NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Patients Groups
- CONV (conventional fractionation): 2 Gy per fraction, 35 fractions, the total dose 70 Gy, delivered once-a-day and 5-days-a-week with a weekend break, for 7 weeks; 55 patients treated with a concurrent CHRT, 7 patients treated with RT only;
- CAIR (continuous accelerated irradiation): 1.8 Gy per fraction, 40 fractions, the total dose 72 Gy, delivered once-a-day and 7-days-a-week, for 6 weeks; 22 patients.
- Manchester scheme (accelerated hypofractionated irradiation): 3 Gy per fraction, 17 fractions, the total dose 51 Gy, delivered once-a-day and 5-days-a-week with a weekend break, for 3.5 weeks; 19 patients.
- SIB (accelerated irradiation with simultaneous integrated boost): 2.2 Gy per fraction to the total dose 66 Gy for gross tumor volume (PTV1), 2.0 Gy per fraction to the total dose 60 Gy for gross tumor volume plus anatomical margins (PTV2), 1.8 Gy per fraction to the total dose 54 Gy for elective fields (PTV3), all in 30 fractions, delivered once-a-day and 5-days-a-week with a weekend break, for 6 weeks; 3 patients.
2.2. Volumes Receiving a Particular Dose of Irradiation
2.3. Serum Samples Collection
2.4. Sample Preparation for NMR Spectroscopy
2.5. Measurement Protocol
2.6. Spectra Post-Processing
2.7. Metabolite Identification
2.8. Metabolite Quantification
2.9. Data Analysis and the Validation of the Multivariate Model
3. Results
3.1. Differences in Volumes Receiving Particular Dose of Irradiation
3.2. Blood Serum Metabolic Profile vs. Radiation Therapy
- Concurrent CHRT (CONV RT fractionation with one to three cycles of CHT administered at weeks 0, 3 and 6 during RT),
- RT with CAIR/CONV/SIB fractionation,
- RT with Manchester fractionation.
- Type I—the metabolic profiles were compared in a weekly increment in RT (e.g., week-0 vs. week-1, week-1 vs. week-2, etc.).
- Type II—the changes in the blood serum during RT were compared to week-0 (e.g., week-0 vs. week-1, week-0 vs. week-2, etc.).
3.2.1. Concurrent CHRT
3.2.2. Radiotherapy with CAIR/CONV/SIB Fractionation
3.2.3. Radiotherapy with Manchester Fractionation
3.2.4. Irradiated Volume Impact on the Metabolic Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sengupta, A. Recent advances in head and neck cancer. Apollo Med. 2012, 9, 96–103. [Google Scholar] [CrossRef]
- Santa Cruz, O.; Tsoutsou, P.; Castella, C.; Khanfir, K.; Anchisi, S.; Bouayed, S.; Matzinger, O.; Ozsahin, M. Locoregional control and toxicity in head and neck carcinoma patients following helical Tomotherapy-delivered intensity-modulated radiation therapy compared with 3D-CRT data. Oncology 2018, 95, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shen, Z.L.; Ward, M.C.; Joshi, N.P.; Koyfman, S.A.; Xia, P. Evolution of treatment planning techniques in external-beam radiation therapy for head and neck cancer. Appl. Radiat. Oncol. 2015, 4, 18–25. [Google Scholar]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maître, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.M.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef]
- Guo, R.; Tang, L.L.; Mao, Y.P.; Zhou, G.Q.; Qi, Z.Y.; Liu, L.Z.; Lin, A.H.; Liu, M.Z.; Ma, J.; Sun, Y. Clinical Outcomes of Volume-Modulated Arc Therapy in 205 Patients with Nasopharyngeal Carcinoma: An Analysis of Survival and Treatment Toxicities. PLoS ONE 2015, 10, e0129679. [Google Scholar] [CrossRef]
- Pignon, J.P.; Bourhis, J.; Domenge, C.; Designé, L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analysis of updated individual data. Lancet 2000, 355, 949–955. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.A.; Welsh, L.C.; Wong, K.H.; Aleksic, A.; Dunne, E.; Islam, M.R.; Patel, A.; Patel, P.; Petkar, I.; Phillips, I.; et al. Normal Tissue Complication Probability (NTCP) Modelling of Severe Acute Mucositis using a Novel Oral Mucosal Surface Organ at Risk. Clin. Oncol. 2017, 29, 263–273. [Google Scholar] [CrossRef]
- Petras, K.G.; Rademaker, A.W.; Refaat, T.; Choi, M.; Thomas, T.O.; Pauloski, B.R.; Mittal, B.B. Dose-volume relationship for laryngeal substructures and aspiration in patients with locally advanced head-and-neck cancer. Radiat. Oncol. 2019, 14, 49. [Google Scholar] [CrossRef]
- Hansen, C.R.; Bertelsen, A.; Zukauskaite, R.; Johnsen, L.; Bernchou, U.; Thwaites, D.I.; Eriksen, J.G.; Johansen, J.; Brink, C. Prediction of radiation-induced mucositis of H&N cancer patients based on a large patient cohort. Radiother. Oncol. 2020, 147, 15–21. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Hajduk, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Składowski, K.; Sokół, M. 1H NMR based metabolomic approach to monitoring of the head and neck cancer treatment toxicity. Metabolomics 2016, 12, 102. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Bieleń, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Wygoda, A.; Kotylak, A.; Składowski, K.; Sokół, M. NMR-based metabolomics in real-time monitoring of treatment induced toxicity and cachexia in head and neck cancer: A method for early detection of high risk patients. Metabolomics 2019, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Zebrowska, A.; Skowronek, A.; Rutkowski, T.; Polanski, K.; Widlak, P.; Marczak, L.; Pietrowska, M. Metabolic Profiles of Whole Serum and Serum-Derived Exosomes Are Different in Head and Neck Cancer Patients Treated by Radiotherapy. J. Pers. Med. 2020, 10, 229. [Google Scholar] [CrossRef]
- Jelonek, K.; Krzywon, A.; Jablonska, P.; Slominska, E.M.; Smolenski, R.T.; Polanska, J.; Rutkowski, T.; Mrochem-Kwarciak, J.; Skladowski, K.; Widlak, P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients-Comparison of Serum Metabolome Profiles. Metabolites 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Widłak, P.; Pietrowska, M.; Polańska, J.; Rutkowski, T.; Jelonek, K.; Kalinowska-Herok, M.; Gdowicz-Kłosok, A.; Wygoda, A.; Tarnawski, R.; Składowski, K. Radiotherapy-related changes in serum proteome patterns of head and neck cancer patients; the effect of low and medium doses of radiation delivered to large volumes of normal tissue. J. Trans. Med. 2013, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Maan, K.; Tyagi, R.; Dutta, A.; Bakhshi, R.; Rana, P. Comparative metabolic profiles of total and partial body radiation exposure in mice using an untargeted metabolomics approach. Metabolomics 2020, 16, 124. [Google Scholar] [CrossRef]
- Satyamitra, M.M.; Cassatt, D.R.; Hollingsworth, B.A.; Price, P.W.; Rios, C.I.; Taliaferro, L.P.; Winters, T.A.; DiCarlo, A.L. Metabolomics in Radiation Biodosimetry: Current Approaches and Advances. Metabolites 2020, 10, 328. [Google Scholar] [CrossRef]
- Ala-Korpela, M.; Korhonen, A.; Keisala, J.; Hörkkö, S.; Korpi, P.; Ingman, L.P.; Jokisaari, J.; Savolainen, M.J.; Kesäniemi, Y.A. 1H NMR-based absolute quantitation of human lipoproteins and their lipid contents directly from plasma. J. Lipid Res. 1994, 35, 2292–2304. [Google Scholar] [CrossRef]
- Baumstark, D.; Kremer, W.; Boettcher, A.; Schreier, C.; Paul Sander, P.; Schmitz, G.; Kirchhoefer, R.; Huber, F.; Kalbitzer, H.R. 1H NMR spectroscopy quantifies visibility of lipoproteins, subclasses, and lipids at varied temperatures and pressures. J. Lipid Res. 2019, 60, 1516–1534. [Google Scholar] [CrossRef]
- Palumbo, E.; Piotto, C.; Calura, E.; Fasanaro, E.; Groff, E.; Busato, F.; El Khouzai, B.; Rigo, M.; Baggio, L.; Romualdi, C.; et al. Individual Radiosensitivity in Oncological Patients: Linking Adverse Normal Tissue Reactions and Genetic Features. Front. Oncol. 2019, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, X.; Pang, Q.; Zhang, H.; Yu, J.; Yang, B.; Zhou, L.; Zhang, F.; Liu, F. Radiosensitivity enhancement by combined treatment of nimotuzumab and celecoxib on nasopharyngeal carcinoma cells. Drug Des. Dev. Ther. 2018, 12, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.D.; Shalaurova, I.; Wolak-Dinsmore, J.; Connelly, M.A.; Mackey, R.H.; Stein, J.H.; Tracy, R.P. GlycA: A Composite Nuclear Magnetic Resonance Biomarker of Systemic Inflammation. Clin. Chem. 2015, 61, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Unione, L.; Ardá, A.; Jiménez-Barbero, J.; Millet, O. NMR of glycoproteins: Profiling, structure, conformation and interactions. Curr. Opin. Struct. Biol. 2021, 68, 9–17. [Google Scholar] [CrossRef]
- Bell, J.D.; Brown, J.C.; Nicholson, J.K.; Sadler, P.J. Assignment of resonances for ‘acute-phase’ glycoproteins in high resolution proton NMR spectra of human blood plasma. FEBS Lett. 1987, 215, 311–315. [Google Scholar] [CrossRef]
- Torri, G.M.; Torri, J.; Gulian, J.M.; Vion-Dury, J.; Viout, P.; Cozzone, P.J. Magnetic resonance spectroscopy of serum and acute-phase proteins revisited: A multiparametric statistical analysis of metabolite variations in inflammatory, infectious and miscellaneous diseases. Clin. Chim. Acta 1999, 279, 77–96. [Google Scholar] [CrossRef]
- Manmadhan, A.; Lin, B.X.; Zhong, J.; Parikh, M.; Berger, J.S.; Fisher, E.A.; Heffron, S.P. Elevated GlycA insevere obesity is normalized by bariatric surgery. Diabetes Obes. Metab. 2019, 21, 178–182. [Google Scholar] [CrossRef]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, inflammation and the immune response in cancer. Mamm. Gen. 2018, 29, 843–865. [Google Scholar] [CrossRef]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef]
- Uraz, S.; Tahan, G.; Aytekin, H.; Tahan, V. N-acetylcysteine expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic acid-induced colitis in rats. Scand. J. Clin. Lab. Investig. 2013, 73, 61–66. [Google Scholar] [CrossRef]
- Gabard, B.; Mascher, H. Endogenous plasma N-acetylcysteine and single dose oral bioavailability from two different formulations as determined by a new analytical method. Biopharm. Drug Dispos. 1991, 12, 343–353. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, N.M.; Beitz, D.C.; Whitehurst, G.B. Effect of Fasting on Free Fatty Acid, Glycerol and Cholesterol Concentrations in Blood Plasma and Lipoprotein Lipase Activity in Adipose Tissue of Cattle. J. Anim. Sci. 1981, 52, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Chandramouli, V.; Schumann, W.C.; Ekberg, K.; Previs, S.F.; Gupta, S.; Landau, B.R. Sources of blood glycerol during fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E998–E1004. [Google Scholar] [CrossRef]
- Knight, J.; Hinsdale, M.; Holmes, R. Glycolate and 2-phosphoglycolate content of tissues measured by ion chromatography coupled to mass spectrometry. Anal. Biochem. 2012, 421, 121–124. [Google Scholar] [CrossRef][Green Version]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Multiple roles of glyoxalase 1-mediated suppression of methylglyoxal glycation in cancer biology—Involvement in tumour suppression, tumour growth, multidrug resistance and target for chemotherapy. Semin. Cancer Biol. 2018, 49, 83–93. [Google Scholar] [CrossRef]
- Thornalley, P.J. The glyoxalase system in health and disease. Mol. Asp. Med. 1993, 14, 287–371. [Google Scholar] [CrossRef]
- Piskorska, D.; Danch, A.; Dróżdż, M.; Kopieczna-Grzebieniak, E.; Namysłowski, G.; Czecior, E.; Nowińska, E. Effect of radiotherapy and surgery on glyoxalase activities in larynx cancer patient erythrocytes. Med. Sci. Monit. 1998, 4, CR840–CR845. [Google Scholar]
- Antognelli, C.; Palumbo, I.; Aristei, C.; Talesa, V.N. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-κB. Br. J. Cancer 2014, 15, 395–406. [Google Scholar] [CrossRef]
- Johnson, C.H.; Patterson, A.D.; Krausz, K.W.; Lanz, C.; Kang, D.W.; Luecke, H.; Gonzalez, F.J.; Idle, J.R. Radiation metabolomics. 4. UPLC-ESI-QTOFMS-Based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat. Res. 2011, 175, 473–484. [Google Scholar] [CrossRef]
- Wolny-Rokicka, E.; Tukiendorf, A.; Wydmański, J.; Brzezniakiewicz-Janus, K.; Zembroń-Łacny, A. The Effect of Radiotherapy on the Concentration of Plasma Lipids in Elderly Prostate Cancer Patients. Am. J. Mens Health 2019, 13, 1557988319846328. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Channa, N.A.; Talpur, F.N.; Younis, M.; Tabassum, N. Radiotherapy improves serum fatty acids and lipid profile in breast cancer. Lipids Health Dis. 2017, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Pietrowska, M.; Ros, M.; Zagdanski, A.; Suchwalko, A.; Polanska, J.; Marczyk, M.; Rutkowski, T.; Skladowski, K.; Clench, M.R.; et al. Radiation-induced changes in serum lipidome of head and neck cancer patients. Int. J. Mol. Sci. 2014, 15, 6609–6624. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Tobacyk, J.; Melnyk, S.; James, S.J.; Cheema, A.K.; Boerma, M.; Hauer-Jensen, M.; Koturbash, I. One-carbon metabolism and ionizing radiation: A multifaceted interaction. Biomol. Concepts 2017, 8, 83–92. [Google Scholar] [CrossRef]
- Melse-Boonstra, A.; Holm, P.I.; Ueland, P.M.; Olthof, M.; Clarke, R.; Verhoef, P. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am. J. Clin. Nutr. 2005, 81, 1378–1382. [Google Scholar] [CrossRef]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef]
- Almanric, K.; Marceau, N.; Cantin, A.; Bertin, É. Risk Factors for Nephrotoxicity Associated with Cisplatin. Can. J. Hosp. Pharm. 2017, 70, 99–106. [Google Scholar] [CrossRef]
- Zhang, P.; Li, W.; Chen, J.; Li, R.; Zhang, Z.; Huang, Y.; Xu, F. Branched-Chain Amino Acids as Predictors for Individual Differences of Cisplatin Nephrotoxicity in Rats: A Pharmacometabonomics Study. J. Proteome Res. 2017, 16, 1753–1762. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef]
- Mayers, J.R.; Torrence, M.E.; Danai, L.V.; Papagiannakopoulos, T.; Davidson, S.M.; Bauer, M.R.; Lau, A.N.; Ji, B.W.; Dixit, P.D.; Hosios, A.M.; et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 2016, 353, 1161–1165. [Google Scholar] [CrossRef]
- Pin, F.; Barreto, R.; Couch, M.E.; Bonetto, A.; O’Connell, T.M. Cachexia induced by cancer and chemotherapy yield distinct perturbations to energy metabolism. J. Cachexia Sarcopenia Muscle 2019, 10, 140–154. [Google Scholar] [CrossRef]
- Cobo-Dols, M.; Pérez-Miranda, E.; Gil-Calle, S.; Alés-Díaz, I.; Villar-Chamorro, E.; Montesa-Pino, A.; Alcaide Garcia, J.; Carabante-Ocón, F.; Benavides-Orgaz, M. Changes in the serum amino acids concentrations after first cycle as a factor predictive of tumor response to chemotherapy. Oncología 2005, 28, 21–28. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0378-48352005000700003&lng=en&tlng=en (accessed on 2 December 2020). [CrossRef]
- Somashekar, B.S.; Kamarajan, P.; Danciu, T.; Kapila, Y.L.; Chinnaiyan, A.M.; Rajendiran, T.M.; Ramamoorthy, A. Magic angle spinning NMR-based metabolic profiling of head and neck squamous cell carcinoma tissues. J. Proteome Res. 2011, 10, 5232–5241. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.M.; Bienholz, A.; Venkatachalam, M.A. The role of glycine in regulated cell death. Cell Mol. Life Sci. 2016, 73, 2285–2308. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Martín, R.; Taverner, D.; Vallvé, J.-C.; Paredes, S.; Masana, L.; Correig Blanchar, X.; Amigó Grau, N. Characterization of 1H NMR Plasma Glycoproteins as a New Strategy To Identify Inflammatory Patterns in Rheumatoid Arthritis. J. Proteome Res. 2018, 17, 3730–3739. [Google Scholar] [CrossRef]
- Wirsdörfer, F.; Jendrossek, V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front. Immunol. 2016, 7, 591. [Google Scholar] [CrossRef]

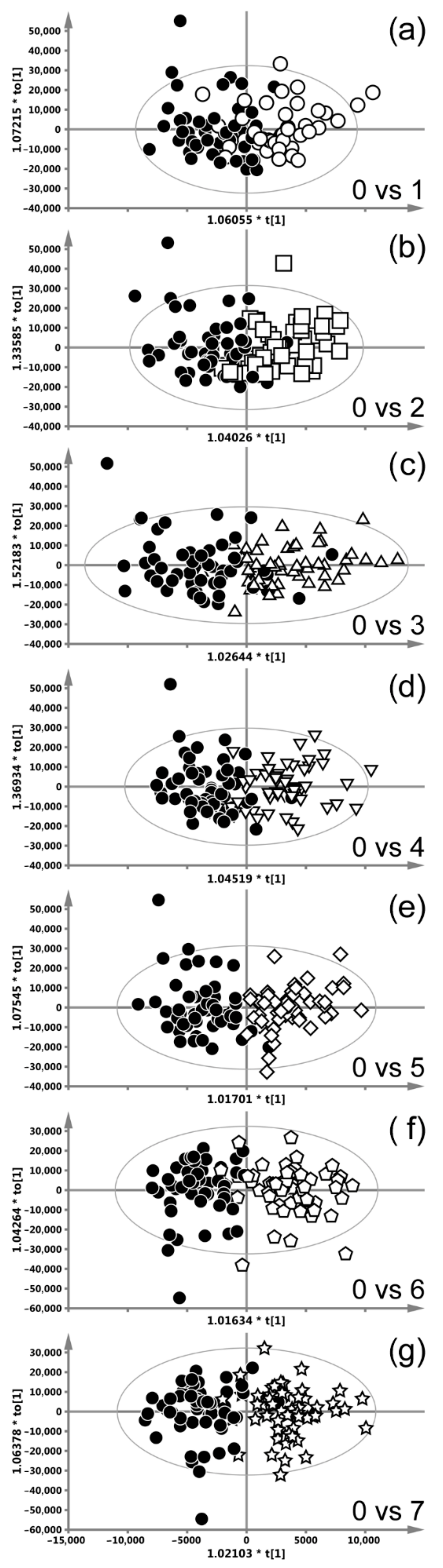
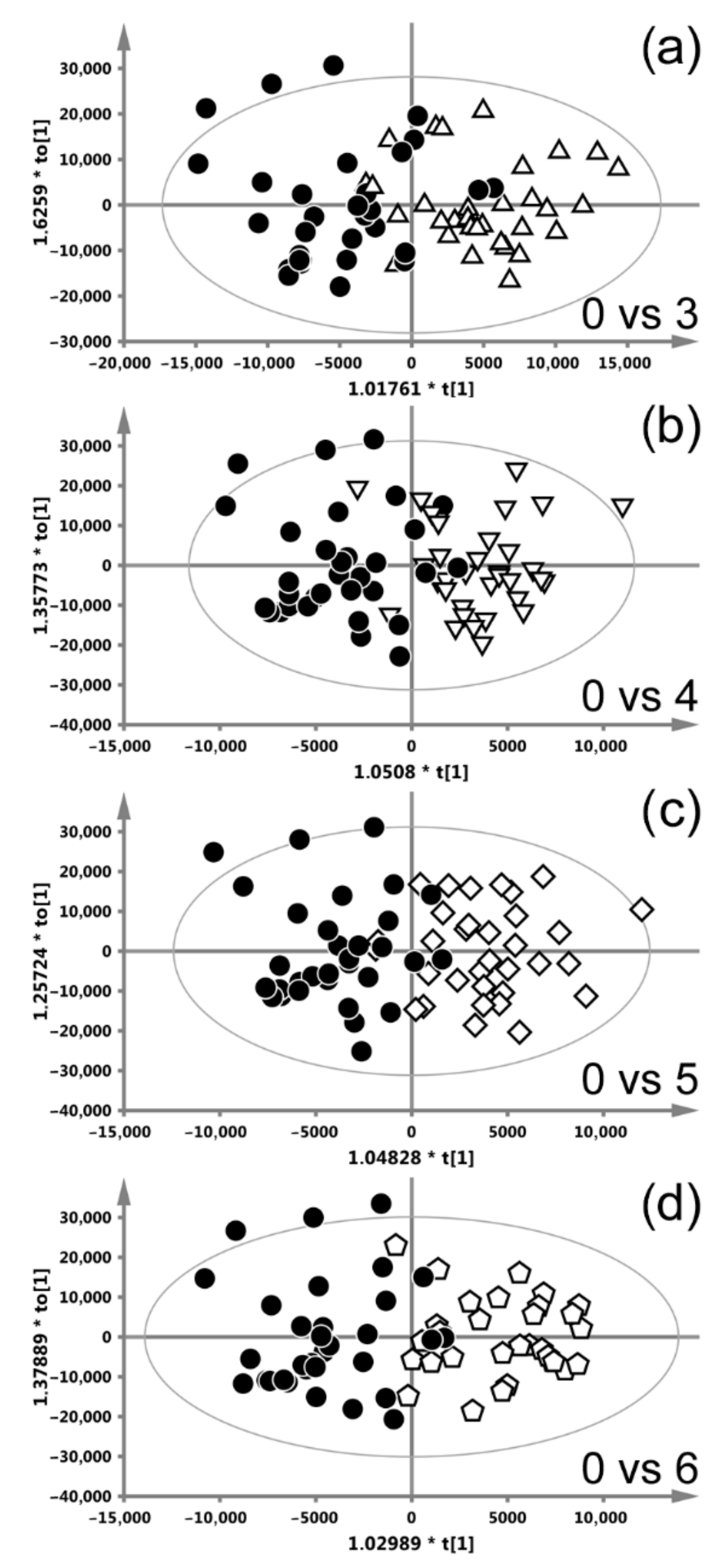

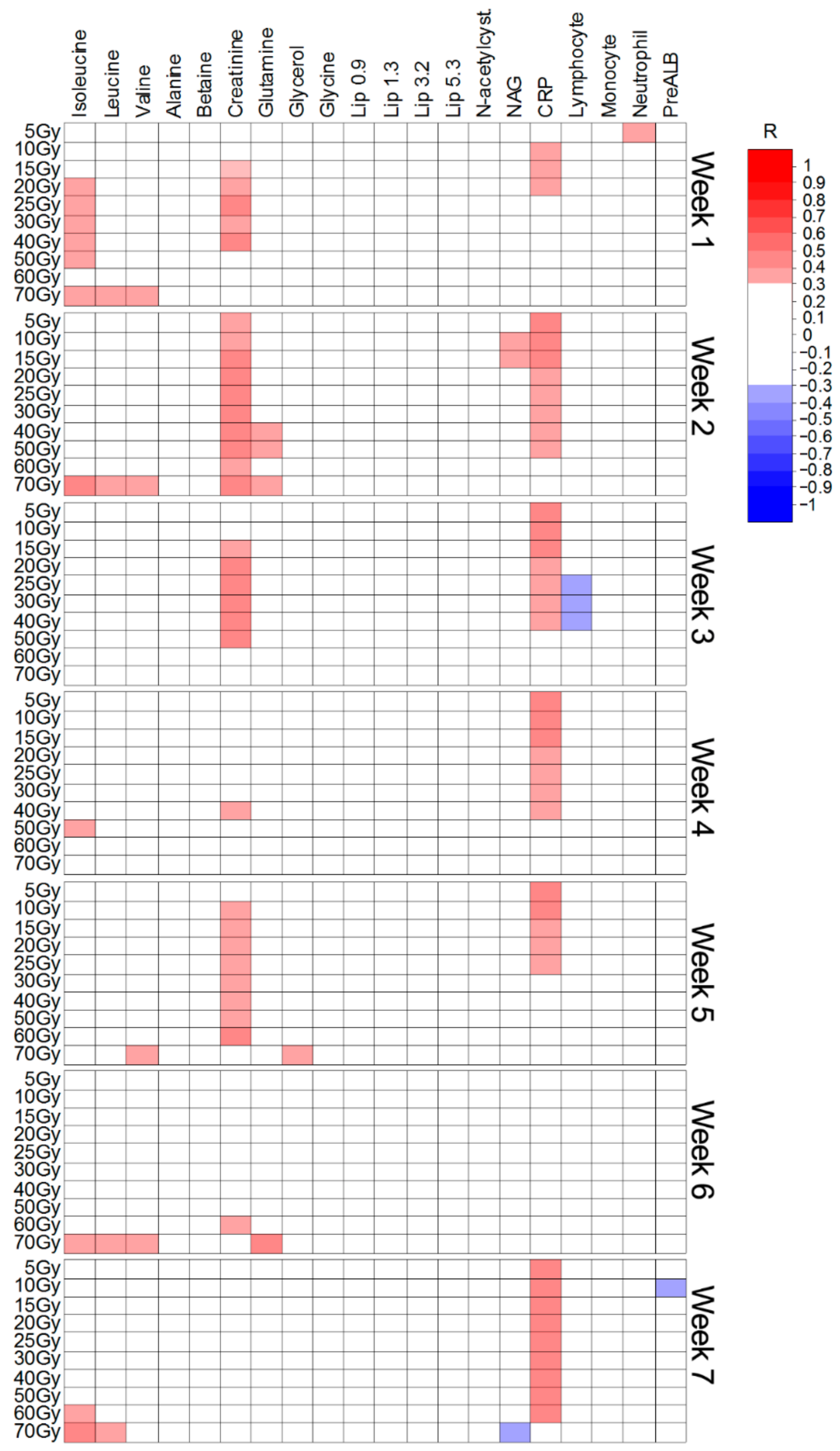

| Weeks of CHRT Treatment | 0 vs. 1 | 0 vs. 2 | 0 vs. 3 | 0 vs. 4 | 0 vs. 5 | 0 vs. 6 | 0 vs. 7 | |
| OPLS-DA Model Quality Parameters | ||||||||
| R2X | 0.05 | 0.05 | 0.1 | 0.06 | 0.06 | 0.06 | 0.06 | |
| R2 | 0.49 | 0.54 | 0.5 | 0.61 | 0.7 | 0.69 | 0.71 | |
| Q2 | 0.33 | 0.35 | 0.34 | 0.54 | 0.56 | 0.61 | 0.63 | |
| Number of orthogonal components | ||||||||
| 2 | 2 | 2 | 2 | 3 | 2 | 2 | ||
| Cumulative R2X of orthogonal components | ||||||||
| R2X(o) | 0.66 | 0.59 | 0.57 | 0.6 | 0.67 | 0.59 | 0.59 | |
| Metabolites increased during radiotherapy | ||||||||
| ppm | p(corr) | p(corr) | p(corr) | p(corr) | p(corr) | p(corr) | p(corr) | |
| Isoleucine | 0.95 | 0.4 | 0.4 | |||||
| Leucine | 0.977 | 0.56 | 0.35 | |||||
| 0.64 | 0.41 | |||||||
| 0.56 | 0.41 | |||||||
| Valine | 1.005 | 0.7 | 0.49 | |||||
| 0.62 | 0.46 | |||||||
| Isoleucine | 1.02 | 0.59 | 0.51 | |||||
| Valine | 1.055 | 0.48 | 0.42 | |||||
| 0.54 | 0.4 | |||||||
| Lipids | 1.3 | >0.4 | >0.45 | >0.5 | ||||
| Low ppm slope | ||||||||
| NAG | 2.057 | 0.43 | 0.4 | 0.6 | 0.43 | 0.53 | 0.59 | |
| N-acetylcysteine | 2.089 | 0.53 | 0.38 | 0.58 | 0.41 | 0.47 | 0.53 | |
| Glutamine | 2.14–2.17 | >0.45 | >0.38 | |||||
| Creatinine | 3.05 | 0.45 | ||||||
| Glycine | 3.57 | 0.54 | 0.6 | 0.57 | 0.6 | 0.52 | ||
| Glycerol | 3.6 | 0.52 | 0.58 | 0.54 | 0.6 | 0.6 | ||
| 0.48 | 0.6 | 0.58 | 0.63 | 0.63 | ||||
| Valine | 3.62 | 0.54 | ||||||
| Glycerol | 3.68 | 0.4 | 0.5 | 0.52 | 0.6 | |||
| 0.36 | 0.4 | 0.47 | 0.15 | 0.49 | ||||
| 0.55 | 0.7 | 0.52 | 0.47 | 0.65 | ||||
| 0.56 | 0.57 | 0.58 | 0.59 | |||||
| Glycolate | 3.96 | 0.66 | 0.72 | 0.66 | 0.64 | 0.65 | ||
| Tyrosine | 6.92 | 0.44 | ||||||
| Tyrosine | 7.21 | 0.4 | ||||||
| Metabolites decreased radiotherapy | ||||||||
| Lipids Low ppm slope | 0.9 | >0.4 | >0.4 | >0.6 | >0.65 | >0.65 | >0.71 | >0.73 |
| Alanine | 1.45 | 0.37 | 0.51 | |||||
| Lipids | 3.2 | >0.45 | >0.5 | >0.6 | >0.65 | >0.67 | >0.67 | >0.64 |
| Low ppm slope | ||||||||
| Betaine | 3.28 | 0.39 | ||||||
| Methanol | 3.38 | 0.41 | 0.5 | 0.43 | ||||
| Glucose | 5.2 | 0.36 | ||||||
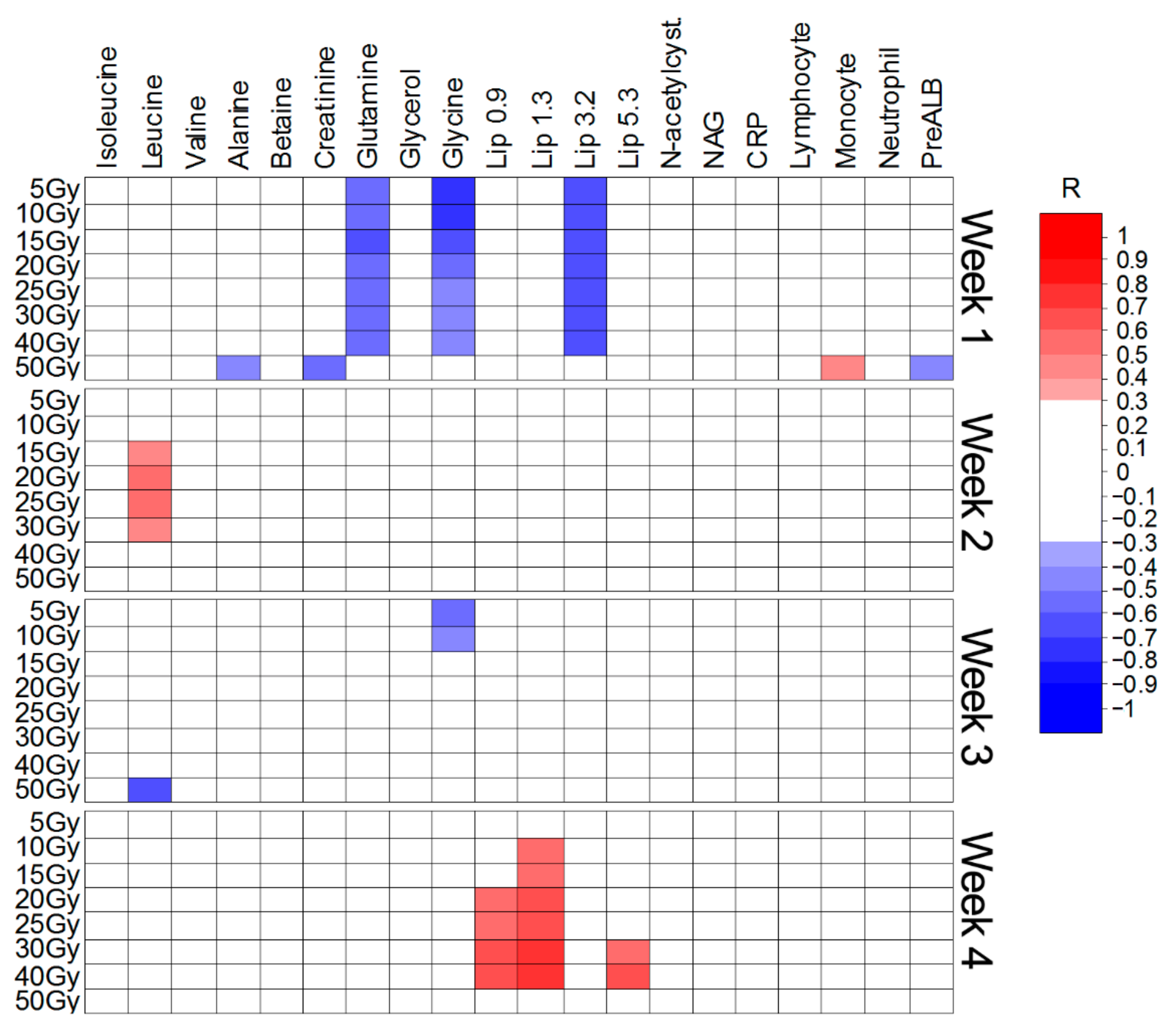
| Weeks of CAIR/CONV/SIB Treatment | 0 vs. 3 | 0 vs. 4 | 0 vs. 5 | 0 vs. 6 | |
| OPLS-DA Model Quality Parameters | |||||
| R2X | 0.145 | 0.062 | 0.07 | 0.09 | |
| R2 | 0.56 | 0.63 | 0.66 | 0.7 | |
| Q2 | 0.29 | 0.48 | 0.43 | 0.56 | |
| Number of orthogonal components | |||||
| 2 | 2 | 2 | 2 | ||
| Cumulative R2X of orthogonal components | |||||
| R2X(o) | 0.51 | 0.59 | 0.59 | 0.57 | |
| Metabolites increased during RT | |||||
| ppm | p(corr) | p(corr) | p(corr) | p(corr) | |
| NAG | 2.057 | 0.31 | 0.43 | 0.58 | 0.38 |
| N-acetylcysteine | 2.089 | 0.32 | 0.43 | 0.57 | 0.37 |
| Glycerol | 3.6 | 0.39 | 0.51 | 0.6 | 0.56 |
| 0.37 | 0.48 | 0.63 | 0.49 | ||
| Glycerol | 3.68 | 0.31 | 0.48 | 0.51 | 0.48 |
| 0.3 | 0.44 | 0.41 | 0.41 | ||
| 0.43 | 0.58 | 0.58 | 0.58 | ||
| 0.49 | 0.51 | 0.65 | 0.57 | ||
| Glycolate | 3.96 | 0.48 | 0.62 | 0.74 | 0.58 |
| Metabolites decreased during RT | |||||
| Lipids Low ppm slope | 0.9 | >0.45 | >0.5 | >0.47 | >0.62 |
| Lipids | 1.3 | >0.44 | 0.34 | 0.34 | |
| Alanine | 1.45 | 0.46 | |||
| Creatinine | 3.05 | 0.44 | 0.45 | 0.57 | |
| Lipids Low ppm slope | 3.2 | >0.5 | 0.54 | >0.49 | >0.62 |
| Betaine | 3.28 | 0.44 | 0.33 | ||
| Lipids | 5.3 | >0.4 | >0.35 | ||
| Weeks of Manchester Treatment | 0 vs. 3 | 0 vs. 4 | |
| OPLS-DA Model Quality Parameters | |||
| R2X | 0.07 | 0.1 | |
| R2 | 0.75 | 0.79 | |
| Q2 | 0.57 | 0.63 | |
| Number of orthogonal components | |||
| 3 | 2 | ||
| Cumulative R2X of orthogonal components | |||
| R2X(o) | 0.77 | 0.7 | |
| Metabolites increased during RT | |||
| ppm | p(corr) | p(corr) | |
| Lysine | 1.7 | 0.48 | |
| NAG | 2.057 | 0.39 | 0.4 |
| N-acetylcysteine | 2.089 | 0.44 | 0.43 |
| Glutamine | 2.14–2.17 | 0.39 | |
| Lysine | 3.02 | 0.45 | |
| Glycerol | 3.6 | 0.61 | 0.5 |
| 0.62 | 0.53 | ||
| Glycerol | 3.68 | 0.54 | 0.45 |
| 0.54 | 0.47 | ||
| 0.58 | 0.51 | ||
| 0.63 | 0.51 | ||
| Glycolate | 3.96 | 0.69 | 0.57 |
| Metabolites decreased during RT | |||
| Lipids Low ppm slope | 0.9 | >0.35 | >0.57 |
| Lipids | 1.3 | >0.32 | >0.34 |
| Lipids Low ppm slope | 3.2 | >0.43 | >0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguszewicz, Ł.; Bieleń, A.; Ciszek, M.; Wendykier, J.; Szczepanik, K.; Skorupa, A.; Mrochem-Kwarciak, J.; Składowski, K.; Sokół, M. NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses. Int. J. Mol. Sci. 2021, 22, 6310. https://doi.org/10.3390/ijms22126310
Boguszewicz Ł, Bieleń A, Ciszek M, Wendykier J, Szczepanik K, Skorupa A, Mrochem-Kwarciak J, Składowski K, Sokół M. NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses. International Journal of Molecular Sciences. 2021; 22(12):6310. https://doi.org/10.3390/ijms22126310
Chicago/Turabian StyleBoguszewicz, Łukasz, Agata Bieleń, Mateusz Ciszek, Jacek Wendykier, Krzysztof Szczepanik, Agnieszka Skorupa, Jolanta Mrochem-Kwarciak, Krzysztof Składowski, and Maria Sokół. 2021. "NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses" International Journal of Molecular Sciences 22, no. 12: 6310. https://doi.org/10.3390/ijms22126310
APA StyleBoguszewicz, Ł., Bieleń, A., Ciszek, M., Wendykier, J., Szczepanik, K., Skorupa, A., Mrochem-Kwarciak, J., Składowski, K., & Sokół, M. (2021). NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses. International Journal of Molecular Sciences, 22(12), 6310. https://doi.org/10.3390/ijms22126310






