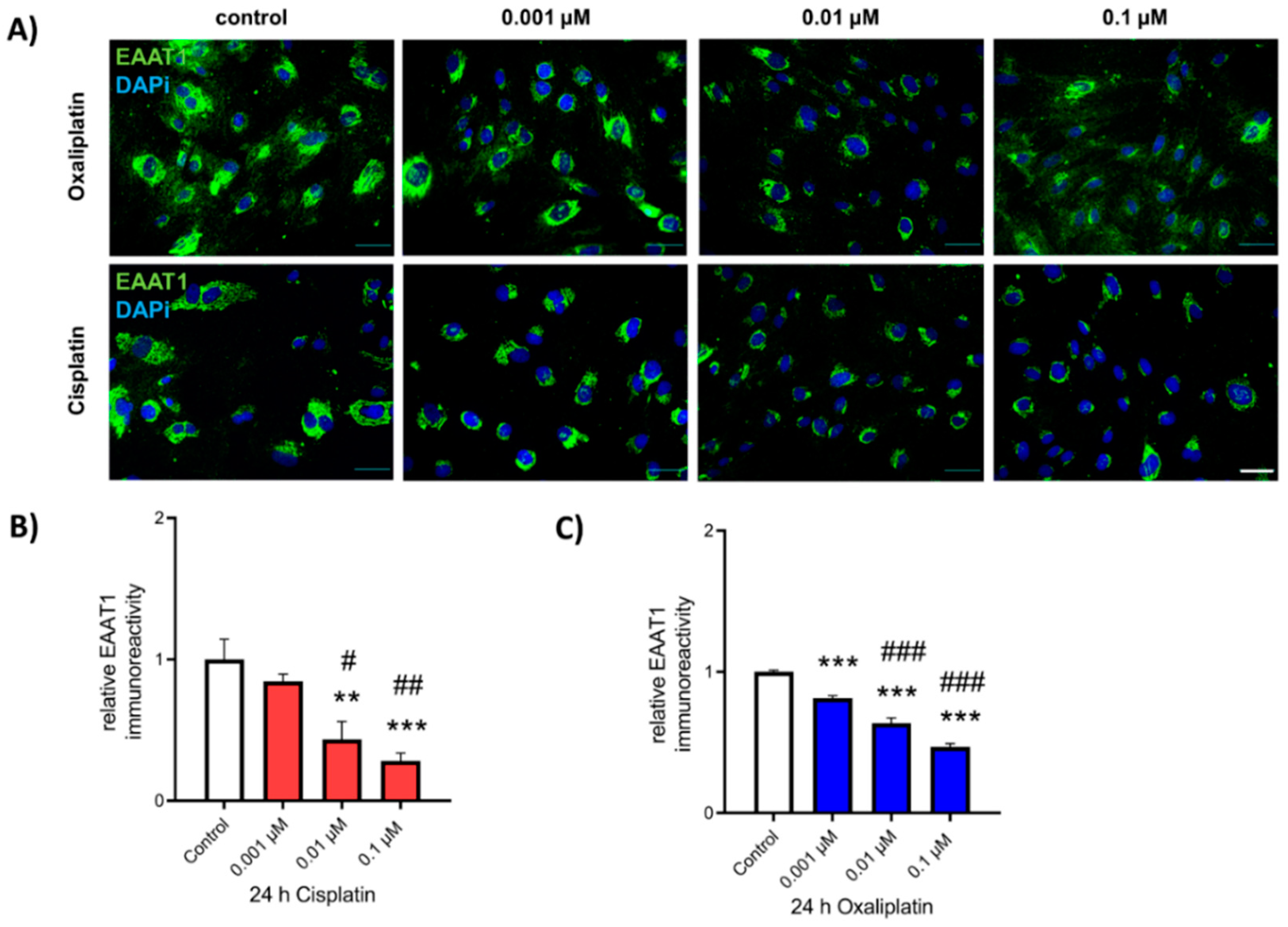
2.4. Reduction of EAAT1 Protein Expression after Exposure to Chemotherapeutics
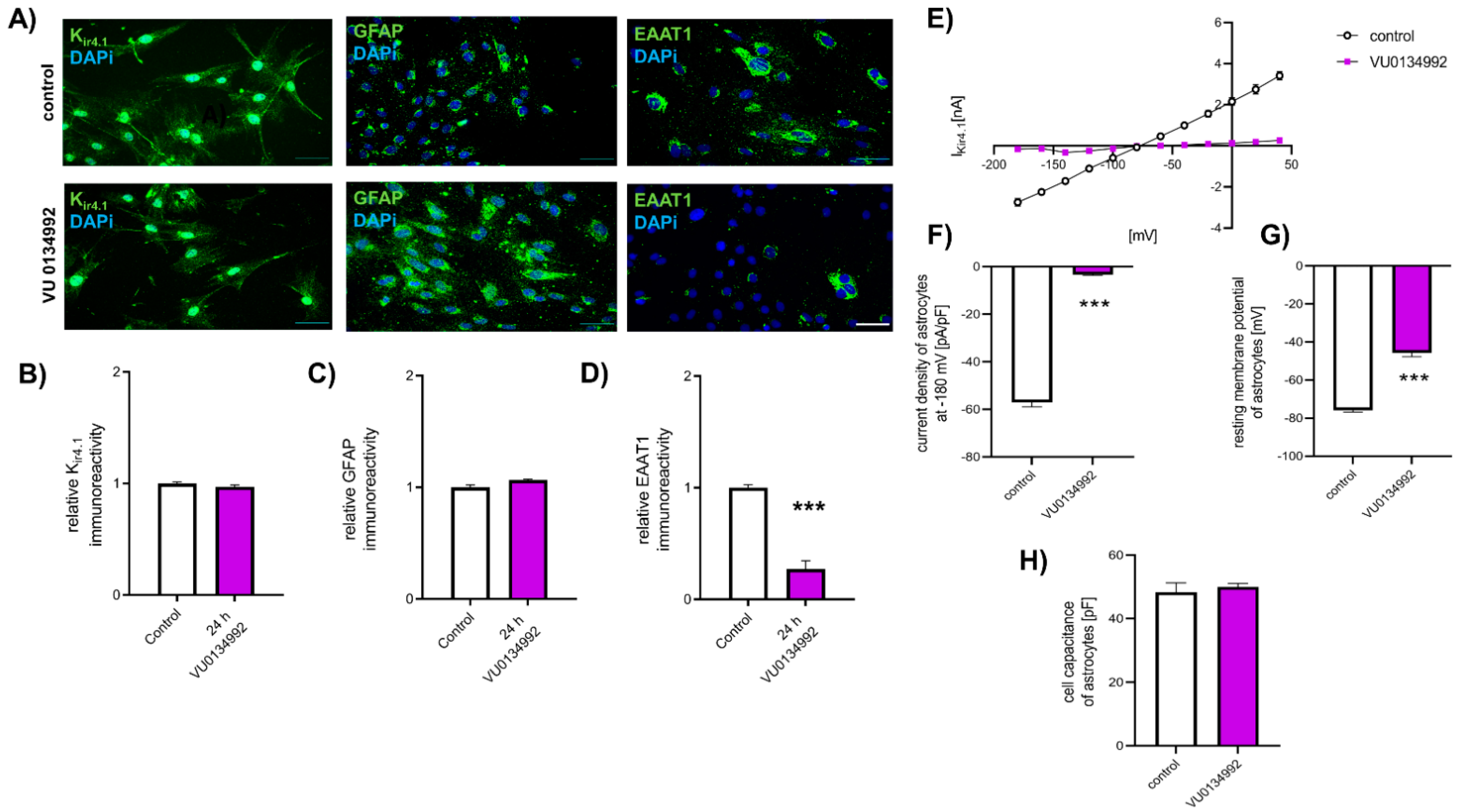
2.5. Reduction in EAAT1 Protein Expression Is Mediated by Kir4.1 Dysfunction
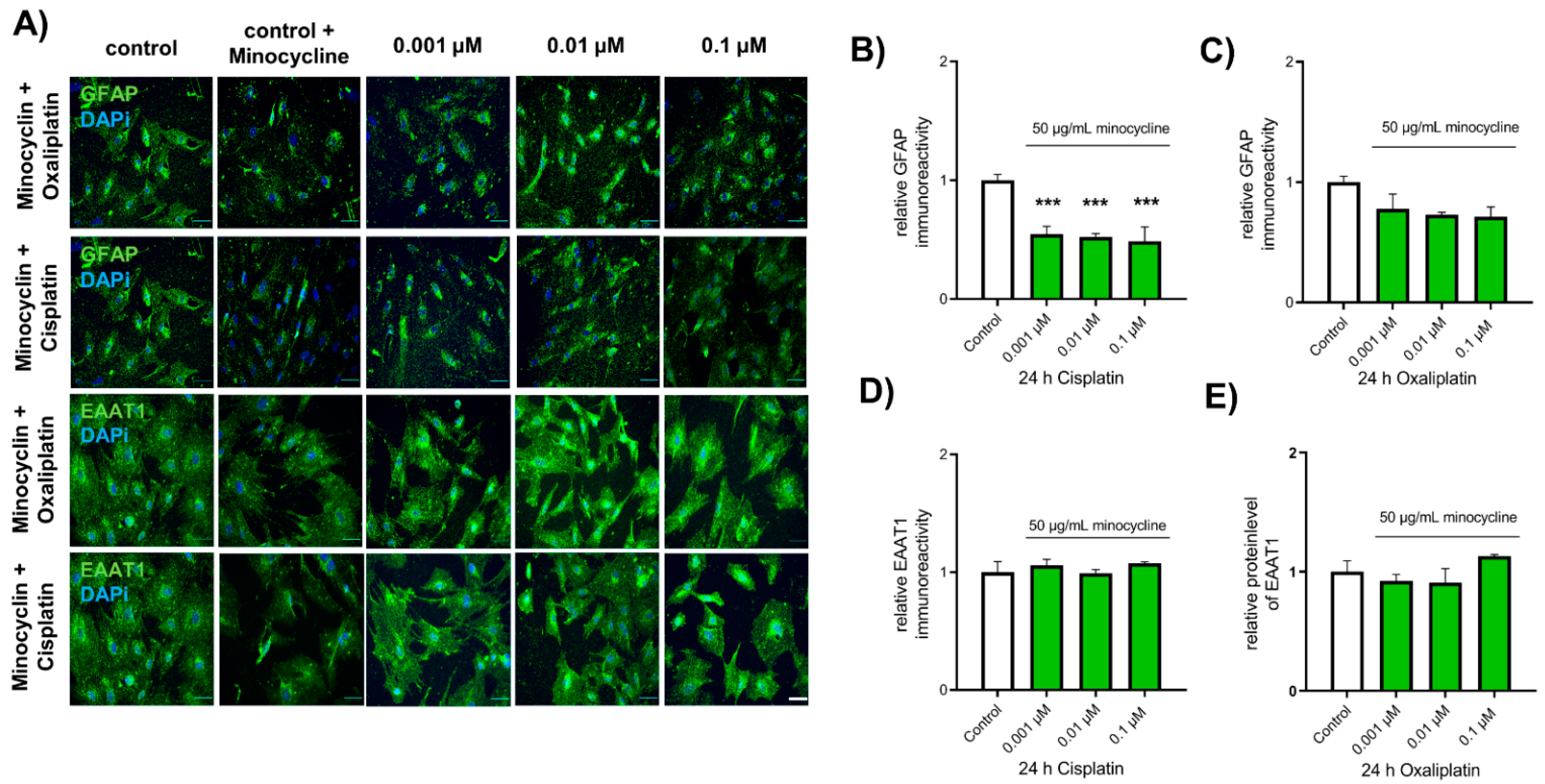
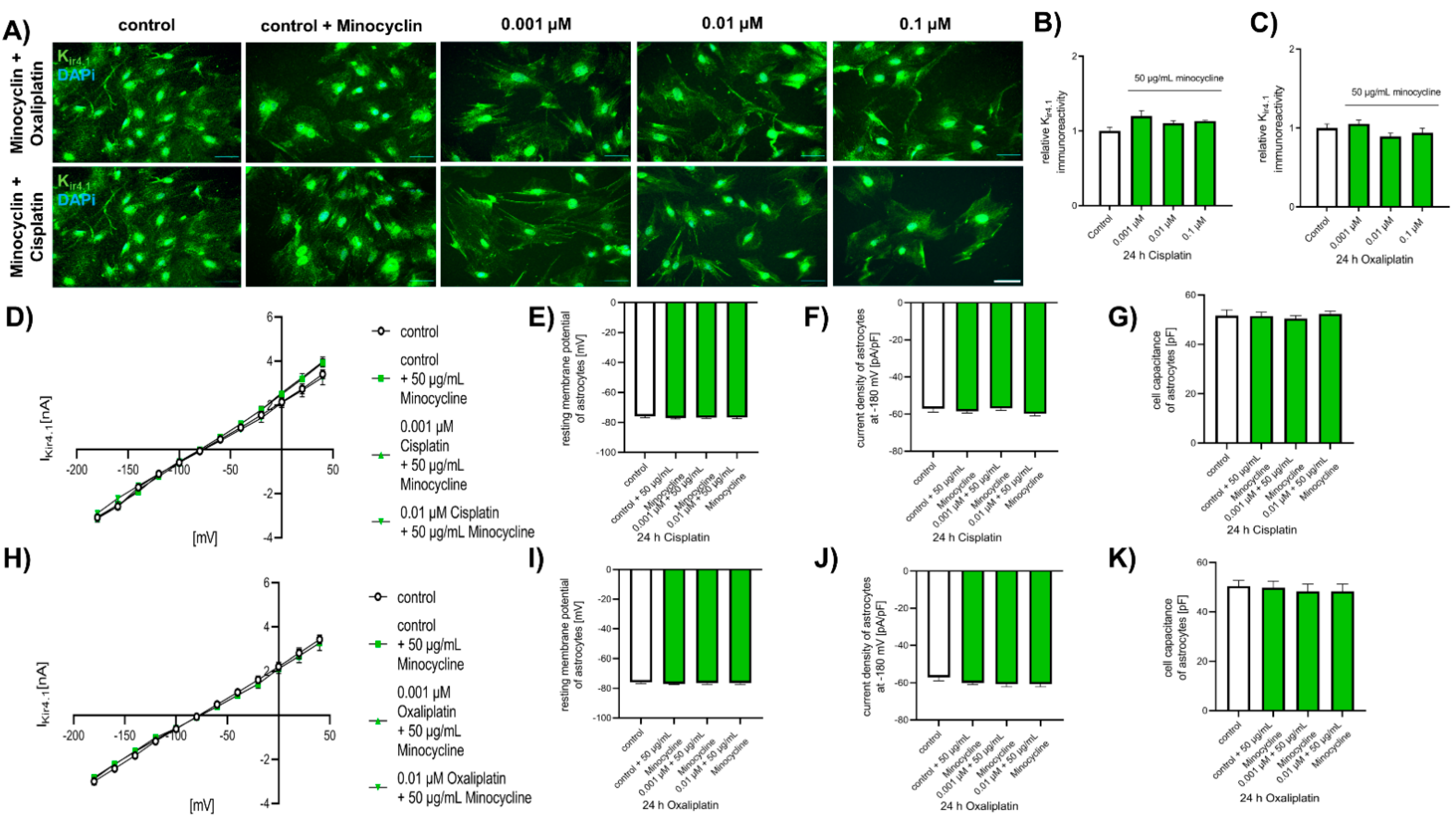
2.6. Chemotherapeutic Effects on Spinal Astrocytes Can Be Prevented by Antibiotic Minocycline
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Astrocytes from Spinal Cord
4.3. Drug Application
4.4. Immunocytochemical Staining of Spinal Astrocytes
4.5. Electrophysiology
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, S.W.; Davis, L.E.; Kornfeld, M.; Hilgers, R.D.; Standefer, J.C. Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer 1984, 54, 1269–1275. [Google Scholar] [CrossRef]
- Cavaletti, G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat. Rev. Cancer 2008, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J. Pain Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Beijers, A.; Mols, F.; Dercksen, W.; Driessen, C.; Vreugdenhil, G. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J. Community Support Oncol. 2014, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Misset, J.L.; Bleiberg, H.; Sutherland, W.; Bekradda, M.; Cvitkovic, E. Oxaliplatin clinical activity: A review. Crit. Rev. Oncol. Hematol. 2000, 35, 75–93. [Google Scholar] [CrossRef]
- Postma, T.J.; Benard, B.A.; Huijgens, P.C.; Ossenkoppele, G.J.; Heimans, J.J. Long-term effects of vincristine on the peripheral nervous system. J. Neurooncol. 1993, 15, 23–27. [Google Scholar] [CrossRef]
- Sahenk, Z.; Barohn, R.; New, P.; Mendell, J.R. Taxol neuropathy. Electrodiagnostic and sural nerve biopsy findings. Arch. Neurol. 1994, 51, 726–729. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Kusterarent, P.; Kutritz, A.; Rassaf, T.; Kleinschnitz, C.; Hendgen-Cotta, U.B.; Hagenacker, T. Platinum-Based Drugs Cause Mitochondrial Dysfunction in Cultured Dorsal Root Ganglion Neurons. Int. J. Mol. Sci. 2020, 21, 8636. [Google Scholar] [CrossRef]
- Leo, M.; Schmitt, L.I.; Kutritz, A.; Kleinschnitz, C.; Hagenacker, T. Cisplatin-induced activation and functional modulation of satellite glial cells lead to cytokine-mediated modulation of sensory neuron excitability. Exp. Neurol. 2021, 341, 113695. [Google Scholar] [CrossRef]
- Dzagnidze, A.; Katsarava, Z.; Makhalova, J.; Liedert, B.; Yoon, M.S.; Kaube, H.; Limmroth, V.; Thomale, J. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J. Neurosci. 2007, 27, 9451–9457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Splettstoesser, F.; Florea, A.M.; Busselberg, D. IP(3) receptor antagonist, 2-APB, attenuates cisplatin induced Ca2+-influx in HeLa-S3 cells and prevents activation of calpain and induction of apoptosis. Br. J. Pharmacol. 2007, 151, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Nat. Rev. Neurol. 2010, 6, 657–666. [Google Scholar] [CrossRef]
- Podratz, J.L.; Knight, A.M.; Ta, L.E.; Staff, N.P.; Gass, J.M.; Genelin, K.; Schlattau, A.; Lathroum, L.; Windebank, A.J. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol. Dis. 2011, 41, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Janes, K.; Wahlman, C.; Little, J.W.; Doyle, T.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav. Immun. 2015, 44, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Makker, P.G.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of synaptic connectivity by glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.; Henneberger, C.; Steinhauser, C. Diversity of astrocyte potassium channels: An update. Brain Res. Bull. 2018, 136, 26–36. [Google Scholar] [CrossRef]
- Kofuji, P.; Newman, E.A. Potassium buffering in the central nervous system. Neuroscience 2004, 129, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Nichols, C.G.; Lopatin, A.N. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997, 59, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Vit, J.P.; Ohara, P.T.; Bhargava, A.; Kelley, K.; Jasmin, L. Silencing the Kir4.1 potassium channel subunit in satellite glial cells of the rat trigeminal ganglion results in pain-like behavior in the absence of nerve injury. J. Neurosci. 2008, 28, 4161–4171. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, L.I.; Leo, M.; Kutritz, A.; Kleinschnitz, C.; Hagenacker, T. Activation and functional modulation of satellite glial cells by oxaliplatin lead to hyperexcitability of sensory neurons in vitro. Mol. Cell Neurosci. 2020, 105, 103499. [Google Scholar] [CrossRef]
- Tang, X.; Schmidt, T.M.; Perez-Leighton, C.E.; Kofuji, P. Inwardly rectifying potassium channel Kir4.1 is responsible for the native inward potassium conductance of satellite glial cells in sensory ganglia. Neuroscience 2010, 166, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Erikson, K.M.; Suber, R.L.; Aschner, M. Glutamate/aspartate transporter (GLAST), taurine transporter and metallothionein mRNA levels are differentially altered in astrocytes exposed to manganese chloride, manganese phosphate or manganese sulfate. Neurotoxicology 2002, 23, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Escartin, C.; Brouillet, E.; Gubellini, P.; Trioulier, Y.; Jacquard, C.; Smadja, C.; Knott, G.W.; Kerkerian-Le Goff, L.; Deglon, N.; Hantraye, P.; et al. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J. Neurosci. 2006, 26, 5978–5989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Watase, K.; Manabe, T.; Yamada, K.; Watanabe, M.; Takahashi, K.; Iwama, H.; Nishikawa, T.; Ichihara, N.; Kikuchi, T.; et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 1997, 276, 1699–1702. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Rothstein, J.D. Glutamate transporters: Animal models to neurologic disease. Neurobiol. Dis. 2004, 15, 461–473. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, S.Y.; Chao, W.; Volsky, D.J. Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): Cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. J. Neurochem. 2003, 87, 1485–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muldoon, L.L.; Soussain, C.; Jahnke, K.; Johanson, C.; Siegal, T.; Smith, Q.R.; Hall, W.A.; Hynynen, K.; Senter, P.D.; Peereboom, D.M.; et al. Chemotherapy delivery issues in central nervous system malignancy: A reality check. J. Clin. Oncol. 2007, 25, 2295–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.S.; Fox, E.; Dennie, C.; Morgan, L.B.; McCully, C.L.; Balis, F.M. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin. Cancer Res. 2005, 11, 1669–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.Z.; Li, D.; Ou-Yang, H.D.; Liu, C.C.; Liu, X.G.; Ma, C.; Wei, J.Y.; Liu, Y.; Xin, W.J. Cerebrospinal Fluid Oxaliplatin Contributes to the Acute Pain Induced by Systemic Administration of Oxaliplatin. Anesthesiology 2016, 124, 1109–1121. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Pacini, A.; Micheli, L.; Tani, A.; Zanardelli, M.; Ghelardini, C. Glial role in oxaliplatin-induced neuropathic pain. Exp. Neurol. 2014, 261, 22–33. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, W. The Role of Satellite Glial Cells, Astrocytes, and Microglia in Oxaliplatin-Induced Neuropathic Pain. Biomedicines 2020, 8, 324. [Google Scholar] [CrossRef]
- Robinson, C.R.; Zhang, H.; Dougherty, P.M. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience 2014, 274, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.Y.; Robinson, C.R.; Zhang, H.; Dougherty, P.M. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J. Pain Off. J. Am. Pain Soc. 2013, 14, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, B.S.; Kim, S.K.; Kim, H.N.; Lee, J.H.; Lee, J.H.; Hwang, D.S.; Bae, H.; Min, B.I.; Kim, S.K. Gyejigachulbu-Tang Relieves Oxaliplatin-Induced Neuropathic Cold and Mechanical Hypersensitivity in Rats via the Suppression of Spinal Glial Activation. Evid. Based Complement. Altern. Med. 2014, 2014, 436482. [Google Scholar] [CrossRef]
- Zhang, H.; Yoon, S.Y.; Zhang, H.; Dougherty, P.M. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J. Pain Off. J. Am. Pain Soc. 2012, 13, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Michetti, F.; Corvino, V.; Geloso, M.C.; Lattanzi, W.; Bernardini, C.; Serpero, L.; Gazzolo, D. The S100B protein in biological fluids: More than a lifelong biomarker of brain distress. J. Neurochem. 2012, 120, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Shashoua, V.E.; Hesse, G.W.; Moore, B.W. Proteins of the brain extracellular fluid: Evidence for release of S-100 protein. J. Neurochem. 1984, 42, 1536–1541. [Google Scholar] [CrossRef]
- Ciccarelli, R.; Di Iorio, P.; Bruno, V.; Battaglia, G.; D’Alimonte, I.; D’Onofrio, M.; Nicoletti, F.; Caciagli, F. Activation of A(1) adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia 1999, 27, 275–281. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Omoaghe, A.O.; Ajonijebu, D.C.; Ojo, A.A.; Fabiyi, T.D.; Olajide, O.J.; Falode, D.T.; Adeniyi, P.A. Glia activation and its role in oxidative stress. Metab. Brain Dis. 2014, 29, 483–493. [Google Scholar] [CrossRef]
- Butt, A.M.; Kalsi, A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: A special role for Kir4.1 in glial functions. J. Cell. Mol. Med. 2006, 10, 33–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez-Gonzalez, M.P.; Rivera-Aponte, D.E.; Benedikt, J.; Maldonado-Martinez, G.; Tejeda-Bayron, F.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Astrocytic Kir4.1 Potassium Channels Is Associated with Hippocampal Neuronal Hyperexcitability in Type 2 Diabetic Mice. Brain Sci. 2020, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Vit, J.P.; Jasmin, L.; Bhargava, A.; Ohara, P.T. Satellite glial cells in the trigeminal ganglion as a determinant of orofacial neuropathic pain. Neuron Glia Biol. 2006, 2, 247–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, K.W.; Ben Haim, L.; Schirmer, L.; Tyzack, G.E.; Tolman, M.; Miller, J.G.; Tsai, H.H.; Chang, S.M.; Molofsky, A.V.; Yang, Y.; et al. Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron 2018, 98, 306–319 e307. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Kinboshi, M.; Shimizu, S. Inwardly Rectifying Potassium Channel Kir4.1 as a Novel Modulator of BDNF Expression in Astrocytes. Int. J. Mol. Sci. 2018, 19, 3313. [Google Scholar] [CrossRef] [Green Version]
- Kucheryavykh, Y.V.; Kucheryavykh, L.Y.; Nichols, C.G.; Maldonado, H.M.; Baksi, K.; Reichenbach, A.; Skatchkov, S.N.; Eaton, M.J. Downregulation of Kir4.1 inward rectifying potassium channel subunits by RNAi impairs potassium transfer and glutamate uptake by cultured cortical astrocytes. Glia 2007, 55, 274–281. [Google Scholar] [CrossRef]
- Kaiser, M.; Maletzki, I.; Hulsmann, S.; Holtmann, B.; Schulz-Schaeffer, W.; Kirchhoff, F.; Bahr, M.; Neusch, C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2006, 99, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Bataveljic, D.; Nikolic, L.; Milosevic, M.; Todorovic, N.; Andjus, P.R. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia 2012, 60, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Howells, D.W.; Porritt, M.J.; Wong, J.Y.; Batchelor, P.E.; Kalnins, R.; Hughes, A.J.; Donnan, G.A. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp. Neurol. 2000, 166, 127–135. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Ushio, S.; Egashira, N.; Kawashiri, T.; Mitsuyasu, S.; Higuchi, H.; Ozawa, N.; Masuguchi, K.; Ono, Y.; Masuda, S. Excessive spinal glutamate transmission is involved in oxaliplatin-induced mechanical allodynia: A possibility for riluzole as a prophylactic drug. Sci. Rep. 2017, 7, 9661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, W.J.; Weng, H.R.; Dougherty, P.M. Plasticity in expression of the glutamate transporters GLT-1 and GLAST in spinal dorsal horn glial cells following partial sciatic nerve ligation. Mol. Pain 2009, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, B.; Brew, H.; Attwell, D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J. Physiol. 1991, 436, 169–193. [Google Scholar] [CrossRef]
- Mennerick, S.; Shen, W.; Xu, W.; Benz, A.; Tanaka, K.; Shimamoto, K.; Isenberg, K.E.; Krause, J.E.; Zorumski, C.F. Substrate turnover by transporters curtails synaptic glutamate transients. J. Neurosci. 1999, 19, 9242–9251. [Google Scholar] [CrossRef] [Green Version]
- Otis, T.S.; Kavanaugh, M.P. Isolation of current components and partial reaction cycles in the glial glutamate transporter EAAT2. J. Neurosci. 2000, 20, 2749–2757. [Google Scholar] [CrossRef]
- D’Ambrosio, R. The role of glial membrane ion channels in seizures and epileptogenesis. Pharmacol. Ther. 2004, 103, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Neusch, C.; Papadopoulos, N.; Muller, M.; Maletzki, I.; Winter, S.M.; Hirrlinger, J.; Handschuh, M.; Bahr, M.; Richter, D.W.; Kirchhoff, F.; et al. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: Impact on extracellular K+ regulation. J. Neurophysiol. 2006, 95, 1843–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.L.; Higashimori, H.; Campbell, S.L.; Hablitz, J.J.; Sontheimer, H. Functional expression of Kir4.1 channels in spinal cord astrocytes. Glia 2006, 53, 516–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.L.; Sontheimer, H. Functional implications for Kir4.1 channels in glial biology: From K+ buffering to cell differentiation. J. Neurochem. 2008, 107, 589–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shultz, R.B.; Zhong, Y. Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen. Res. 2017, 12, 702–713. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Yang, Y.; Chen, W.; Du, N.; Du, Y.; Gu, H.; Liu, Q. Minocycline, but not doxycycline attenuates NMDA-induced [Ca2+]i and excitotoxicity. NeuroReport 2021, 32, 38–43. [Google Scholar] [CrossRef]
- Hu, L.Y.; Zhou, Y.; Cui, W.Q.; Hu, X.M.; Du, L.X.; Mi, W.L.; Chu, Y.X.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef]
- Poulsen, J.N.; Larsen, F.; Duroux, M.; Gazerani, P. Primary culture of trigeminal satellite glial cells: A cell-based platform to study morphology and function of peripheral glia. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 1–12. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leo, M.; Schmitt, L.-I.; Steffen, R.; Kutritz, A.; Kleinschnitz, C.; Hagenacker, T. Modulation of Glutamate Transporter EAAT1 and Inward-Rectifier Potassium Channel Kir4.1 Expression in Cultured Spinal Cord Astrocytes by Platinum-Based Chemotherapeutics. Int. J. Mol. Sci. 2021, 22, 6300. https://doi.org/10.3390/ijms22126300
Leo M, Schmitt L-I, Steffen R, Kutritz A, Kleinschnitz C, Hagenacker T. Modulation of Glutamate Transporter EAAT1 and Inward-Rectifier Potassium Channel Kir4.1 Expression in Cultured Spinal Cord Astrocytes by Platinum-Based Chemotherapeutics. International Journal of Molecular Sciences. 2021; 22(12):6300. https://doi.org/10.3390/ijms22126300
Chicago/Turabian StyleLeo, Markus, Linda-Isabell Schmitt, Rebecca Steffen, Andrea Kutritz, Christoph Kleinschnitz, and Tim Hagenacker. 2021. "Modulation of Glutamate Transporter EAAT1 and Inward-Rectifier Potassium Channel Kir4.1 Expression in Cultured Spinal Cord Astrocytes by Platinum-Based Chemotherapeutics" International Journal of Molecular Sciences 22, no. 12: 6300. https://doi.org/10.3390/ijms22126300






