The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide
Abstract
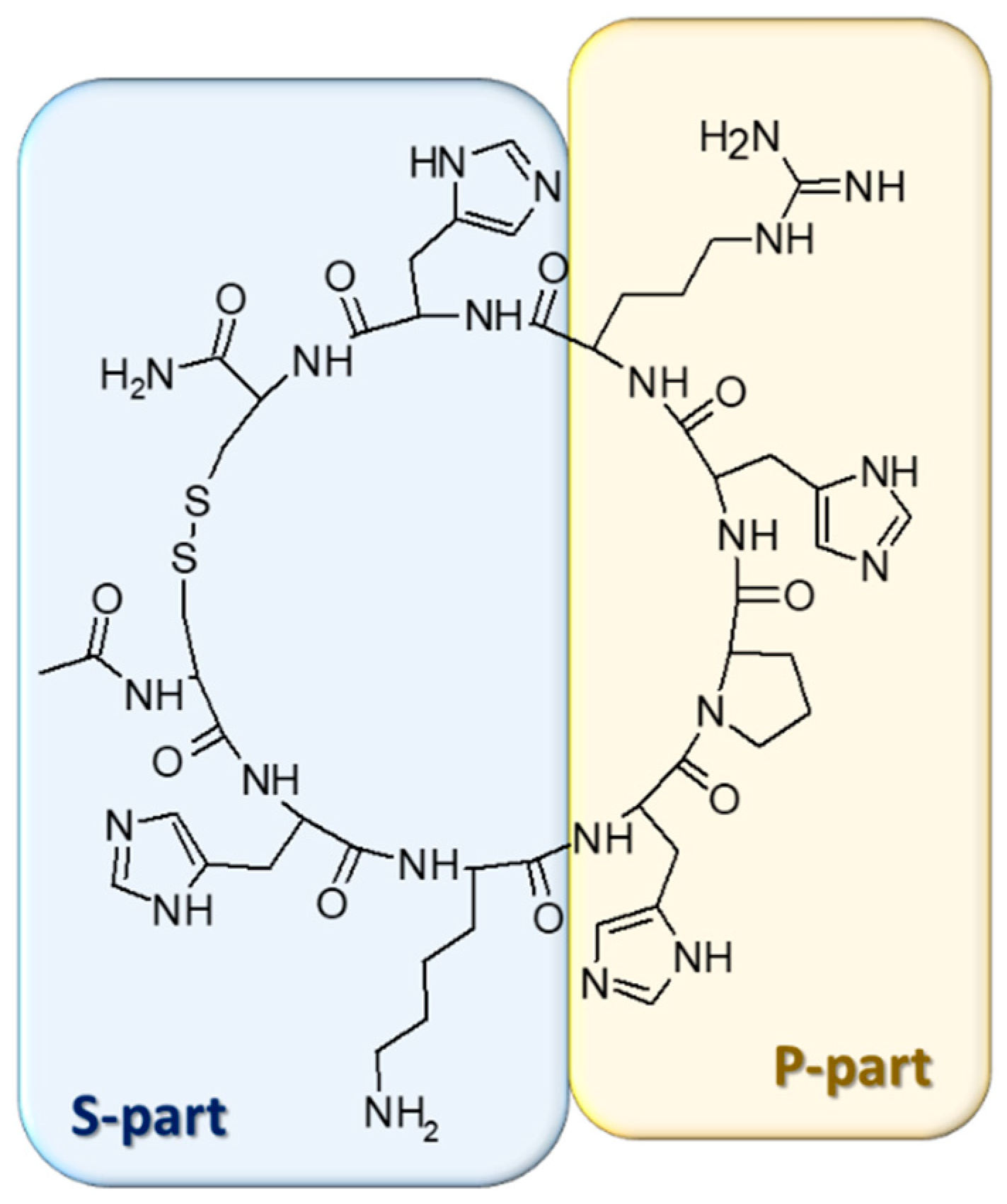
:1. Introduction
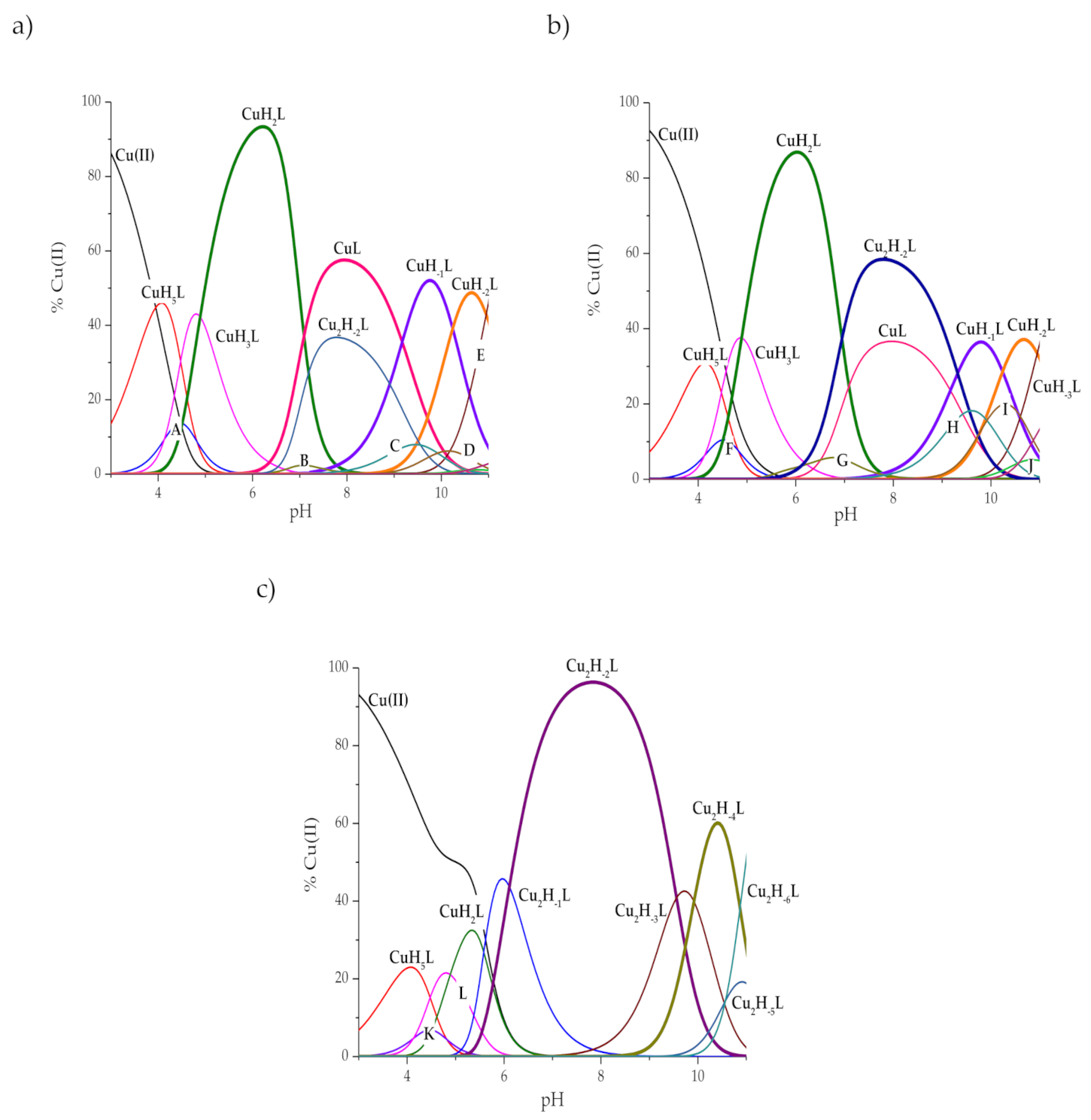
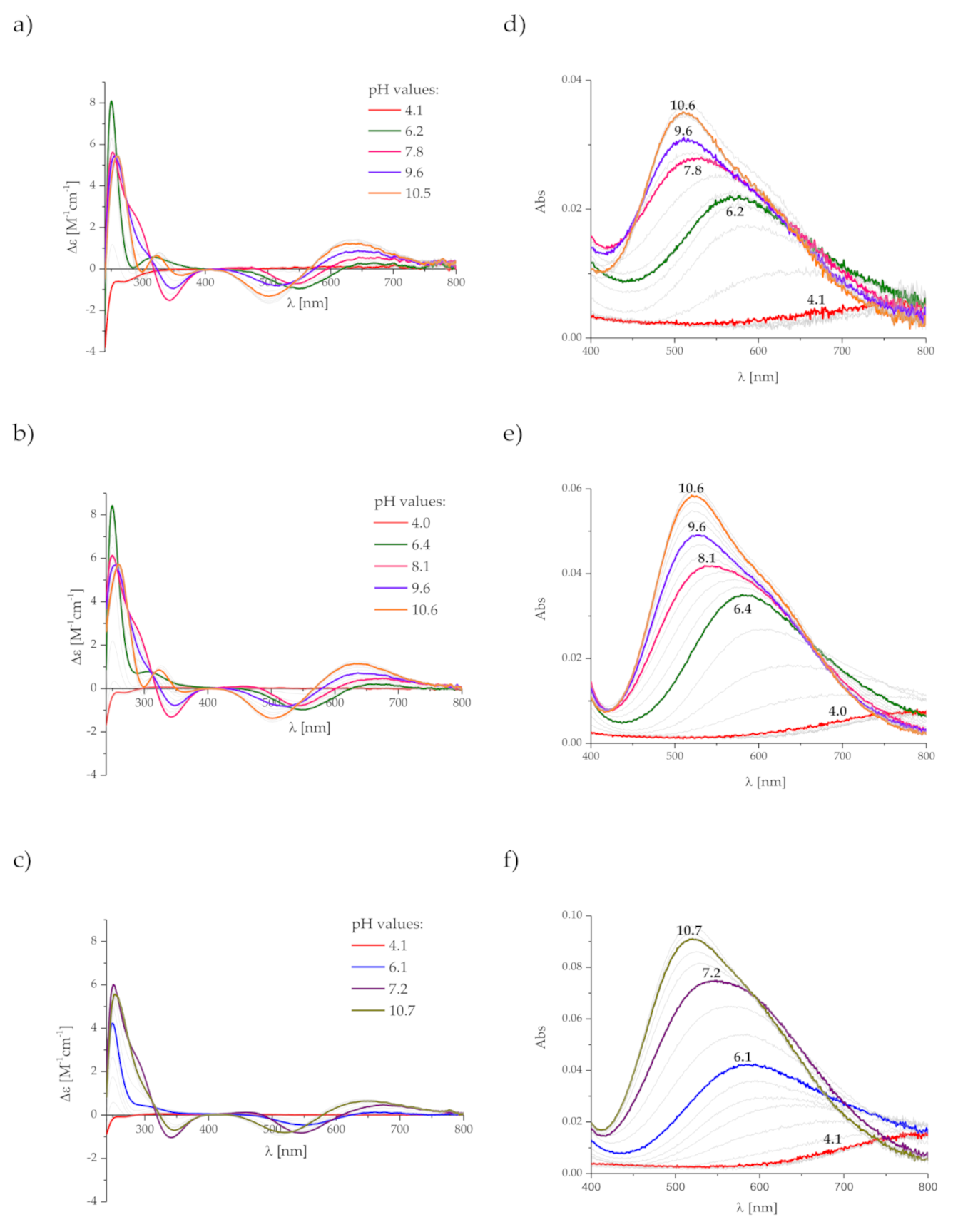
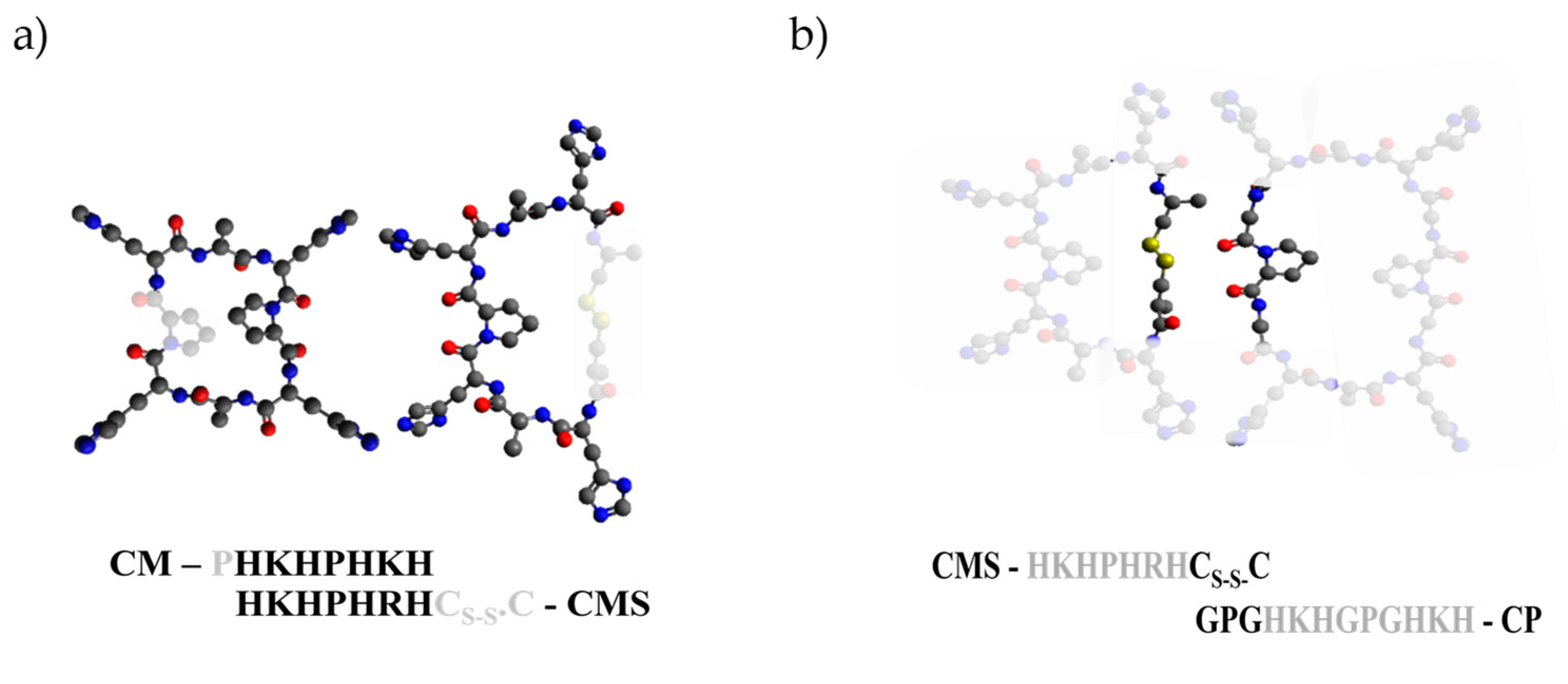
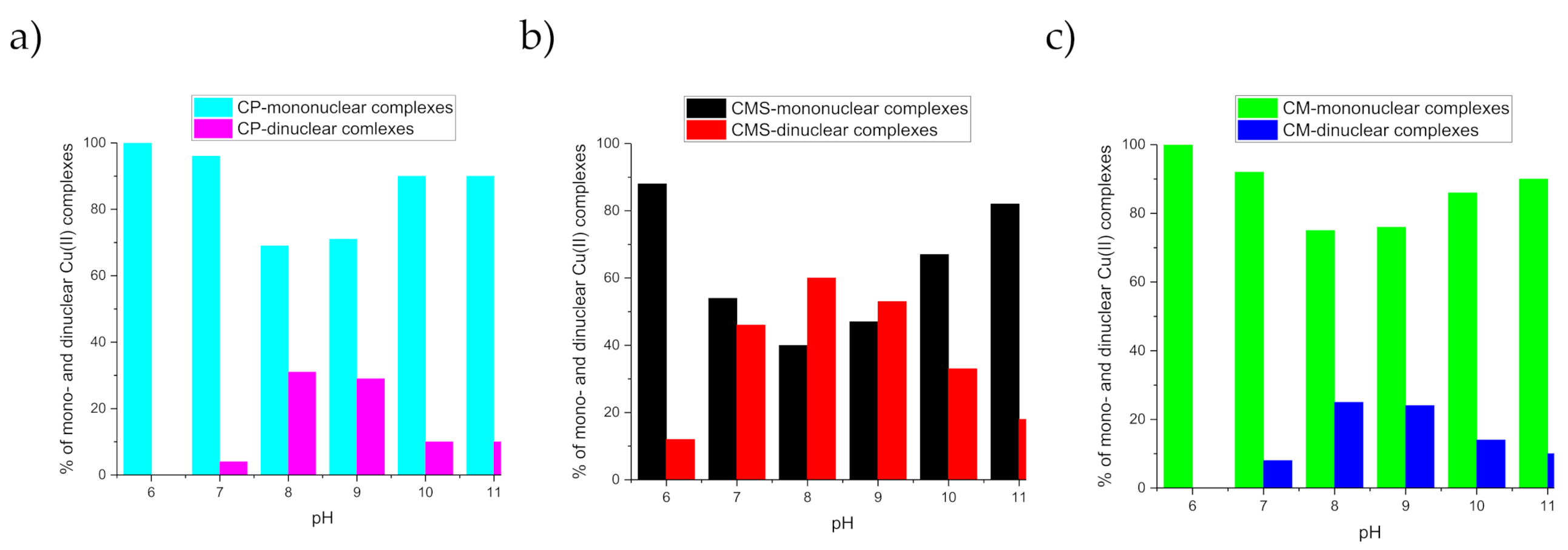
2. Results and Discussion
3. Materials and Methods
3.1. The Synthesis of the Ligand
3.2. Potentiometric Measurements
3.3. Spectroscopic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ovchinnikov, Y.A.; Ivanov, V.T. Conformational states and biological activity of cyclic peptides. Tetrahedron 1975, 31, 2177–2209. [Google Scholar] [CrossRef]
- Brasuń, J.; Matera, A.; Ołdziej, S.; Światek-Kozłowska, J.; Messori, L.; Gabbiani, C.; Orfei, M.; Ginanneschi, M. The copper (II) coordination abilities of three novel cyclic tetrapeptides with -His-Xaa-His- motif. J. Inorg. Biochem. 2007, 101, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, R.P.; Impellizzeri, G.; Pappalardo, G.; Purrello, R.; Rizzarelli, E.; Tabbì, G. Co-ordinating properties of cyclopeptides. Thermodynamic and spectroscopic study on the formation of copper (II) complexes with cyclo (Gly-His)4 and cyclo(Gly-His-Gly)2 and their superoxide dismutase-like activity. J. Chem. Soc. Dalt. Trans. 1998, 2, 3851–3857. [Google Scholar] [CrossRef]
- Laussac, J.P.; Robert, A.; Haran, R.; Sarkar, B. Complexation of Copper (II) with a Macrocyclic Peptide Containing Histidyl Residues: Novel Observation of NMR Spectra of Paramagnetic Copper (II) Compounds. Inorg. Chem. 1986, 25, 2760–2765. [Google Scholar] [CrossRef]
- Kozlowski, H.; Radomska, B.; Kupryszewski, G.; Lammek, B.; Livera, C.; Pettit, L.D.; Pyburn, S. The unusual co-ordination ability of vasopressin-like peptides; potentiometric and spectroscopic studies of some copper (II) and nickel (II) complexes. J. Chem. Soc. Dalt. Trans. 1989, 173–177. [Google Scholar] [CrossRef]
- Bal, W.; Kozlowski, H.; Lammek, B.; Rolka, K.; Pettit, L.D. Potentiometric and spectroscopic studies of the Cu (II) complexes of Ala-Arg8-vasopressin and oxytocin: Two vasopressin-like peptides. J. Inorg. Biochem. 1992. [Google Scholar] [CrossRef]
- Kotynia, A.; Bielińska, S.; Kamysz, W.; Brasuń, J. The coordination abilities of the multiHis-cyclopeptide with two metal-binding centers - Potentiometric and spectroscopic investigation. Dalt. Trans. 2012, 41, 12114–12120. [Google Scholar] [CrossRef] [PubMed]
- Kotynia, A.; Czyznikowska, Z.; Bielińska, S.; Szyrwiel, Ł.; Kamysz, W.; Malinka, W.; Brasuń, J. The impact of two -GlyProGly-motifs on formation of di-copper complexes by His4-cyclopeptides. New J. Chem. 2014, 38, 5198–5206. [Google Scholar] [CrossRef]
- Kozłowski, H.; Bal, W.; Dyba, M.; Kowalik-Jankowska, T. Specific structure-stability relations in metallopeptides. Coord. Chem. Rev. 1999, 184, 319–346. [Google Scholar] [CrossRef]
- Formicka-Kozlowska, G.; Kozlowska, H.; Siemion, I.Z.; Sobczyk, K.; Nawrocka, E. Copper (II) interaction with proline-containing tetrapeptides. J. Inorg. Biochem. 1981, 15, 201–212. [Google Scholar] [CrossRef]
- Brasun, J.; Matera-Witkiewicz, A.; Kamysz, E.; Kamysz, W.; Ołdziej, S. The influence of the cyclopeptide sequence on its coordination abilities towards Cu (II). Polyhedron 2010, 29, 1535–1542. [Google Scholar] [CrossRef]
- Kállay, C.; Várnagy, K.; Malandrinos, G.; Hadjiliadis, N.; Sanna, D.; Sóvágó, I. Thermodynamic and structural characterization of the macrochelates formed in the reactions of copper (II) and zinc (II) ions with peptides of histidine. Inorg. Chim. Acta 2009, 362, 935–945. [Google Scholar] [CrossRef]
- Brasun, J.; Gabbiani, C.; Ginanneschi, M.; Messori, L.; Orfei, M.; Swiatek-Kozlowska, J. The copper (II) binding properties of the cyclic peptide c(HGHK). J. Inorg. Biochem. 2004, 98, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Chakrabarti, P. Stereospecific interactions of proline residues in protein structures and complexes. J. Mol. Biol. 2003, 331, 925–940. [Google Scholar] [CrossRef]
- Kállay, C.; Nagy, Z.; Várnagy, K.; Malandrinos, G.; Hadjiliadis, N.; Sóvágó, I. Thermodynamic and structural characterization of the copper (II) complexes of peptides containing both histidyl and aspartyl residues. Bioinorg. Chem. Appl. 2007, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kállay, C.; Várnagy, K.; Micera, G.; Sanna, D.; Sóvágó, I. Copper (II) complexes of oligopeptides containing aspartyl and glutamyl residues. Potentiometric and spectroscopic studies. J. Inorg. Biochem. 2005. [Google Scholar] [CrossRef] [PubMed]
- Prenesti, E.; Daniele, P.G.; Prencipe, M.; Ostacoli, G. Spectrum-structure correlation for visible absorption spectra of copper (II) complexes in aqueous solution. Polyhedron 1999, 18, 3233–3241. [Google Scholar] [CrossRef]
- Irving, H.M.; Miles, M.G.; Pettit, L.D. A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: An improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalt. Trans. 1985, 1195–1200. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
| CMS—HKHPHRHC-S-S-C | |||||||
|---|---|---|---|---|---|---|---|
| HL | H2L | H3L | H4L | H5L | H6L | ||
| logβ | 11.12 ± 0.01 | 20.83 ± 0.01 | 27.61 ± 0.02 | 33.69 ± 0.02 | 39.36 ± 0.02 | 44.34 ± 0.02 | |
| logK | 11.12 | 9.71 | 6.78 | 6.08 | 5.67 | 4.98 | |
| Mononuclear complexes | Dinuclear complexes | ||||||
| logβ | logβ | ||||||
| Species | Species | ||||||
| CuH5L | 43.28 ± 0.04 | Cu2H-1L | 15.58 ± 0.03 | ||||
| CuH4L | 38.46 ± 0.10 | Cu2H-2L | 9.47 ± 0.02 | ||||
| CuH3L | 34.33 ± 0.03 | Cu2H-3L | −0.09 ± 0.05 | ||||
| CuH2L | 29.44 ± 0.02 | Cu2H-4L | −10.01 ± 0.04 | ||||
| CuL | 15.26 ± 0.04 | Cu2H-5L | −21.18 ± 0.11 | ||||
| CuH-1L | 6.09 ± 0.05 | Cu2H-6L | −31.75 ± 0.04 | ||||
| CuH-2L | −4.15 ± 0.04 | ||||||
| CuH-3L | −15.10 ± 0.04 | ||||||
| logK | logK | ||||||
| logβCuH5L—logβCuH4L | 4.82 | logβCu2H-1L—logβCu2H-2L | 6.11 | ||||
| logβCuH4L—logβCuH3L | 4.13 | logβCu2H-2L—logβCu2H-3L | 9.56 | ||||
| logβCuH3L—logβCuH2L | 4.89 | logβCu2H-3L—logβCu2H-4L | 9.92 | ||||
| logβCuH2L—logβCuL | 14.18 | logβCu2H-4L—logβCu2H-5L | 11.17 | ||||
| logβCuL—logβCuH-1L | 9.17 | logβCu2H-5L—logβCu2H-6L | 10.57 | ||||
| logβCuH-1L—logβCuH-2L | 10.24 | ||||||
| logβCuH-2L—logβCuH-3L | 10.95 | ||||||
| The Binding Manner | CMS | CM [7] | CP [8] |
|---|---|---|---|
| Mononuclear complexes | |||
| {2NIm} {3NIm} {4NIm} {2NIm,2N-am} {1NIm,3N-am} | 17.63 13.50 8.61 −5.57 −15.10 | 17.20 - 8.46− 6.34− 15.81 | 19.02 - 9.81 −5.41 −14.69 |
| Dinuclear complexes | |||
| {2NIm,2N-am}{3NIm,1N-am} {2NIm,2N-am}{2NIm,2N-am} {1NIm,3N-am}{2NIm,3N-am} {1NIm,3N-am}{1NIm,3N-am} | −5.25 −11.36 −20.92 −31.75 | −7.13 −14.23 −22.87 - | −5.64 −11.64 - - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieniężna, A.; Witak, W.; Szymańska, A.; Brasuń, J. The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide. Int. J. Mol. Sci. 2021, 22, 6458. https://doi.org/10.3390/ijms22126458
Pieniężna A, Witak W, Szymańska A, Brasuń J. The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide. International Journal of Molecular Sciences. 2021; 22(12):6458. https://doi.org/10.3390/ijms22126458
Chicago/Turabian StylePieniężna, Aleksandra, Weronika Witak, Aneta Szymańska, and Justyna Brasuń. 2021. "The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide" International Journal of Molecular Sciences 22, no. 12: 6458. https://doi.org/10.3390/ijms22126458
APA StylePieniężna, A., Witak, W., Szymańska, A., & Brasuń, J. (2021). The Role of the Disulfide Bridge in the Copper (II) Binding by the Cyclic His4-Peptide. International Journal of Molecular Sciences, 22(12), 6458. https://doi.org/10.3390/ijms22126458






