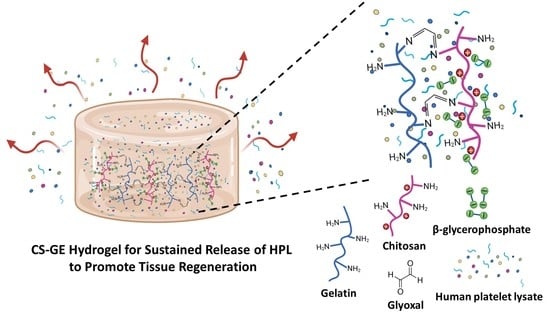
Developing a Glyoxal-Crosslinked Chitosan/Gelatin Hydrogel for Sustained Release of Human Platelet Lysate to Promote Tissue Regeneration
Abstract
1. Introduction
2. Results
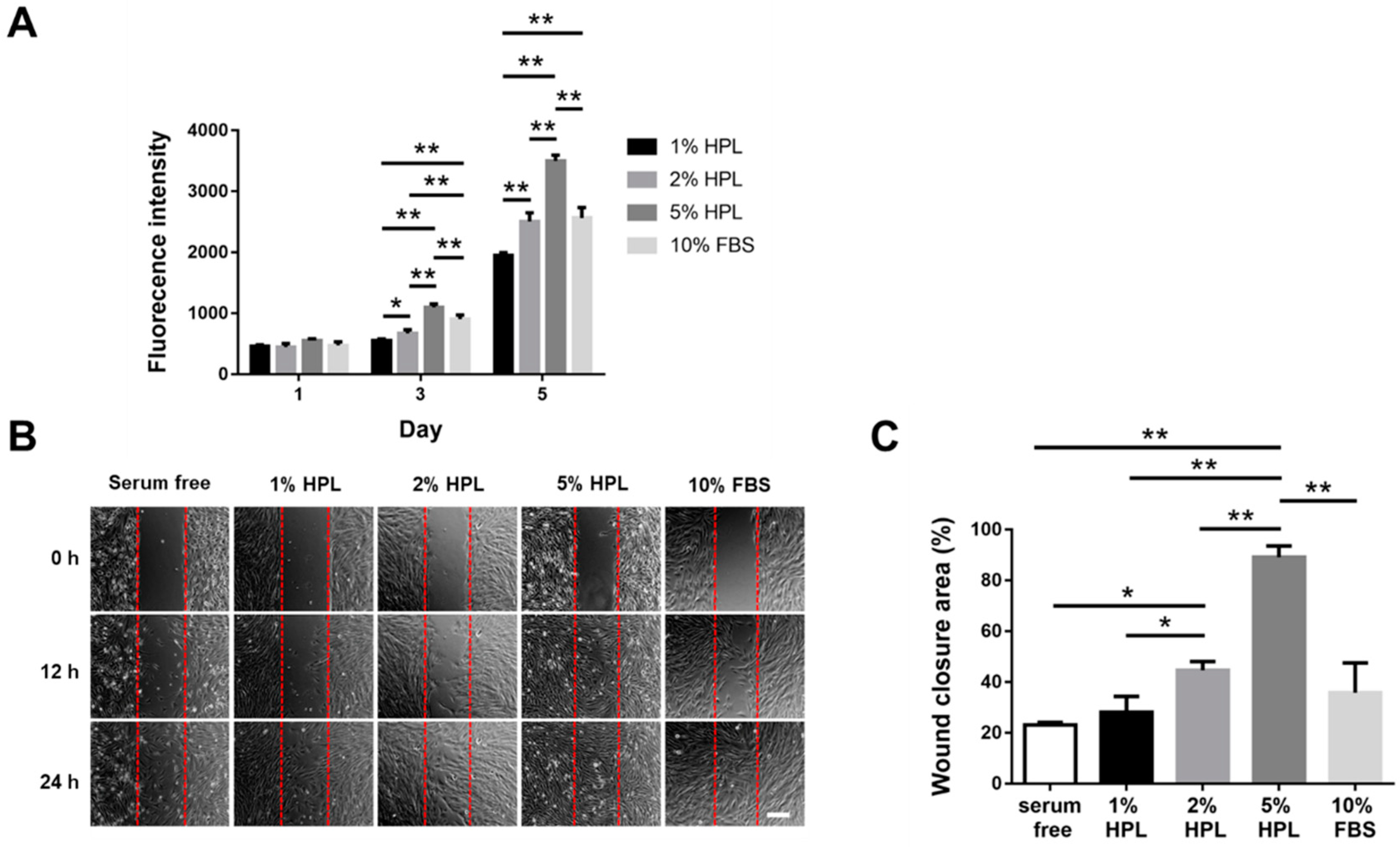
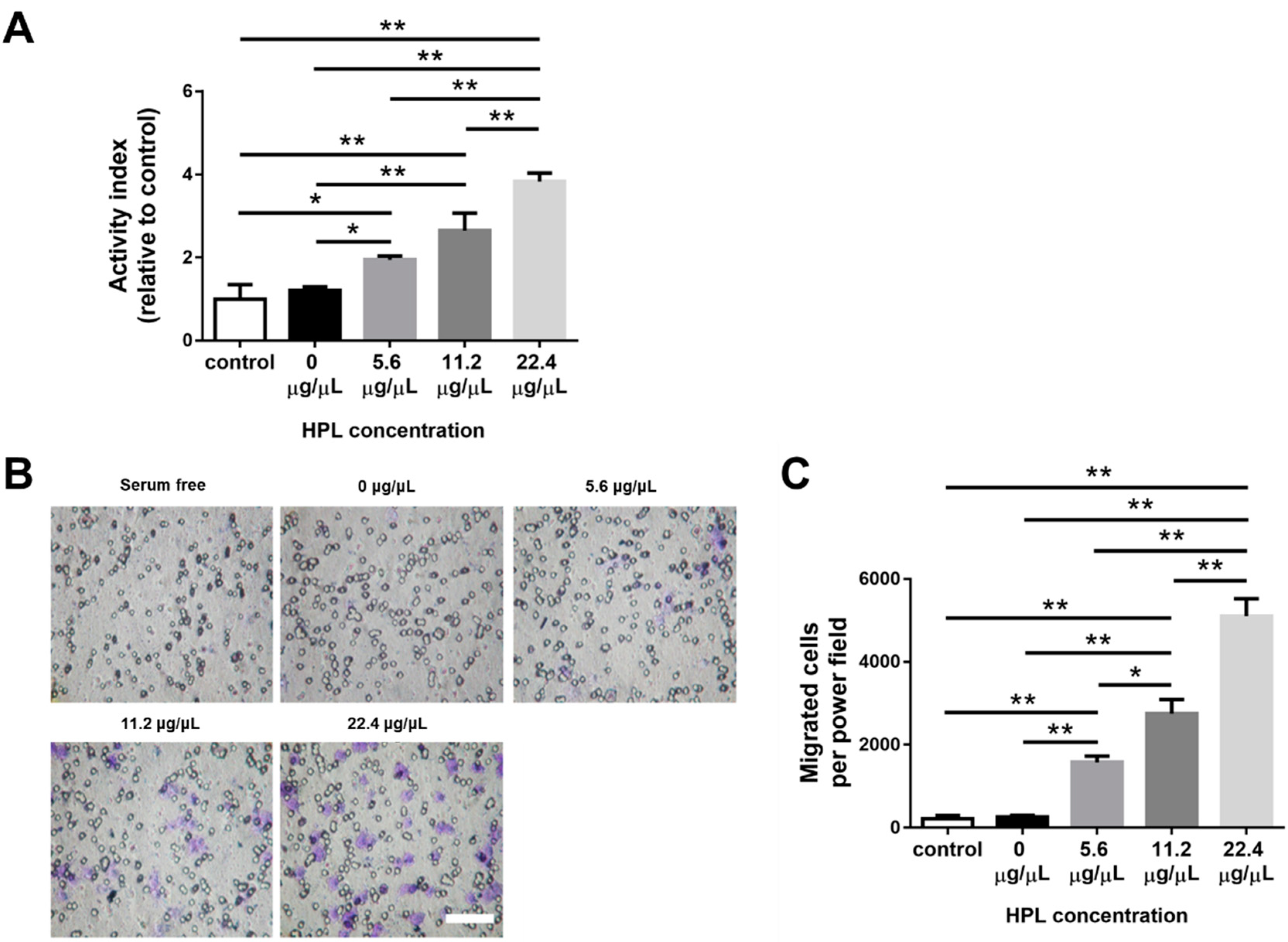
2.1. HPL Enhanced the Proliferation and Migration of Hs68 Fibroblasts
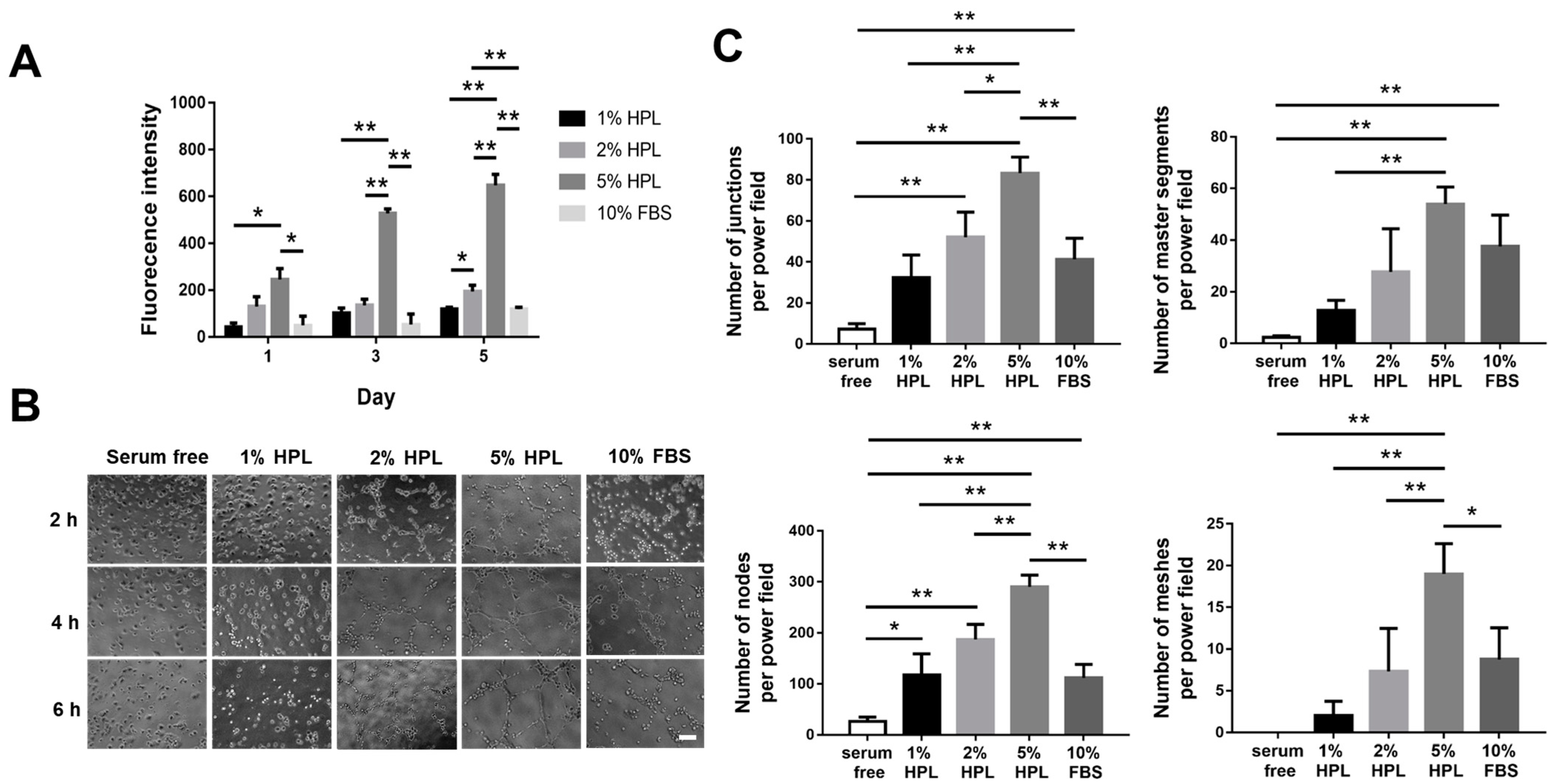
2.2. HPL Enhanced the Proliferation and Tube Formation of Endothelial Cells
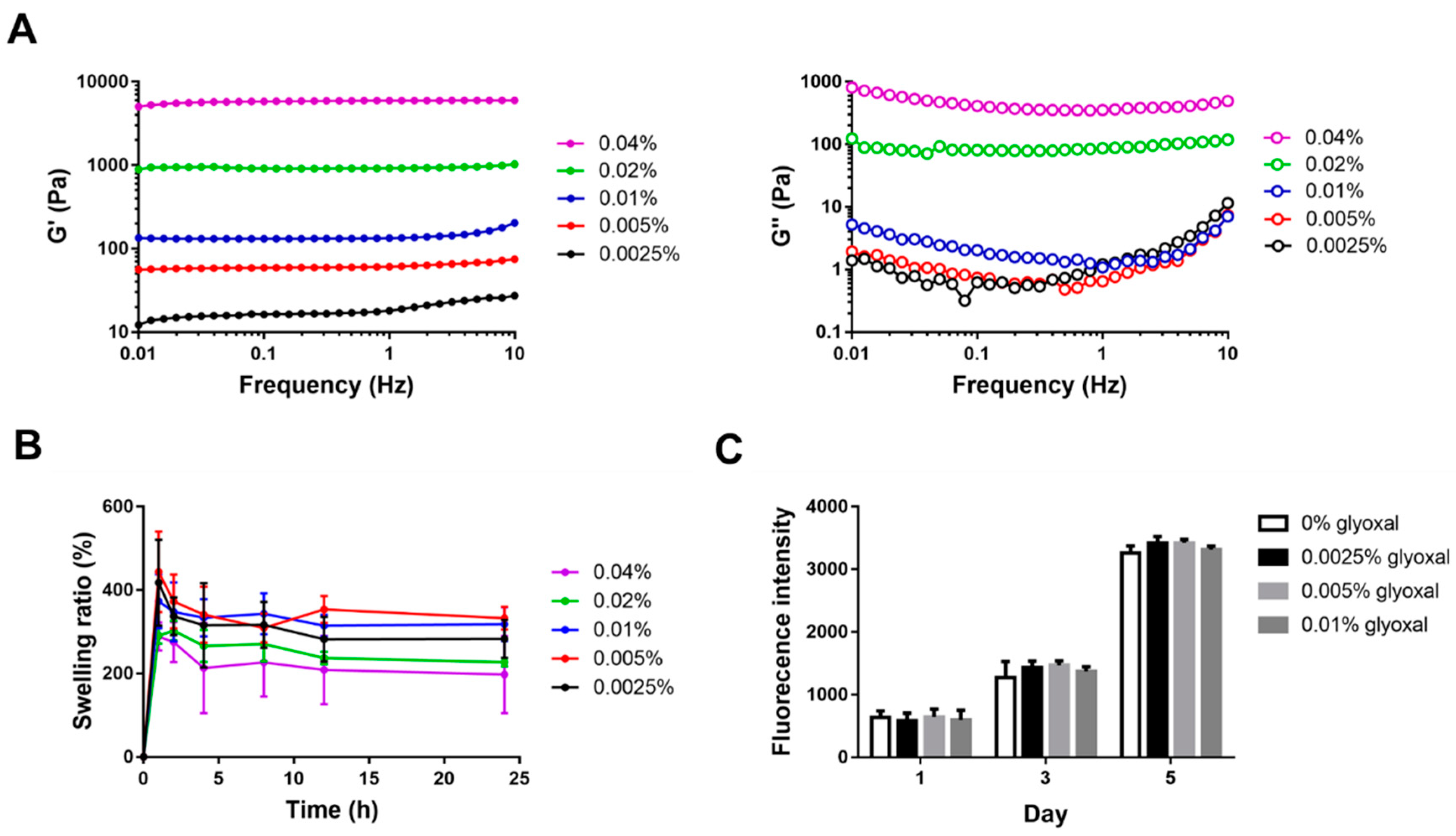
2.3. Characterization of CS-GE Hydrogel
2.4. In Vitro Cytotoxicity Assay of Glyoxal
2.5. Scanning Electron Micrographs and Pore Size Measurement
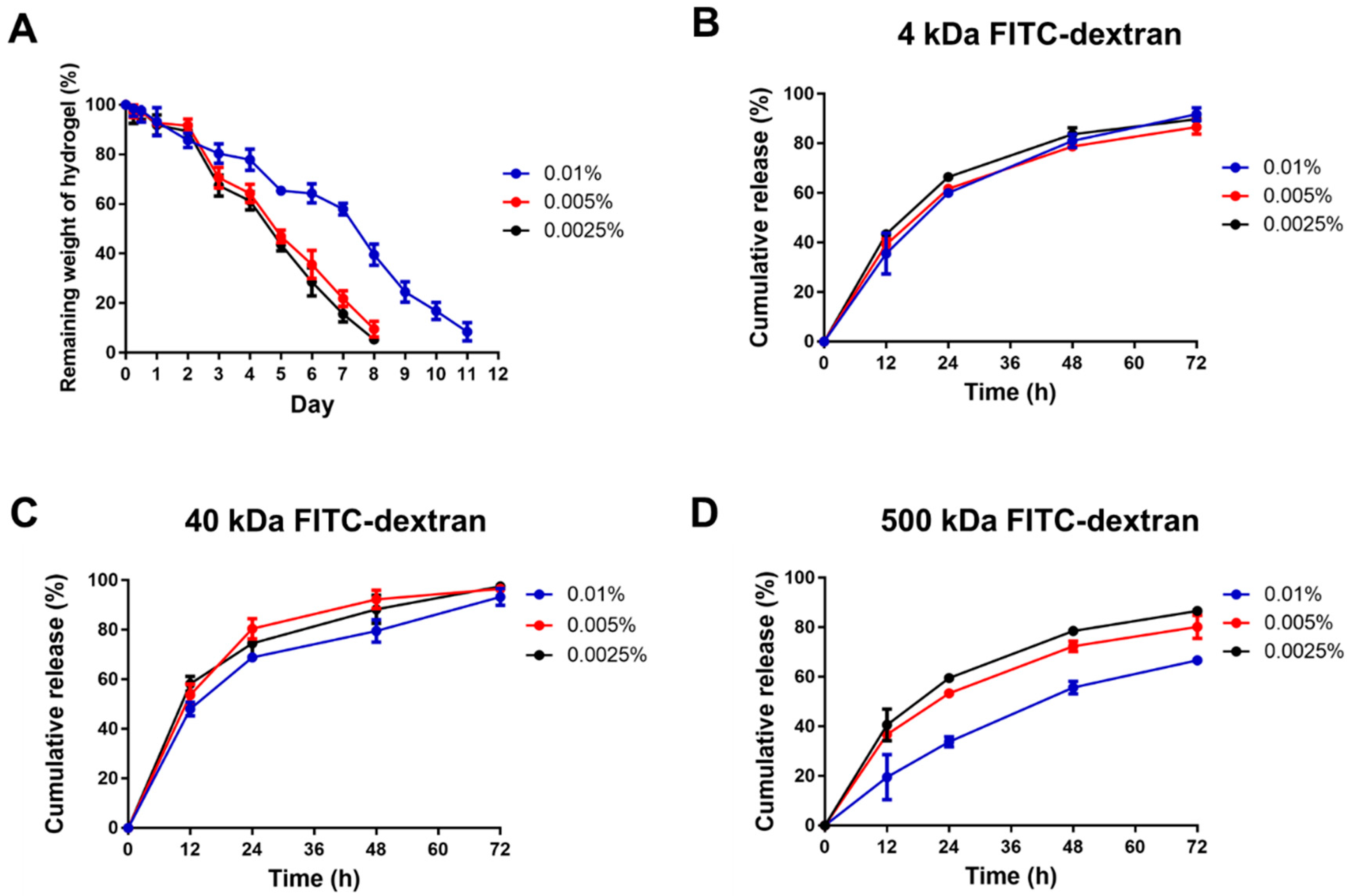
2.6. Enzymatic Degradation Assay
2.7. Release Pattern of FITC-Dextran from Hydrogels
2.8. HPL-Incorporated Hydrogel Enhanced the Cell Performance
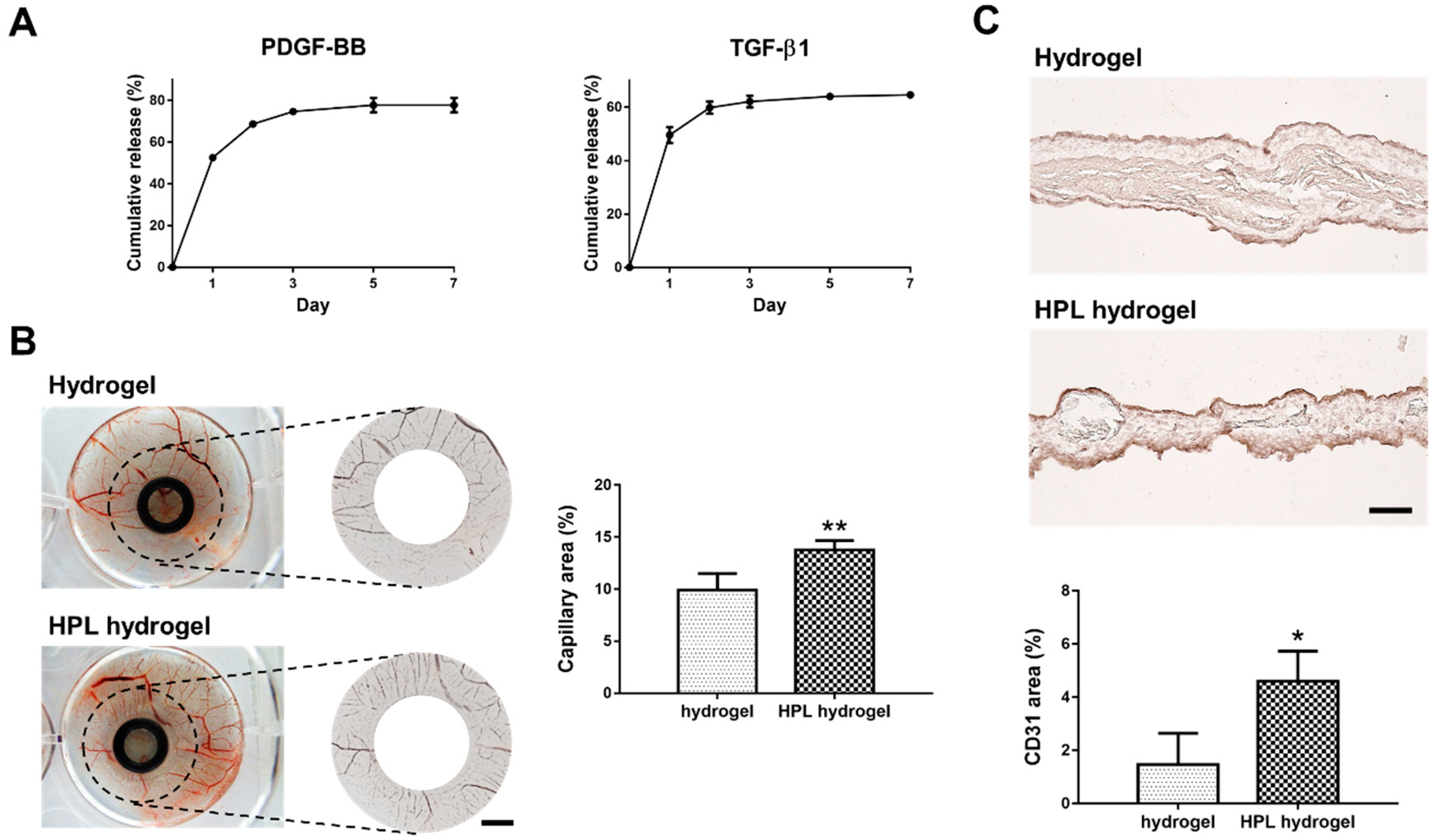
2.9. Growth Factor Release from HPL-Incorporated Hydrogel
2.10. HPL-Incorporated Hydrogel Enhanced Angiogenesis In Ovo
3. Discussion
4. Materials and Methods
4.1. Culture of Hs68 Cells and HUVECs
4.2. The Effect of HPL on Cell Proliferation
4.3. In Vitro Wound Healing Assay with Hs68 Cells
4.4. In Vitro Tube Formation Assay with HUVECs
4.5. Preparation of Crosslinked CS-GE Hydrogels
4.6. Gelation Time and Rheological Studies
4.7. Swelling Test
4.8. Cytotoxicity Study of the Crosslinked Hydrogels
4.9. Scanning Electron Microscopy
4.10. In Vitro Enzymatic Degradation Test
4.11. FITC-Dextran Release
4.12. HPL-Incorporated Hydrogel Enhanced Hs68 Cell Proliferation
4.13. HPL-Incorporated Hydrogel Enhanced HUVEC Migration
4.14. In Vitro Growth Factor Release into the Medium
4.15. Angiogenesis Assay in the CAM Model
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 25, 3747–3755. [Google Scholar] [CrossRef]
- Mi, B.; Liu, G.; Zhou, W.; Lv, H.; Liu, Y.; Wu, Q.; Liu, J. Platelet rich plasma versus steroid on lateral epicondylitis: Meta-analysis of randomized clinical trials. Physician Sportsmed. 2017, 45, 97–104. [Google Scholar] [CrossRef]
- Bennardo, F.; Bennardo, L.; Del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef] [PubMed]
- Hemeda, H.; Giebel, B.; Wagner, W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014, 16, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Shanskii, Y.D.; Sergeeva, N.; Sviridova, I.; Kirakozov, M.; Kirsanova, V.; Akhmedova, S.; Antokhin, A.; Chissov, V. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull. Exp. Biol. Med. 2013, 156, 146–151. [Google Scholar] [CrossRef]
- Chen, M.-S.; Wang, T.-J.; Lin, H.-C.; Burnouf, T. Four types of human platelet lysate, including one virally inactivated by solvent-detergent, can be used to propagate Wharton jelly mesenchymal stromal cells. New Biotechnol. 2019, 49, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Huang, C.-J.; Tu, W.-H.; Lu, C.-J.; Sun, Y.-C.; Lin, S.-Y.; Chen, W.-L. The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates. PLoS ONE 2018, 13, e0194345. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Varon, D.; Hayon, Y.; Dashevsky, O.; Shai, E. Involvement of platelet derived microparticles in tumor metastasis and tissue regeneration. Thromb. Res. 2012, 130, S98–S99. [Google Scholar] [CrossRef] [PubMed]
- Boilard, E.; Nigrovic, P.A.; Larabee, K.; Watts, G.F.; Coblyn, J.S.; Weinblatt, M.E.; Massarotti, E.M.; Remold-O’Donnell, E.; Farndale, R.W.; Ware, J. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 2010, 327, 580–583. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Tu, Y.-K.; Lee, N.-H.; Young, T.-H. Influence of Human Platelet Lysate on Extracellular Matrix Deposition and Cellular Characteristics in Adipose-Derived Stem Cell Sheets. Front. Cell Dev. Biol. 2020, 8, 1122. [Google Scholar] [CrossRef]
- Schallmoser, K.; Strunk, D. Generation of a pool of human platelet lysate and efficient use in cell culture. In Basic Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2013; pp. 349–362. [Google Scholar]
- Barro, L.; Nebie, O.; Chen, M.-S.; Wu, Y.-W.; Koh, M.B.; Knutson, F.; Watanabe, N.; Takahara, M.; Burnouf, T. Nanofiltration of growth media supplemented with human platelet lysates for pathogen-safe xeno-free expansion of mesenchymal stromal cells. Cytotherapy 2020, 22, 458–472. [Google Scholar] [CrossRef]
- Hayon, Y.; Dashevsky, O.; Shai, E.; Varon, D.; Leker, R.R. Platelet lysates stimulate angiogenesis, neurogenesis and neuroprotection after stroke. Thromb. Haemost. 2013, 110, 323–330. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Pirraco, R.P.; Marques, A.P.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Platelet lysate-based pro-angiogenic nanocoatings. Acta Biomater. 2016, 32, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Babo, P.S.; Pires, R.L.; Santos, L.; Franco, A.; Rodrigues, F.; Leonor, I.; Reis, R.L.; Gomes, M.E. Platelet lysate-loaded photocrosslinkable hyaluronic acid hydrogels for periodontal endogenous regenerative technology. ACS Biomater. Sci. Eng. 2017, 3, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.T.; Douglas, A.M.; Chadid, T.; Kuo, K.; Rajabalan, A.; Li, H.; Copland, I.B.; Barker, T.H.; Galipeau, J.; Brewster, L.P. A novel platelet lysate hydrogel for endothelial cell and mesenchymal stem cell-directed neovascularization. Acta Biomater. 2016, 36, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiao, R.; Cao, Y.-L.; Yin, H.-Y. Evaluation of human platelet lysate and dimethyl sulfoxide as cryoprotectants for the cryopreservation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2017, 491, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.T.; Dass, C.R.; Dunstan, D.E. Injectable chitosan hydrogels for localised cancer therapy. J. Control Release 2008, 126, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Viyoch, J.; Sudedmark, T.; Srema, W.; Suwongkrua, W. Development of hydrogel patch for controlled release of alpha-hydroxy acid contained in tamarind fruit pulp extract. Int. J. Cosmet. Sci. 2005, 27, 89–99. [Google Scholar] [CrossRef]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan–glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan–glycerol phosphate hydrogels. J. Biomed. Mater. Res. Part A 2008, 86, 824–832. [Google Scholar] [CrossRef]
- Cheng, N.-C.; Lin, W.-J.; Ling, T.-Y.; Young, T.-H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nimni, M.E.; Yang, Z.; Han, B. Chitosan/gelatin–based films crosslinked by proanthocyanidin. J. Biomed. Mater. Res. Part B 2005, 75, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.C.; Chang, H.H.; Tu, Y.K.; Young, T.H. Efficient transfer of human adipose-derived stem cells by chitosan/gelatin blend films. J. Biomed. Mater. Res. Part B 2012, 100, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Dessì, M.; Borzacchiello, A.; Mohamed, T.H.; Abdel-Fattah, W.I.; Ambrosio, L. Novel biomimetic thermosensitive β-tricalcium phosphate/chitosan-based hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Stegemann, J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011, 7, 2410–2417. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Chenite, A.; Sun, J.; Hurtig, M.; Serreqi, A.; Lu, Z.; Rossomacha, E.; Buschmann, M.D. Cytocompatible gel formation of chitosan-glycerol phosphate solutions supplemented with hydroxyl ethyl cellulose is due to the presence of glyoxal. J. Biomed. Mater. Res. A 2007, 83, 521–529. [Google Scholar] [CrossRef]
- Jawad, A.H.; Norrahma, S.S.A.; Hameed, B.; Ismail, K. Chitosan-glyoxal film as a superior adsorbent for two structurally different reactive and acid dyes: Adsorption and mechanism study. Int. J. Biol. Macromol. 2019, 135, 569–581. [Google Scholar] [CrossRef]
- Boccardo, S.; Gaudiello, E.; Melly, L.; Cerino, G.; Ricci, D.; Martin, I.; Eckstein, F.; Banfi, A.; Marsano, A. Engineered mesenchymal cell-based patches as controlled VEGF delivery systems to induce extrinsic angiogenesis. Acta Biomater. 2016, 42, 127–135. [Google Scholar] [CrossRef]
- Ranzato, E.; Mazzucco, L.; Patrone, M.; Burlando, B. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: Different roles of cell calcium, P38, ERK and PI3K/AKT. J. Cell. Mol. Med. 2009, 13, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Griffith, G.L.; Holt, A.W.; Eriksson, E.; Johnson, A.J.; McDaniel, J.S. Human platelet lysate delivered via an ocular wound chamber for the treatment of corneal epithelial injuries. Exp. Eye Res. 2021, 206, 108493. [Google Scholar] [CrossRef]
- Jafar, H.; Hasan, M.; Al-Hattab, D.; Saleh, M.; Ameereh, L.A.; Khraisha, S.; Younes, N.; Awidi, A. Platelet lysate promotes the healing of long-standing diabetic foot ulcers: A report of two cases and in vitro study. Heliyon 2020, 6, e03929. [Google Scholar] [CrossRef] [PubMed]
- Rozman, P.; Bolta, Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol. Alp. Panon. Et Adriat. 2007, 16, 156. [Google Scholar]
- Al-Ajlouni, J.; Awidi, A.; Samara, O.; Al-Najar, M.; Tarwanah, E.; Saleh, M.; Awidi, M.; Hassan, F.A.; Samih, M.; Bener, A. Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans: A prospective open-label study. Clin. J. Sport Med. 2015, 25, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Altaie, A.; Owston, H.; Jones, E. Use of platelet lysate for bone regeneration—Are we ready for clinical translation? World J. Stem Cells 2016, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- G Tahrir, F.; Ganji, F.; M Ahooyi, T. Injectable thermosensitive chitosan/glycerophosphate-based hydrogels for tissue engineering and drug delivery applications: A review. Recent Pat. Drug Deliv. Formul. 2015, 9, 107–120. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef]
- Jarry, C.; Chaput, C.; Chenite, A.; Renaud, M.A.; Buschmann, M.; Leroux, J.C. Effects of steam sterilization on thermogelling chitosan-based gels. J. Biomed. Mater. Res. 2001, 58, 127–135. [Google Scholar] [CrossRef]
- Vaz, C.M.; De Graaf, L.A.; Reis, R.L.; Cunha, A.M. In vitro degradation behaviour of biodegradable soy plastics: Effects of crosslinking with glyoxal and thermal treatment. Polym. Degrad. Stab. 2003, 81, 65–74. [Google Scholar] [CrossRef]
- Shangari, N.; O’Brien, P.J. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem. Pharmacol. 2004, 68, 1433–1442. [Google Scholar] [CrossRef]
- Jin, J.; Song, M.; Hourston, D. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Chiono, V.; Pulieri, E.; Vozzi, G.; Ciardelli, G.; Ahluwalia, A.; Giusti, P. Genipin-crosslinked chitosan/gelatin blends for biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.-J.; Song, Y.S.; Kim, H.-J.; Lee, Y.-H.; Hong, S.-M.; Kim, S.-J.; Kim, B.-C.; Jin, C.; Lim, C.-J.; Park, E.-H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. 2004, 495, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly (vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Tang, Q.; Zhu, Z.; Wei, X.; Zhang, C. Sustained release of human platelet lysate growth factors by thermosensitive hydroxybutyl chitosan hydrogel promotes skin wound healing in rats. J. Biomed. Mater. Res. Part. A 2020, 108, 2111–2122. [Google Scholar] [CrossRef]
- Schallmoser, K.; Bartmann, C.; Rohde, E.; Reinisch, A.; Kashofer, K.; Stadelmeyer, E.; Drexler, C.; Lanzer, G.; Linkesch, W.; Strunk, D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 2007, 47, 1436–1446. [Google Scholar] [CrossRef]
- Mojica-Henshaw, M.P.; Morris, J.; Kelley, L.; Pierce, J.; Boyer, M.; Reems, J.-A. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy 2013, 15, 1458–1468. [Google Scholar] [CrossRef]
- Li, W.; Fan, J.; Chen, M.; Guan, S.; Sawcer, D.; Bokoch, G.M.; Woodley, D.T. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol. Biol. Cell 2004, 15, 294–309. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.D.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Ribatti, D.; Annese, T.; Tamma, R. The use of the chick embryo CAM assay in the study of angiogenic activiy of biomaterials. Microvasc. Res. 2020, 131, 104026. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Hong, Y.-J.; Lee, R.J.; Cheng, N.-C.; Yu, J. Enhancement of human adipose-derived stem cell spheroid differentiation in an in situ enzyme-crosslinked gelatin hydrogel. J. Mater. Chem. B 2019, 7, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Yu, J.; Tsai, W. Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable crosslinking for controlled drug release. J. Mater. Chem. B 2016, 4, 2304–2313. [Google Scholar] [CrossRef] [PubMed]

| Glyoxal (%) | 0.0025% | 0.005% | 0.01% | 0.02% | 0.04% |
| Gelation time (s) | 63.0 ± 7.1 | 39.7 ± 2.9 | 21.0 ± 1.7 | 15.7 ± 3.8 | 13.7 ± 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Young, T.-H.; Chen, G.-S.; Cheng, N.-C. Developing a Glyoxal-Crosslinked Chitosan/Gelatin Hydrogel for Sustained Release of Human Platelet Lysate to Promote Tissue Regeneration. Int. J. Mol. Sci. 2021, 22, 6451. https://doi.org/10.3390/ijms22126451
Tsai C-C, Young T-H, Chen G-S, Cheng N-C. Developing a Glyoxal-Crosslinked Chitosan/Gelatin Hydrogel for Sustained Release of Human Platelet Lysate to Promote Tissue Regeneration. International Journal of Molecular Sciences. 2021; 22(12):6451. https://doi.org/10.3390/ijms22126451
Chicago/Turabian StyleTsai, Ching-Cheng, Tai-Horng Young, Guang-Shih Chen, and Nai-Chen Cheng. 2021. "Developing a Glyoxal-Crosslinked Chitosan/Gelatin Hydrogel for Sustained Release of Human Platelet Lysate to Promote Tissue Regeneration" International Journal of Molecular Sciences 22, no. 12: 6451. https://doi.org/10.3390/ijms22126451
APA StyleTsai, C.-C., Young, T.-H., Chen, G.-S., & Cheng, N.-C. (2021). Developing a Glyoxal-Crosslinked Chitosan/Gelatin Hydrogel for Sustained Release of Human Platelet Lysate to Promote Tissue Regeneration. International Journal of Molecular Sciences, 22(12), 6451. https://doi.org/10.3390/ijms22126451