Synthesis of Caffeoyl-Prolyl-Histidyl-Xaa Derivatives and Evaluation of Their Activities and Stability upon Long-Term Storage
Abstract
1. Introduction
2. Results and Discussion
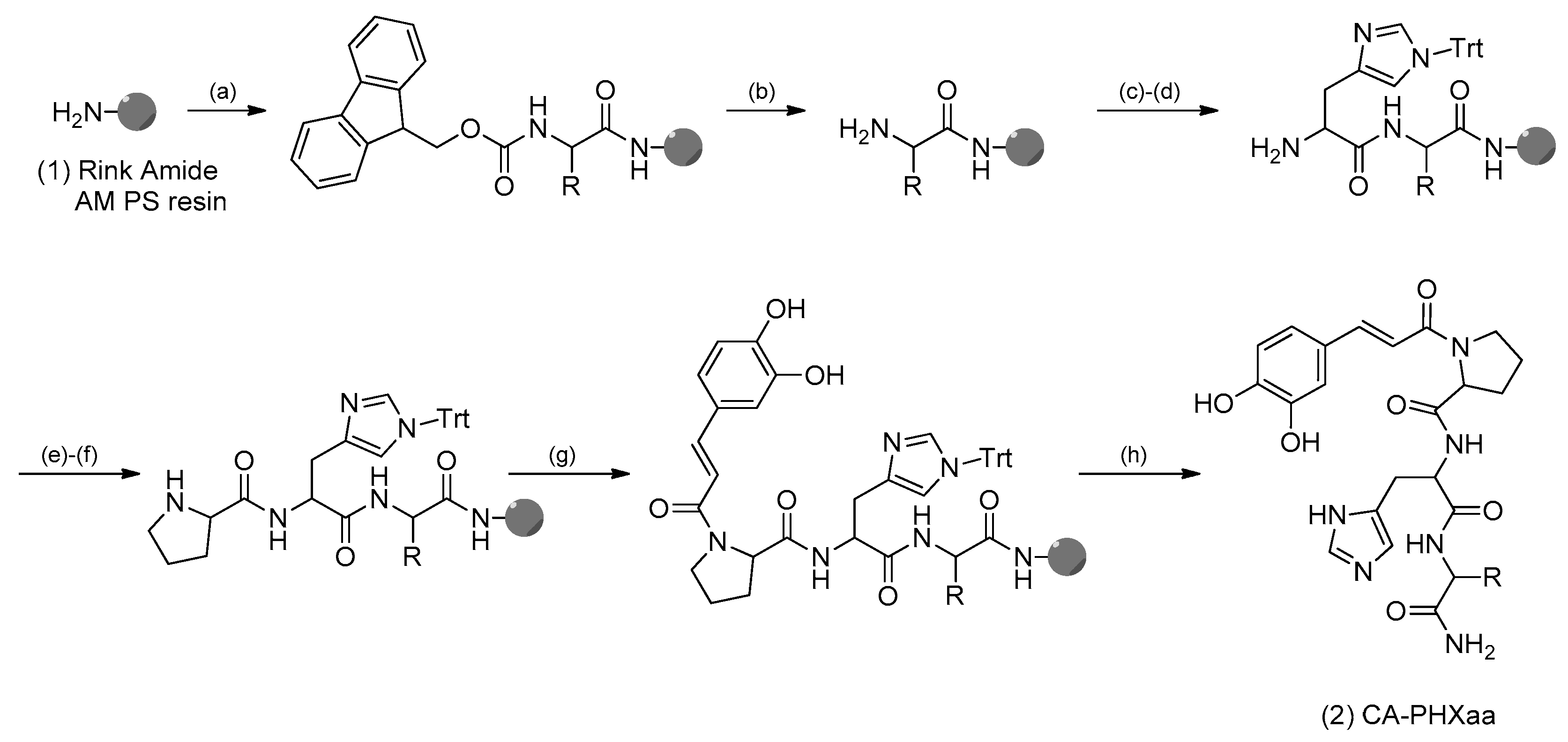
2.1. Synthesis of CA-PHX-NH2
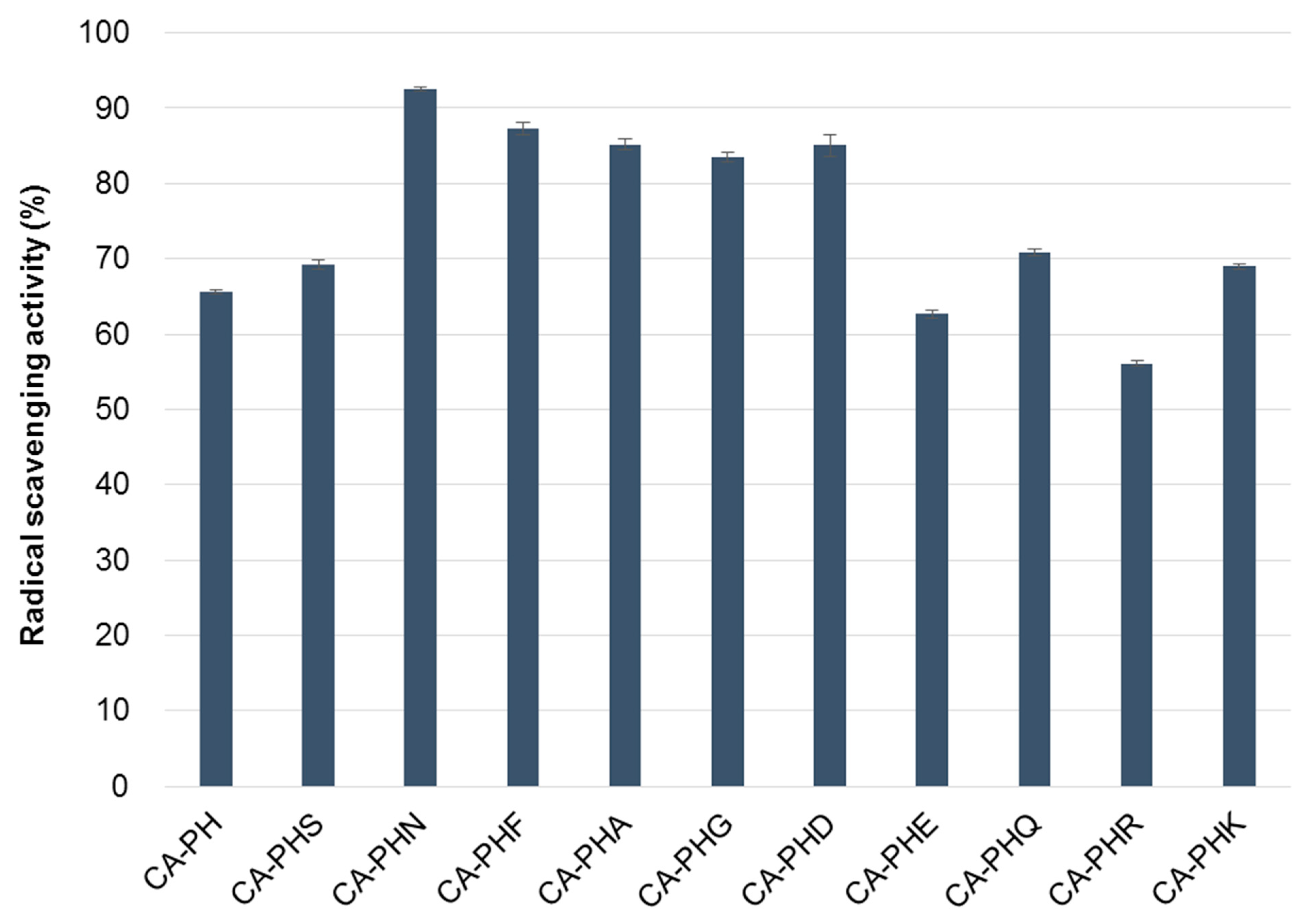
2.2. Antioxidative Activities of the CA-PHX-NH2 Derivatives
2.2.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Test
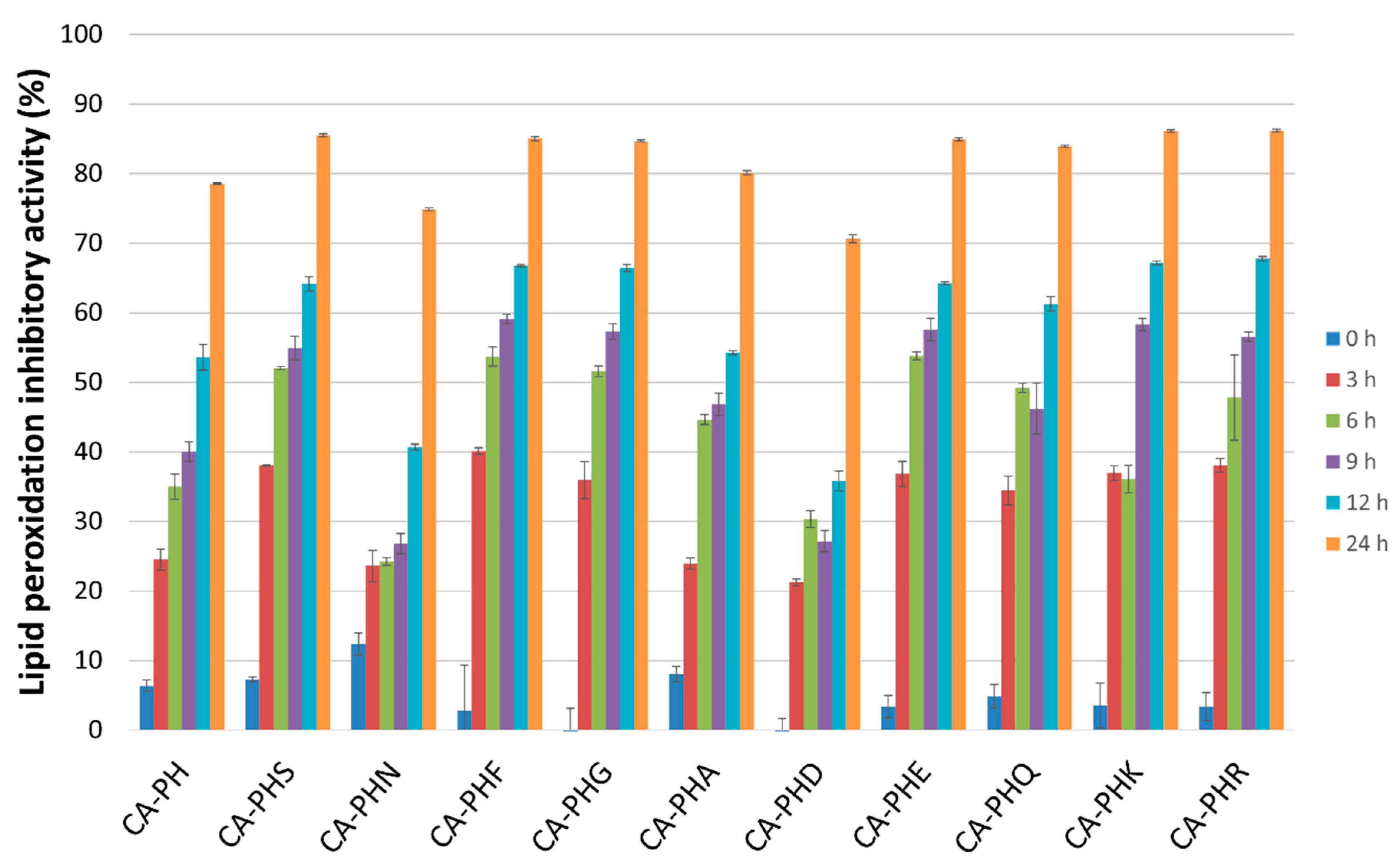
2.2.2. Lipid Peroxidation (LPO) Test
2.3. Cytotoxicity of the CA-PHX-NH2 Derivatives
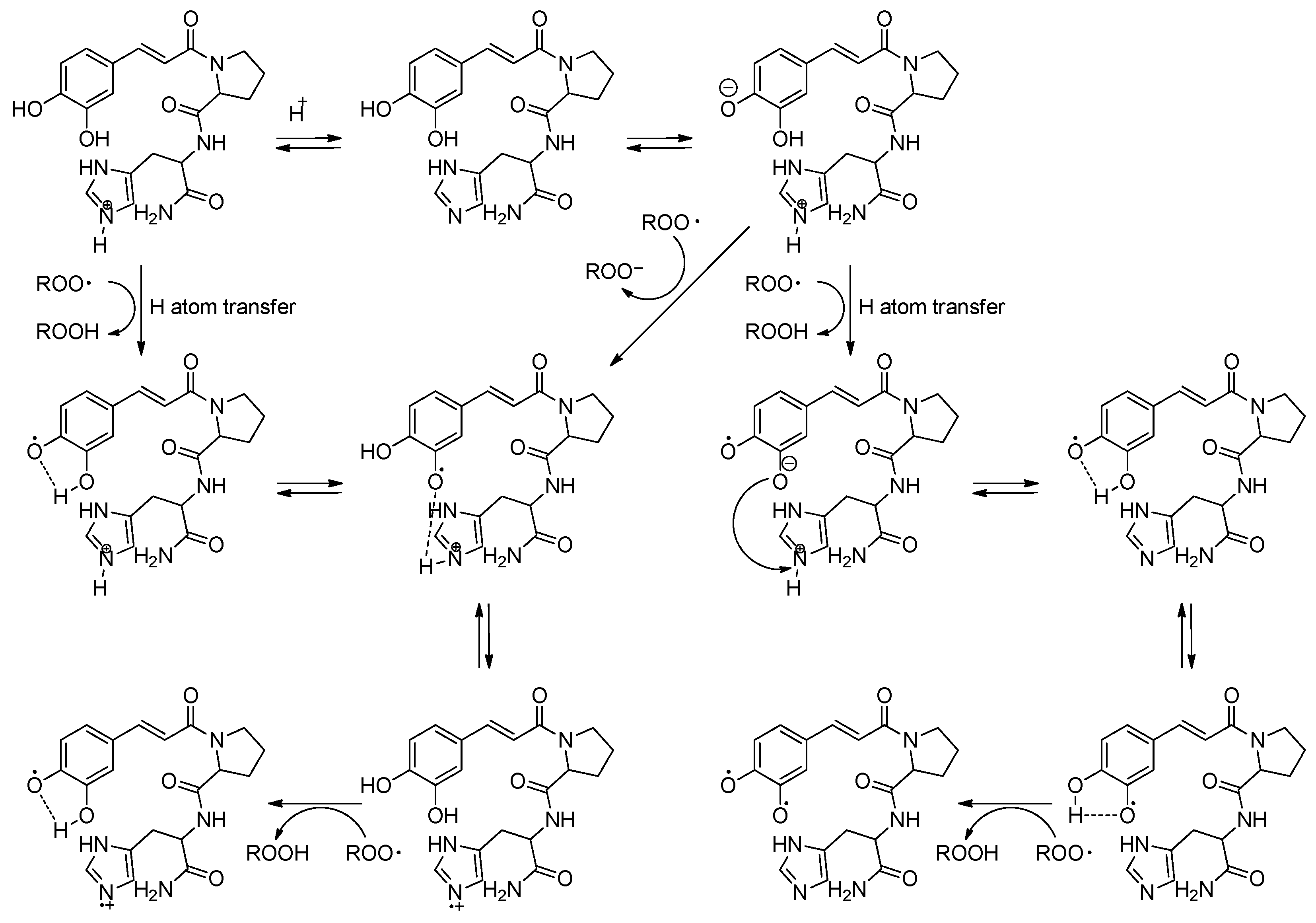
2.4. Stability Evaluation Using 1H-NMR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Solid-Phase Synthesis of CA-PHX-NH2
3.3. Antioxidant Activities of CA-PHX-NH2
3.3.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Test
3.3.2. LPO Test
3.4. Cytotoxicity Assay for CA-PHX-NH2
3.5. Long-Term Stability Test Using 1H-NMR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CA-PHX-NH2 | Caffeoyl-prolyl-histidyl-X amino acid amide |
| AM PS | Aminomethyl polystyrene |
| MBHA | 4-Methylbenzhydrylamine |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| LPO | Lipid peroxidation |
References
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Benzi, G.; Moretti, A. Are reactive oxygen species involved in Alzheimer’s disease? Neurobiol. Aging 1995, 16, 661–674. [Google Scholar] [CrossRef]
- Perry, G.; Castellani, R.J.; Hirai, K.; Smith, M.A. Reactive oxygen species mediate cellular damage in Alzheimer disease. J. Alzheimer’s Dis. 1998, 1, 45–55. [Google Scholar] [CrossRef]
- Zhao, B. Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem. Res. 2009, 34, 630–638. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Chen, Z.; Chan, P.; Kwan, K.; Zhang, A. Reassessment of the antioxidant activity of conjugated linoleic acids. J. Am. Oil Chem. Soc. 1997, 74, 749–753. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants in food and food antioxidants. Food/Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Guan, Y.; Chu, Q.; Fu, L.; Ye, J. Determination of antioxidants in cosmetics by micellar electrokinetic capillary chromatography with electrochemical detection. J. Chromatogr. A 2005, 1074, 201–204. [Google Scholar] [CrossRef]
- Rayner, B.S.; Duong, T.H.; Myers, S.J.; Witting, P.K. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J. Neurochem. 2006, 97, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Branen, A. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J. Am. Oil Chem. Soc. 1975, 52, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure—activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Ren, J.; Li, Y.; Chang, W.; Chen, Z. Study on the multiple mechanisms underlying the reaction between hydroxyl radical and phenolic compounds by qualitative structure and activity relationship. Bioorganic Med. Chem. 2002, 10, 4067–4073. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Doiron, J.A.; Métayer, B.; Richard, R.R.; Desjardins, D.; Boudreau, L.H.; Levesque, N.A.; Jean-François, J.; Poirier, S.J.; Surette, M.E.; Touaibia, M. Clicked cinnamic/caffeic esters and amides as radical scavengers and 5-lipoxygenase inhibitors. Int. J. Med. Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Marinova, E.; Georgiev, L.; Totseva, I.; Seizova, K.; Milkova, T. Antioxidant activity and mechanism of action of some synthesised phenolic acid amides of aromatic amines. Czech J. Food Sci. 2013, 31, 5–13. [Google Scholar] [CrossRef]
- Rajan, P.; Vedernikova, I.; Cos, P.; Berghe, D.V.; Augustyns, K.; Haemers, A. Synthesis and evaluation of caffeic acid amides as antioxidants. Bioorganic Med. Chem. Lett. 2001, 11, 215–217. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Seo, H.S.; Lee, Y.S. Synergistic antioxidative activities of hydroxycinnamoyl-peptides. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2009, 15, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Kwak, S.-Y.; Lee, Y.-S. Antioxidative activities of histidine containing caffeic acid-dipeptides. Bioorganic Med. Chem. Lett. 2010, 20, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Kortenska-Kancheva, V.; Totseva, I.; Ivanova, G.; Georgiev, L.; Milkova, T. Synthesis of cinnamoyl and hydroxycinnamoyl amino acid conjugates and evaluation of their antioxidant activity. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2006, 12, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Stankova, I.; Chuchkov, K.; Shishkov, S.; Kostova, K.; Mukova, L.; Galabov, A.S. Synthesis, antioxidative and antiviral activity of hydroxycinnamic acid amides of thiazole containing amino acid. Amino Acids 2009, 37, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Lee, H.J.; Yang, J.-K.; Lee, E.J.; Seo, M.; Lee, Y.-S. Antioxidant activity of caffeoyl-prolyl-histidine amide and its effects on PDGF-induced proliferation of vascular smooth muscle cells. Amino Acids 2014, 46, 2777–2785. [Google Scholar] [CrossRef]
- Matsueda, G.R.; Stewart, J.M. A p-methylbenzhydrylamine resin for improved solid-phase synthesis of peptide amides. Peptides 1981, 2, 45–50. [Google Scholar] [CrossRef]
- Mitsuda, H. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo Shokuryo 1966, 19, 210–221. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F. Structural analysis of antioxidative peptides from Soybean. beta.-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]

| Tripeptide | Rink Amide MBHA Resin | Rink Amide AM PS Resin | ||
|---|---|---|---|---|
| Yield (%) | Purity (%) | Yield (%) | Purity (%) | |
| PHS-NH2 | 49.4 | 89.7 | 89.6 | 96.2 |
| PHA-NH2 | 57.5 | 94.0 | 82.2 | 97.4 |
| PHN-NH2 | 49.5 | 90.0 | >99 | 95.4 |
| PHD-NH2 | 47.8 | 89.5 | 97.3 | 93.7 |
| CA-PHX-NH2 | Crude Purity (%) | Refinement Purity (%) | ESI-MS | |
|---|---|---|---|---|
| Calculated | Found | |||
| [M + H]+ | [M + H]+ | |||
| CA-PH-NH2 | 74.6 | 98.2 | 414.43 | 414.02 |
| CA-PHS-NH2 | 77.6 | 98.5 | 501.49 | 501.07 |
| CA-PHR-NH2 | 76.3 | 97.4 | 570.60 | 570.00 |
| CA-PHD-NH2 | 72.6 | 99.5 | 529.5 | 529.04 |
| CA-PHG-NH2 | 73.4 | 97.1 | 471.46 | 471.03 |
| CA-PHN-NH2 | 76.9 | 98.7 | 528.51 | 528.09 |
| CA-PHF-NH2 | 79.9 | 99.2 | 561.59 | 561.02 |
| CA-PHK-NH2 | 79.7 | 98.5 | 542.58 | 542.14 |
| CA-PHE-NH2 | 75.9 | 97.7 | 543.53 | 543.04 |
| CA-PHA-NH2 | 73.4 | 98.1 | 485.49 | 485.07 |
| CA-PHQ-NH2 | 75.1 | 96.1 | 542.54 | 541.96 |
| CA-PHN-NH2 | Relative Integral Intensity (%) | ||
|---|---|---|---|
| (Ratio of s-cis Form) | |||
| CH= Proton | His Cε1-H Proton | Average | |
| 0 h | 94.38 | 94.61 | 94.50 |
| 1 week | 93.84 | 94.01 | 93.93 |
| 1 month | 93.40 | 94.22 | 93.81 |
| 2 months | 95.04 | 93.82 | 94.43 |
| 3 months | 93.69 | 95.53 | 94.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Jeon, Y.-E.; Yang, J.-K.; Kim, J.; Chung, W.-J.; Lee, Y.-S.; Shin, D.-S. Synthesis of Caffeoyl-Prolyl-Histidyl-Xaa Derivatives and Evaluation of Their Activities and Stability upon Long-Term Storage. Int. J. Mol. Sci. 2021, 22, 6301. https://doi.org/10.3390/ijms22126301
Jeong H, Jeon Y-E, Yang J-K, Kim J, Chung W-J, Lee Y-S, Shin D-S. Synthesis of Caffeoyl-Prolyl-Histidyl-Xaa Derivatives and Evaluation of Their Activities and Stability upon Long-Term Storage. International Journal of Molecular Sciences. 2021; 22(12):6301. https://doi.org/10.3390/ijms22126301
Chicago/Turabian StyleJeong, Hyeri, Young-Eun Jeon, Jin-Kyoung Yang, Jaehi Kim, Woo-Jae Chung, Yoon-Sik Lee, and Dong-Sik Shin. 2021. "Synthesis of Caffeoyl-Prolyl-Histidyl-Xaa Derivatives and Evaluation of Their Activities and Stability upon Long-Term Storage" International Journal of Molecular Sciences 22, no. 12: 6301. https://doi.org/10.3390/ijms22126301
APA StyleJeong, H., Jeon, Y.-E., Yang, J.-K., Kim, J., Chung, W.-J., Lee, Y.-S., & Shin, D.-S. (2021). Synthesis of Caffeoyl-Prolyl-Histidyl-Xaa Derivatives and Evaluation of Their Activities and Stability upon Long-Term Storage. International Journal of Molecular Sciences, 22(12), 6301. https://doi.org/10.3390/ijms22126301






