The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity
Abstract
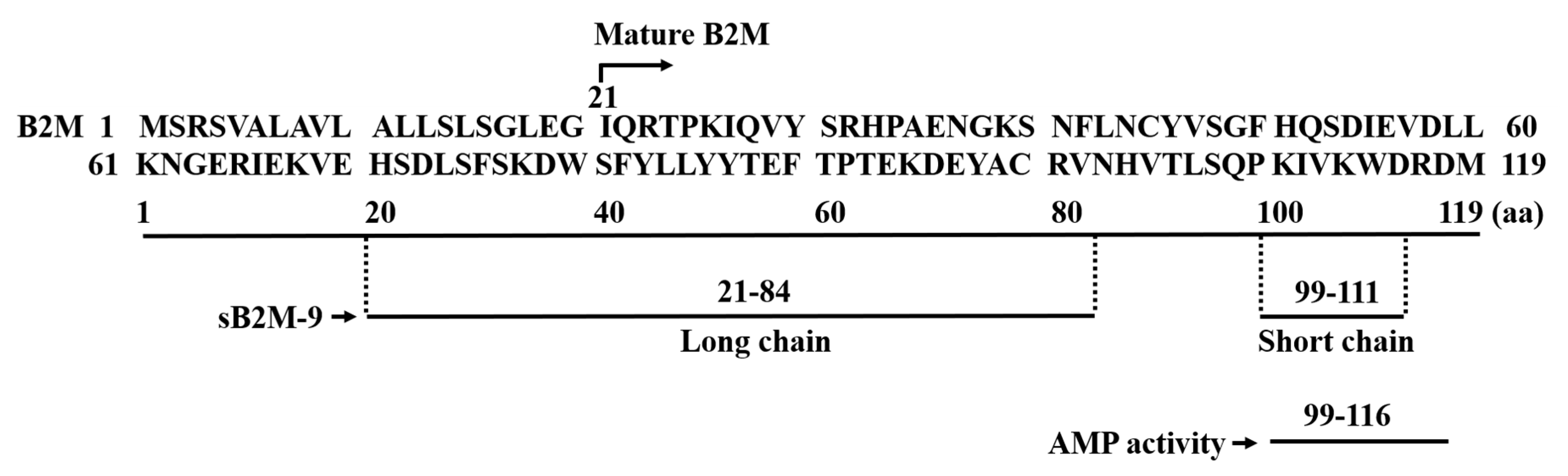
1. Background of Beta-2 Microglobulin
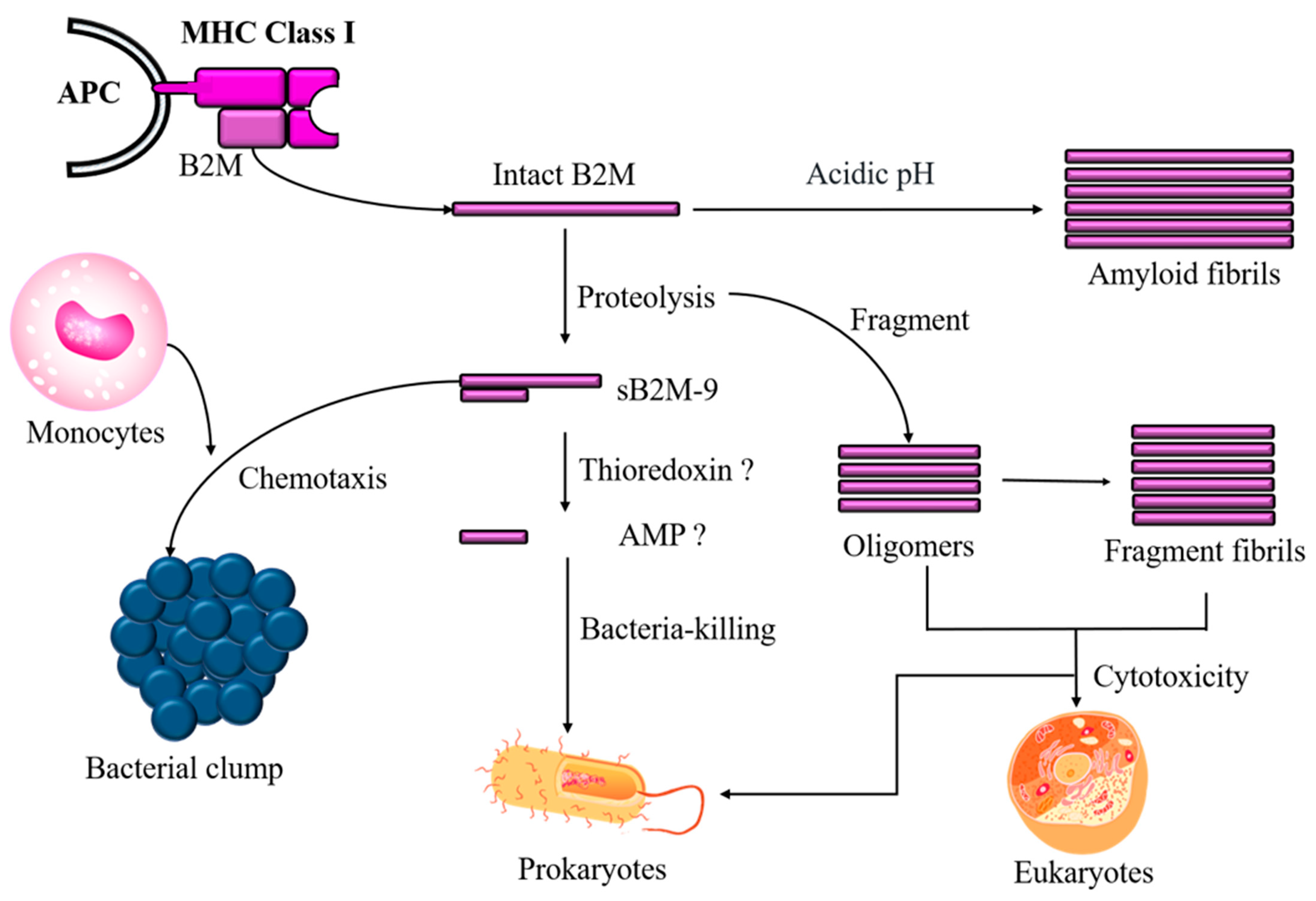
2. Discovery of B2M’s Antimicrobial Activity
3. pH-Dependent Antimicrobial Activity of B2M
| Source | AMP | Antimicrobial Activity at Acidic pH | References |
|---|---|---|---|
| Human | B2M | Increase | [25] |
| Human | LL-37 | Decrease | [51] |
| Human | β-defensin 3 | Decrease | [51] |
| Human | β-microseminoprotein | Increase | [52] |
| Human | Hepcidin-20, -25 | Increase | [53] |
| Human | Lysozyme | Decrease | [54] |
| Human | Lactoferrin | Increase | [55] |
| Human | Psoriasin (S100A7) | Increase | [56] |
| Human | Phagocytin | Increase | [57] |
| Human | Dermcidin-1L (DCD-1L) | Increase | [58] |
| Human | Hemoglobin β subunit (aa 112–147) | Increase | [22] |
| Human | Calprotectin | Decrease | [59] |
| Synthetic | A cationic, amphiphilic random copolymer | Decrease | [60] |
| Synthetic | Histidine-rich peptides (histatin) | Increase | [61] |
4. Role of Aggregated B2M in Antimicrobial Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halenius, A.; Gerke, C.; Hengel, H. Classical and non-classical MHC I molecule manipulation by human cytomegalovirus: So many targets—but how many arrows in the quiver? Cell Mol. Immunol. 2015, 12, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Pérarnau, B.; Siegrist, C.A.; Gillet, A.; Vincent, C.; Kimura, S.; Lemonnier, F.A. Beta 2-microglobulin restriction of antigen presentation. Nature 1990, 346, 751–754. [Google Scholar] [CrossRef]
- Berko, D.; Carmi, Y.; Cafri, G.; Ben-Zaken, S.; Sheikhet, H.M.; Tzehoval, E.; Eisenbach, L.; Margalit, A.; Gross, G. Membrane-anchored beta 2-microglobulin stabilizes a highly receptive state of MHC class I molecules. J. Immunol. 2005, 174, 2116. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.A.; Tan, R. Xenogeneic beta 2-microglobulin substitution affects functional binding of MHC class I molecules by CD8+ T cells. J. Immunol. 2007, 179, 3588. [Google Scholar] [CrossRef]
- Cooper, J.C.; Dealtry, G.B.; Ahmed, M.A.; Arck, P.C.; Klapp, B.F.; Blois, S.M.; Fernández, N. An impaired breeding phenotype in mice with a genetic deletion of beta-2 microglobulin and diminished MHC class I expression: Role in reproductive fitness. Biol. Reprod. 2007, 77, 274–279. [Google Scholar] [CrossRef]
- Kapadia, D.; Sadikovic, A.; Vanloubbeeck, Y.; Brockstedt, D.; Fong, L. Interplay between CD8α+ dendritic cells and monocytes in response to Listeria monocytogenes infection attenuates T cell responses. PLoS ONE 2011, 6, e19376. [Google Scholar] [CrossRef] [PubMed]
- Street, S.E.; Hayakawa, Y.; Zhan, Y.; Lew, A.M.; MacGregor, D.; Jamieson, A.M.; Diefenbach, A.; Yagita, H.; Godfrey, D.I.; Smyth, M.J. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J. Exp. Med. 2004, 199, 879–884. [Google Scholar] [CrossRef]
- Terpos, E.; Mihou, D.; Szydlo, R.; Tsimirika, K.; Karkantaris, C.; Politou, M.; Voskaridou, E.; Rahemtulla, A.; Dimopoulos, M.A.; Zervas, K. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia 2005, 19, 1969–1976. [Google Scholar] [CrossRef]
- Chen, C.H.; Su, C.Y.; Chien, C.Y.; Huang, C.C.; Chuang, H.C.; Fang, F.M.; Huang, H.Y.; Chen, C.M.; Chiou, S.J. Overexpression of β2-microglobulin is associated with poor survival in patients with oral cavity squamous cell carcinoma and contributes to oral cancer cell migration and invasion. Br. J. Cancer 2008, 99, 1453–1461. [Google Scholar] [CrossRef]
- Naiki, H.; Okoshi, T.; Ozawa, D.; Yamaguchi, I.; Hasegawa, K. Molecular pathogenesis of human amyloidosis: Lessons from β2 -microglobulin-related amyloidosis. Pathol. Int. 2016, 66, 193–201. [Google Scholar] [CrossRef]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Smith, L.K.; He, Y.; Park, J.-S.; Bieri, G.; Snethlage, C.E.; Lin, K.; Gontier, G.; Wabl, R.; Plambeck, K.E.; Udeochu, J.; et al. β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat. Med. 2015, 21, 932–937. [Google Scholar] [CrossRef]
- Roch, P.; Valembois, P.; Vaillier, J. Amino acid compositions and relationships of five earthworm defense proteins. Comp. Biochem. Physiol. Part B Comp. Biochem. 1986, 85, 747–751. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Park, S.-C.; Lee, J.-K.; Choi, S.J.; Hahm, K.-S.; Park, Y. Novel Antibacterial Activity of β2-Microglobulin in Human Amniotic Fluid. PLoS ONE 2012, 7, e47642. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.D.; Ordway, D.J.; Orme, I.M. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect. Immun. 1993, 61, 1113–1116. [Google Scholar] [CrossRef]
- D’Souza, C.D.; Cooper, A.M.; Frank, A.A.; Ehlers, S.; Turner, J.; Bendelac, A.; Orme, I.M. A novel nonclassic beta2-microglobulin-restricted mechanism influencing early lymphocyte accumulation and subsequent resistance to tuberculosis in the lung. Am. J. Respir. Cell Mol. Biol. 2000, 23, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rolph, M.S.; Raupach, B.; Köbernick, H.H.; Collins, H.L.; Pérarnau, B.; Lemonnier, F.A.; Kaufmann, S.H. MHC class Ia-restricted T cells partially account for beta2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur. J. Immunol. 2001, 31, 1944–1949. [Google Scholar] [CrossRef]
- Cogen, A.L.; Moore, T.A. Beta2-microglobulin-dependent bacterial clearance and survival during murine Klebsiella pneumoniae bacteremia. Infect. Immun. 2009, 77, 360–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hou, F.F.; Owen, W.F., Jr. Beta 2-microglobulin amyloidosis: Role of monocytes/macrophages. Curr. Opin. Nephrol. Hypertens 2002, 11, 417–421. [Google Scholar] [CrossRef]
- Chiou, S.J.; Wang, C.C.; Tseng, Y.S.; Lee, Y.J.; Chen, S.C.; Chou, C.H.; Chuang, L.Y.; Hong, Y.R.; Lu, C.Y.; Chiu, C.C.; et al. A novel role for β2-microglobulin: A precursor of antibacterial chemokine in respiratory epithelial cells. Sci. Rep. 2016, 6, 31035. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Groß, R.; Bauer, R.; Krüger, F.; Rücker-Braun, E.; Olari, L.R.; Ständker, L.; Preising, N.; Rodríguez, A.A.; Conzelmann, C.; Gerbl, F.; et al. A Placenta Derived C-Terminal Fragment of β-Hemoglobin With Combined Antibacterial and Antiviral Activity. Front. Microbiol. 2020, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Liao, H.I.; Stuchlik, O.; Tilan, J.; Pohl, J.; Ganz, T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J. Immunol. 2002, 169, 6985–6991. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Liu, W.Y.; Kwok, S.C. Cloning and nucleotide sequence analysis of the human beta-microseminoprotein gene. Biochem. Biophys. Res. Commun. 1990, 167, 1184–1190. [Google Scholar] [CrossRef]
- Holch, A.; Bauer, R.; Olari, L.R.; Rodriguez, A.A.; Ständker, L.; Preising, N.; Karacan, M.; Wiese, S.; Walther, P.; Ruiz-Blanco, Y.B.; et al. Respiratory ß-2-Microglobulin exerts pH dependent antimicrobial activity. Virulence 2020, 11, 1402–1414. [Google Scholar] [CrossRef]
- Wolf, M.; Moser, B. Antimicrobial activities of chemokines: Not just a side-effect? Front. Immunol. 2012, 3, 213. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Chan, D.I.; Boszhard, L.; Zaat, S.A.J.; Vogel, H.J. Structure–function studies of chemokine-derived carboxy-terminal antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1062–1072. [Google Scholar] [CrossRef]
- Wendler, J.; Schroeder, B.O.; Ehmann, D.; Koeninger, L.; Mailänder-Sánchez, D.; Lemberg, C.; Wanner, S.; Schaller, M.; Stange, E.F.; Malek, N.P.; et al. Proteolytic Degradation of reduced Human Beta Defensin 1 generates a Novel Antibiotic Octapeptide. Sci. Rep. 2019, 9, 3640. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sasaki, K.; Minamino, N. Peptidomics-Based Discovery of an Antimicrobial Peptide Derived from Insulin-Like Growth Factor-Binding Protein 5. J. Proteome Res. 2011, 10, 1870–1880. [Google Scholar] [CrossRef]
- Bertini, R.; Howard, O.M.; Dong, H.F.; Oppenheim, J.J.; Bizzarri, C.; Sergi, R.; Caselli, G.; Pagliei, S.; Romines, B.; Wilshire, J.A.; et al. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 1999, 189, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Peaper, D.R.; Wearsch, P.A.; Cresswell, P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. Embo J. 2005, 24, 3613–3623. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.N.; Brüggemann, H. Vimentin in Bacterial Infections. Cells 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Chan, L.Y.; Tang, S.C.; Yiu, W.H.; Li, R.; Lai, K.N.; Leung, J.C. BMP-7 represses albumin-induced chemokine synthesis in kidney tubular epithelial cells through destabilization of NF-κB-inducing kinase. Immunol. Cell Biol. 2014, 92, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Linge, H.M.; Collin, M.; Nordenfelt, P.; Mörgelin, M.; Malmsten, M.; Egesten, A. The human CXC chemokine granulocyte chemotactic protein 2 (GCP-2)/CXCL6 possesses membrane-disrupting properties and is antibacterial. Antimicrob. Agents Chemother. 2008, 52, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; McNinch, J.; Basu, R.; Simonet, S. Cloning and characterization of the human neutrophil-activating peptide (ENA-78) gene. J. Biol. Chem. 1994, 269, 25277–25282. [Google Scholar] [CrossRef]
- Lee, M.M.; Yoon, B.-J.; Osiewicz, K.; Preston, M.; Bundy, B.; van Heeckeren, A.M.; Werb, Z.; Soloway, P.D. Tissue inhibitor of metalloproteinase 1 regulates resistance to infection. Infect. Immun. 2005, 73, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Clahsen, T.; Schaper, F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008, 84, 1521–1529. [Google Scholar] [CrossRef]
- Björstad, A.; Fu, H.; Karlsson, A.; Dahlgren, C.; Bylund, J. Interleukin-8-derived peptide has antibacterial activity. Antimicrob. Agents Chemother. 2005, 49, 3889–3895. [Google Scholar] [CrossRef]
- Potapovich, A.I.; Pastore, S.; Kostyuk, V.A.; Lulli, D.; Mariani, V.; De Luca, C.; Dudich, E.I.; Korkina, L.G. alpha-Fetoprotein as a modulator of the pro-inflammatory response of human keratinocytes. Br. J. Pharm. 2009, 158, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Bistoni, O.; Manetti, M.; Cafaro, G.; Valentini, V.; Bartoloni, E.; Gerli, R.; Liso, A. Insulin-Like Growth Factor Binding Protein 6 in Rheumatoid Arthritis: A Possible Novel Chemotactic Factor? Front. Immunol. 2017, 8, 554. [Google Scholar] [CrossRef]
- Liou, H.C.; Jin, Z.; Tumang, J.; Andjelic, S.; Smith, K.A.; Liou, M.L. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int. Immunol. 1999, 11, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals (Basel) 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef]
- Ng, A.W.; Bidani, A.; Heming, T.A. Innate Host Defense of the Lung: Effects of Lung-lining Fluid pH. Lung 2004, 182, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Coakley, R.D.; Grubb, B.R.; Paradiso, A.M.; Gatzy, J.T.; Johnson, L.G.; Kreda, S.M.; O’Neal, W.K.; Boucher, R.C. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. USA 2003, 100, 16083–16088. [Google Scholar] [CrossRef]
- Nakayama, K.; Jia, Y.X.; Hirai, H.; Shinkawa, M.; Yamaya, M.; Sekizawa, K.; Sasaki, H. Acid stimulation reduces bactericidal activity of surface liquid in cultured human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2002, 26, 105–113. [Google Scholar] [CrossRef]
- Tipping, K.W.; Karamanos, T.K.; Jakhria, T.; Iadanza, M.G.; Goodchild, S.C.; Tuma, R.; Ranson, N.A.; Hewitt, E.W.; Radford, S.E. pH-induced molecular shedding drives the formation of amyloid fibril-derived oligomers. Proc. Natl. Acad. Sci. USA 2015, 112, 5691–5696. [Google Scholar] [CrossRef]
- Okoshi, T.; Yamaguchi, I.; Ozawa, D.; Hasegawa, K.; Naiki, H. Endocytosed 2-Microglobulin Amyloid Fibrils Induce Necrosis and Apoptosis of Rabbit Synovial Fibroblasts by Disrupting Endosomal/Lysosomal Membranes: A Novel Mechanism on the Cytotoxicity of Amyloid Fibrils. PLoS ONE 2015, 10, e0139330. [Google Scholar] [CrossRef]
- Nissen, M.H.; Bjerrum, O.J.; Plesner, T.; Wilken, M.; Rørth, M. Modification of beta-2-microglobulin in sera from patients with small cell lung cancer: Evidence for involvement of a serine protease. Clin. Exp. Immunol. 1987, 67, 425–432. [Google Scholar]
- Monti, M.; Amoresano, A.; Giorgetti, S.; Bellotti, V.; Pucci, P. Limited proteolysis in the investigation of beta2-microglobulin amyloidogenic and fibrillar states. Biochim. Biophys. Acta 2005, 1753, 44–50. [Google Scholar] [CrossRef]
- Abou Alaiwa, M.H.; Reznikov, L.R.; Gansemer, N.D.; Sheets, K.A.; Horswill, A.R.; Stoltz, D.A.; Zabner, J.; Welsh, M.J. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc. Natl. Acad. Sci. USA 2014, 111, 18703. [Google Scholar] [CrossRef] [PubMed]
- Edström Hägerwall, A.M.L.; Rydengård, V.; Fernlund, P.; Mörgelin, M.; Baumgarten, M.; Cole, A.M.; Malmsten, M.; Kragelund, B.B.; Sørensen, O.E. β-Microseminoprotein Endows Post Coital Seminal Plasma with Potent Candidacidal Activity by a Calcium- and pH-Dependent Mechanism. PLoS Pathog. 2012, 8, e1002625. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Vitali, A.; Scorciapino, M.A.; Rinaldi, A.C.; Petruzzelli, R.; Brancatisano, F.L.; Esin, S.; Stringaro, A.; Colone, M.; Luzi, C.; et al. pH-dependent disruption of Escherichia coli ATCC 25922 and model membranes by the human antimicrobial peptides hepcidin 20 and 25. FEBS J. 2013, 280, 2842–2854. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, H.; Okakura, Y.; Ichikawa, M.; Kuda, T.; Kimura, B. Impact of pH and protein hydrophobicity on norovirus inactivation by heat-denatured lysozyme. PLoS ONE 2020, 15, e0237888. [Google Scholar] [CrossRef]
- Baker, H.M.; Baker, E.N. Lactoferrin and Iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef]
- Michalek, M.; Gelhaus, C.; Hecht, O.; Podschun, R.; Schröder, J.M.; Leippe, M.; Grötzinger, J. The human antimicrobial protein psoriasin acts by permeabilization of bacterial membranes. Dev. Comp. Immunol. 2009, 33, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.G. Studies of the bactericidal action of phagocytin. J. Exp. Med. 1956, 103, 613–621. [Google Scholar] [CrossRef]
- Mohanan, G.; Nair, K.S.; Nampoothiri, K.M.; Bajaj, H. Engineering bio-mimicking functional vesicles with multiple compartments for quantifying molecular transport. Chem. Sci. 2020, 11, 4669–4679. [Google Scholar] [CrossRef]
- Rosen, T.; Nolan, E.M. Metal Sequestration and Antimicrobial Activity of Human Calprotectin Are pH-Dependent. Biochemistry 2020, 59, 2468–2478. [Google Scholar] [CrossRef]
- Hong, S.; Takahashi, H.; Nadres, E.T.; Mortazavian, H.; Caputo, G.A.; Younger, J.G.; Kuroda, K. A Cationic Amphiphilic Random Copolymer with pH-Responsive Activity against Methicillin-Resistant Staphylococcus aureus. PLoS ONE 2017, 12, e0169262. [Google Scholar] [CrossRef]
- Kacprzyk, L.; Rydengård, V.; Mörgelin, M.; Davoudi, M.; Pasupuleti, M.; Malmsten, M.; Schmidtchen, A. Antimicrobial activity of histidine-rich peptides is dependent on acidic conditions. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2667–2680. [Google Scholar] [CrossRef]
- Myhre, E.B.; Kronvall, G. Heterogeneity of nonimmune immunoglobulin Fc reactivity among gram-positive cocci: Description of three major types of receptors for human immunoglobulin G. Infect. Immun. 1977, 17, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Kronvall, G.; Myhre, E.B.; Björck, L.; Berggård, I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect. Immun. 1978, 22, 136–142. [Google Scholar] [CrossRef]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.X.; et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, N.H. beta(2)-microglobulin: From physiology to amyloidosis. Amyloid 2009, 16, 151–173. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, B.; Zhou, Y.; Wang, X.; Wu, W.; Wang, Z.; Dai, Z.; Cheng, Q.; Yang, K. B2M overexpression correlates with malignancy and immune signatures in human gliomas. Sci. Rep. 2021, 11, 5045. [Google Scholar] [CrossRef]
- Foster, M.C.; Coresh, J.; Hsu, C.-Y.; Xie, D.; Levey, A.S.; Nelson, R.G.; Eckfeldt, J.H.; Vasan, R.S.; Kimmel, P.L.; Schelling, J.; et al. Serum β-Trace Protein and β2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2016, 68, 68–76. [Google Scholar] [CrossRef]
- Hirakura, Y.; Kagan, B.L. Pore formation by beta-2-microglobulin: A mechanism for the pathogenesis of dialysis associated amyloidosis. Amyloid 2001, 8, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, R.; Mendoza, V.L.; Bridgewater, J.D.; Zhang, G.; Vachet, R.W. Copper binding to beta-2-microglobulin and its pre-amyloid oligomers. Biochemistry 2009, 48, 9871–9881. [Google Scholar] [CrossRef]
- Goodchild, S.C.; Sheynis, T.; Thompson, R.; Tipping, K.W.; Xue, W.F.; Ranson, N.A.; Beales, P.A.; Hewitt, E.W.; Radford, S.E. β2-Microglobulin amyloid fibril-induced membrane disruption is enhanced by endosomal lipids and acidic pH. PLoS ONE 2014, 9, e104492. [Google Scholar] [CrossRef]
- Jakhria, T.; Hellewell, A.L.; Porter, M.Y.; Jackson, M.P.; Tipping, K.W.; Xue, W.F.; Radford, S.E.; Hewitt, E.W. β2-microglobulin amyloid fibrils are nanoparticles that disrupt lysosomal membrane protein trafficking and inhibit protein degradation by lysosomes. J. Biol. Chem. 2014, 289, 35781–35794. [Google Scholar] [CrossRef] [PubMed]
- Soscia, S.J.; Kirby, J.E.; Washicosky, K.J.; Tucker, S.M.; Ingelsson, M.; Hyman, B.; Burton, M.A.; Goldstein, L.E.; Duong, S.; Tanzi, R.E.; et al. The Alzheimer’s Disease-Associated Amyloid β-Protein Is an Antimicrobial Peptide. PLoS ONE 2010, 5, e9505. [Google Scholar] [CrossRef]
- Kagan, B.L.; Jang, H.; Capone, R.; Teran Arce, F.; Ramachandran, S.; Lal, R.; Nussinov, R. Antimicrobial Properties of Amyloid Peptides. Mol. Pharm. 2012, 9, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.A.; Wibell, L.; Evrin, P.E. beta 2-Microglobulin in clinical medicine. Scand. J. Clin. Lab. Invest. Suppl. 1980, 154, 27–37. [Google Scholar]
- Hetz, C.; Bono, M.R.; Barros, L.F.; Lagos, R. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc. Natl. Acad. Sci. USA 2002, 99, 2696–2701. [Google Scholar] [CrossRef]
- Xue, W.F.; Hellewell, A.L.; Gosal, W.S.; Homans, S.W.; Hewitt, E.W.; Radford, S.E. Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 2009, 284, 34272–34282. [Google Scholar] [CrossRef]
- Sokolowski, F.; Modler, A.J.; Masuch, R.; Zirwer, D.; Baier, M.; Lutsch, G.; Moss, D.A.; Gast, K.; Naumann, D. Formation of critical oligomers is a key event during conformational transition of recombinant syrian hamster prion protein. J. Biol. Chem. 2003, 278, 40481–40492. [Google Scholar] [CrossRef]
- Conway, K.A.; Lee, S.J.; Rochet, J.C.; Ding, T.T.; Williamson, R.E.; Lansbury, P.T., Jr. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: Implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. USA 2000, 97, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Arrasate, M.; Mitra, S.; Schweitzer, E.S.; Segal, M.R.; Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 2004, 431, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.; Simon, R.; Schubert, D.; et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Zeth, K. Structure and evolution of mitochondrial outer membrane proteins of β-barrel topology. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
| UniProt Entry | Protein Hit | Cytokine/Chemokine/AMP |
|---|---|---|
| VIME_human | Vimentin | Modulating cytokine [32] |
| ALBU_human | Serum albumin | Inducing chemokine synthesis [33] |
| IBP1_human | Insulin-like growth factor-binding protein 1 | None |
| CXCL6_human | C-X-C motif chemokine 6 | Chemokine/AMP [34] |
| THIO_human | Thioredoxin | Chemokine [30] |
| CXCL5_human | C-X-C motif chemokine 5 | Chemokine [35] |
| TIMP1_human | Metalloproteinase inhibitor 1 | MMP1 inhibitor: Regulating AMP shedding [36] |
| TPM1_human | Tropomyosin alpha-1 chain | None |
| TPM3_human | Tropomyosin alpha-3 chain | None |
| TPM4_human | Tropomyosin alpha-4 chain | None |
| K2C8_human | Keratin, type II cytoskeletal 8 | None |
| IL6_human | Interleukin-6 | Cytokine/Chemokine [37] |
| TMEM2_human | Transmembrane protein 2 | None |
| B2MG_human | β-2-microglobulin | Chemokine/AMP [20,25] |
| LUZP1_human | Leucine zipper protein 1 | None |
| IL8_human | Interleukin-8 | Cytokine/chemokine/AMP [27,38] |
| FETA_human | α-fetoprotein | A modulator of the pro-inflammatory response [39] |
| IBP6_human | Insulin-like growth factor-binding protein 6 | Chemokine [40] |
| CH10_human | 10 kDa heat shock protein, mitochondrial | None |
| REL_human | Proto-oncogene c-Rel | Cytokine regulator [41] |
| DEST_human | Destrin | None |
| COF2_human | Cofilin-2 | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, S.-J.; Ko, H.-J.; Hwang, C.-C.; Hong, Y.-R. The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 6330. https://doi.org/10.3390/ijms22126330
Chiou S-J, Ko H-J, Hwang C-C, Hong Y-R. The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity. International Journal of Molecular Sciences. 2021; 22(12):6330. https://doi.org/10.3390/ijms22126330
Chicago/Turabian StyleChiou, Shean-Jaw, Huey-Jiun Ko, Chi-Ching Hwang, and Yi-Ren Hong. 2021. "The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity" International Journal of Molecular Sciences 22, no. 12: 6330. https://doi.org/10.3390/ijms22126330
APA StyleChiou, S.-J., Ko, H.-J., Hwang, C.-C., & Hong, Y.-R. (2021). The Double-Edged Sword of Beta2-Microglobulin in Antibacterial Properties and Amyloid Fibril-Mediated Cytotoxicity. International Journal of Molecular Sciences, 22(12), 6330. https://doi.org/10.3390/ijms22126330






