Insights into the Role of the Discontinuous TM7 Helix of Human Ferroportin through the Prism of the Asp325 Residue
Abstract
:1. Introduction
2. Results
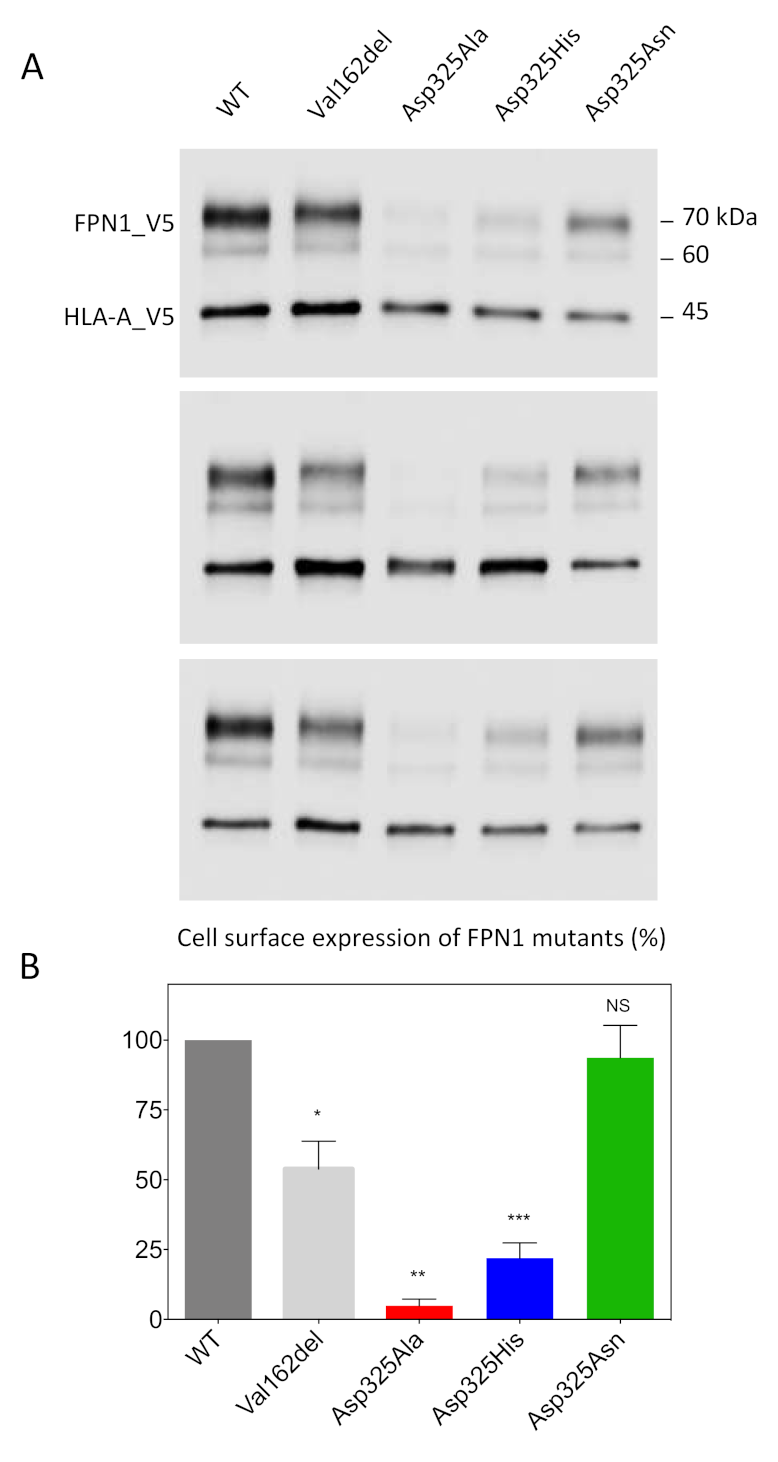
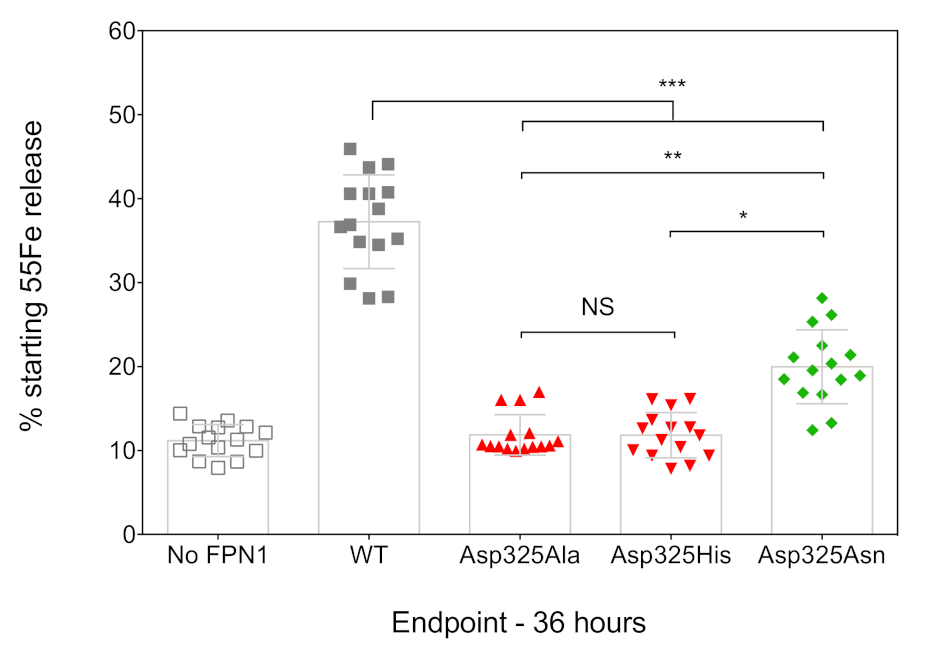
2.1. Effects of the Asp325Ala, His, and Asn Mutants on the Cell Surface Expression of Human FPN1 and Its Ability to Export Iron
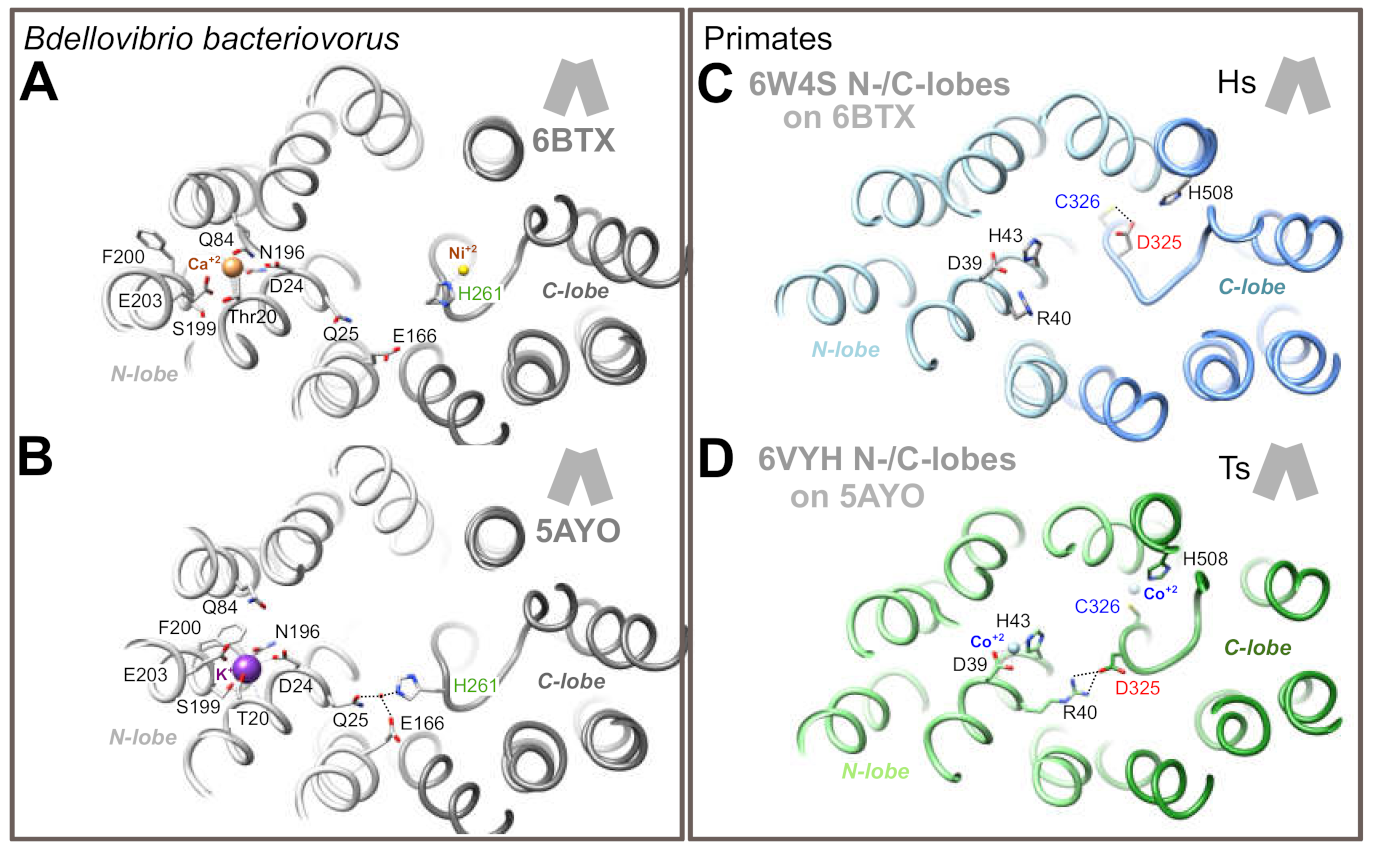
2.2. 3D Structure Analysis
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructs
4.2. Culture and Transfection of Human Epithelial Kidney (HEK)293T Cells
4.3. Isolation of Cell-Surface Proteins and Western Blot Analysis
4.4. Densitometry Study
4.5. 55Fe Release Measurements
4.6. 3D Structure Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MFS | Major Facilitator Superfamily |
| OF | Outward-facing |
| IF | Inward-facing |
| HsFPN1 | Human Ferroportin |
| BbFpn | Bdellovibrio bacteriovorus Ferroportin |
| TsFpn | primate Philippine Tarsier Ferroportin |
References
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The Iron Exporter Ferroportin/Slc40a1 Is Essential for Iron Homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girelli, D.; Nemeth, E.; Swinkels, D.W. Hepcidin in the Diagnosis of Iron Disorders. Blood 2016, 127, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. The Hepcidin-Ferroportin System as a Therapeutic Target in Anemias and Iron Overload Disorders. Hematol. Educ. Program Am. Soc. Hematol. 2011, 2011, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.; Hu, L.; Lu, K.; Wang, Y.; Xie, Y.; Gao, L.; Yang, G.; Xie, B.; He, W.; Chen, G.; et al. Ferroportin Downregulation Promotes Cell Proliferation by Modulating the Nrf2–MiR-17-5p Axis in Multiple Myeloma. Cell Death Dis. 2019, 10, 624. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev. MMBR 1998, 62, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.S.; Shlykov, M.A.; Castillo, R.; Sun, E.I.; Saier, M.H. The Major Facilitator Superfamily (MFS) Revisited: The Major Facilitator Family Revisited. FEBS J. 2012, 279, 2022–2035. [Google Scholar] [CrossRef]
- Quistgaard, E.M.; Löw, C.; Guettou, F.; Nordlund, P. Understanding Transport by the Major Facilitator Superfamily (MFS): Structures Pave the Way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123–132. [Google Scholar] [CrossRef]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and General Transport Mechanisms by the Major Facilitator Superfamily (MFS). Chem. Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jardetzky, O. Simple Allosteric Model for Membrane Pumps. Nature 1966, 211, 969–970. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lemieux, M.J.; Song, J.; Auer, M.; Wang, D.-N. Structure and Mechanism of the Glycerol-3-Phosphate Transporter from Escherichia coli. Science 2003, 301, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Law, C.J.; Maloney, P.C.; Wang, D.-N. Ins and Outs of Major Facilitator Superfamily Antiporters. Annu. Rev. Microbiol. 2008, 62, 289–305. [Google Scholar] [CrossRef] [Green Version]
- Fowler, P.W.; Orwick-Rydmark, M.; Radestock, S.; Solcan, N.; Dijkman, P.M.; Lyons, J.A.; Kwok, J.; Caffrey, M.; Watts, A.; Forrest, L.R.; et al. Gating Topology of the Proton-Coupled Oligopeptide Symporters. Struct. Lond. Engl. 2015, 23, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.F.; Harris, J.M.; Subramaniam, V.N. Functional Analysis and Theoretical Modeling of Ferroportin Reveals Clustering of Mutations According to Phenotype. Am. J. Physiol. Cell Physiol. 2010, 298, C75–C84. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi di Patti, M.C.; Polticelli, F.; Cece, G.; Cutone, A.; Felici, F.; Persichini, T.; Musci, G. A Structural Model of Human Ferroportin and of Its Iron Binding Site. FEBS J. 2014, 281, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Le Gac, G.; Ka, C.; Joubrel, R.; Gourlaouen, I.; Lehn, P.; Mornon, J.-P.; Férec, C.; Callebaut, I. Structure-Function Analysis of the Human Ferroportin Iron Exporter (SLC40A1): Effect of Hemochromatosis Type 4 Disease Mutations and Identification of Critical Residues. Hum. Mutat. 2013, 34, 1371–1380. [Google Scholar] [CrossRef]
- Taniguchi, R.; Kato, H.E.; Font, J.; Deshpande, C.N.; Wada, M.; Ito, K.; Ishitani, R.; Jormakka, M.; Nureki, O. Outward- and Inward-Facing Structures of a Putative Bacterial Transition-Metal Transporter with Homology to Ferroportin. Nat. Commun. 2015, 6, 8545. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, C.N.; Ruwe, T.A.; Shawki, A.; Xin, V.; Vieth, K.R.; Valore, E.V.; Qiao, B.; Ganz, T.; Nemeth, E.; Mackenzie, B.; et al. Calcium Is an Essential Cofactor for Metal Efflux by the Ferroportin Transporter Family. Nat. Commun. 2018, 9, 3075. [Google Scholar] [CrossRef]
- Billesbølle, C.B.; Azumaya, C.M.; Kretsch, R.C.; Powers, A.S.; Gonen, S.; Schneider, S.; Arvedson, T.; Dror, R.O.; Cheng, Y.; Manglik, A. Structure of Hepcidin-Bound Ferroportin Reveals Iron Homeostatic Mechanisms. Nature 2020. [Google Scholar] [CrossRef]
- Pan, Y.; Ren, Z.; Gao, S.; Shen, J.; Wang, L.; Xu, Z.; Yu, Y.; Bachina, P.; Zhang, H.; Fan, X.; et al. Structural Basis of Ion Transport and Inhibition in Ferroportin. Nat. Commun. 2020, 11, 5686. [Google Scholar] [CrossRef]
- Drakesmith, H.; Schimanski, L.M.; Ormerod, E.; Merryweather-Clarke, A.T.; Viprakasit, V.; Edwards, J.P.; Sweetland, E.; Bastin, J.M.; Cowley, D.; Chinthammitr, Y.; et al. Resistance to Hepcidin Is Conferred by Hemochromatosis-Associated Mutations of Ferroportin. Blood 2005, 106, 1092–1097. [Google Scholar] [CrossRef]
- Mayr, R.; Griffiths, W.J.H.; Hermann, M.; McFarlane, I.; Halsall, D.J.; Finkenstedt, A.; Douds, A.; Davies, S.E.; Janecke, A.R.; Vogel, W.; et al. Identification of Mutations in SLC40A1 That Affect Ferroportin Function and Phenotype of Human Ferroportin Iron Overload. Gastroenterology 2011, 140, 2056–2063.e1. [Google Scholar] [CrossRef]
- Callebaut, I.; Joubrel, R.; Pissard, S.; Kannengiesser, C.; Gérolami, V.; Ged, C.; Cadet, E.; Cartault, F.; Ka, C.; Gourlaouen, I.; et al. Comprehensive Functional Annotation of 18 Missense Mutations Found in Suspected Hemochromatosis Type 4 Patients. Hum. Mol. Genet. 2014, 23, 4479–4490. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Suades, A.; Matsuoka, R.; Brock, J.; McComas, S.E.; Nji, E.; Orellana, L.; Claesson, M.; Delemotte, L.; Drew, D. The Molecular Basis for Sugar Import in Malaria Parasites. Nature 2020, 578, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, K.; Sargsyan, K.; Grauffel, C.; Dudev, T.; Lim, C. Preferred Hydrogen-Bonding Partners of Cysteine: Implications for Regulating Cys Functions. J. Phys. Chem. B 2016, 120, 10288–10296. [Google Scholar] [CrossRef]
- Mazmanian, K.; Sargsyan, K.; Lim, C. How the Local Environment of Functional Sites Regulates Protein Function. J. Am. Chem. Soc. 2020, 142, 9861–9871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Zhao, Y.; Heng, J.; Jiang, D. Energy Coupling Mechanisms of MFS Transporters. Protein Sci. Publ. Protein Soc. 2015, 24, 1560–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yang, Y.; Li, W. Human Ferroportin Mediates Proton-Coupled Active Transport of Iron. Blood Adv. 2020, 4, 4758–4768. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Finer-Moore, J.S.; Kaback, H.R.; Stroud, R.M. Structure of LacY with an α-Substituted Galactoside: Connecting the Binding Site to the Protonation Site. Proc. Natl. Acad. Sci. USA 2015, 112, 9004–9009. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.V.; Singh, H.; Raturi, S.; Neuberger, A.; Tong, Z.; Ding, N.; Agboh, K.; van Veen, H.W. Relocation of Active Site Carboxylates in Major Facilitator Superfamily Multidrug Transporter LmrP Reveals Plasticity in Proton Interactions. Sci. Rep. 2016, 6, 38052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martens, C.; Shekhar, M.; Borysik, A.J.; Lau, A.M.; Reading, E.; Tajkhorshid, E.; Booth, P.J.; Politis, A. Direct Protein-Lipid Interactions Shape the Conformational Landscape of Secondary Transporters. Nat. Commun. 2018, 9, 4151. [Google Scholar] [CrossRef] [PubMed]
- Guellec, J.; Elbahnsi, A.; Le Tertre, M.; Uguen, K.; Gourlaouen, I.; Férec, C.; Ka, C.; Callebaut, I.; Le Gac, G. Molecular Model of the Ferroportin Intracellular Gate and Implications for the Human Iron Transport Cycle and Hemochromatosis Type 4A. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 14625–14635. [Google Scholar] [CrossRef] [Green Version]
- Tortosa, V.; Bonaccorsi di Patti, M.C.; Iacovelli, F.; Pasquadibisceglie, A.; Falconi, M.; Musci, G.; Polticelli, F. Dynamical Behavior of the Human Ferroportin Homologue from Bdellovibrio Bacteriovorus: Insight into the Ligand Recognition Mechanism. Int. J. Mol. Sci. 2020, 21, 6785. [Google Scholar] [CrossRef] [PubMed]
- Ka, C.; Le Gac, G.; Dupradeau, F.-Y.; Rochette, J.; Férec, C. The Q283P Amino-Acid Change in HFE Leads to Structural and Functional Consequences Similar to Those Described for the Mutated 282Y HFE Protein. Hum. Genet. 2005, 117, 467–475. [Google Scholar] [CrossRef]
- Schimanski, L.M.; Drakesmith, H.; Merryweather-Clarke, A.T.; Viprakasit, V.; Edwards, J.P.; Sweetland, E.; Bastin, J.M.; Cowley, D.; Chinthammitr, Y.; Robson, K.J.H.; et al. In Vitro Functional Analysis of Human Ferroportin (FPN) and Hemochromatosis-Associated FPN Mutations. Blood 2005, 105, 4096–4102. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Tertre, M.; Elbahnsi, A.; Ka, C.; Callebaut, I.; Le Gac, G. Insights into the Role of the Discontinuous TM7 Helix of Human Ferroportin through the Prism of the Asp325 Residue. Int. J. Mol. Sci. 2021, 22, 6412. https://doi.org/10.3390/ijms22126412
Le Tertre M, Elbahnsi A, Ka C, Callebaut I, Le Gac G. Insights into the Role of the Discontinuous TM7 Helix of Human Ferroportin through the Prism of the Asp325 Residue. International Journal of Molecular Sciences. 2021; 22(12):6412. https://doi.org/10.3390/ijms22126412
Chicago/Turabian StyleLe Tertre, Marlène, Ahmad Elbahnsi, Chandran Ka, Isabelle Callebaut, and Gérald Le Gac. 2021. "Insights into the Role of the Discontinuous TM7 Helix of Human Ferroportin through the Prism of the Asp325 Residue" International Journal of Molecular Sciences 22, no. 12: 6412. https://doi.org/10.3390/ijms22126412





