1. Introduction
The promotion of fluid transport in porous media has important applications in energy, soil and biological science and other fields. How to construct functional molecules to modify pore surfaces and facilitate fluid flow in narrow spaces is recognized as a worldwide problem, e.g., for ultra-low permeability reservoirs (<5 mD). Given the current contradiction between the rising energy demand and falling oil production [
1], petroleum engineers pay more attention to the exploitation of unconventional oil reservoirs. A growing number of ultra-low permeability reservoirs has been proven and they account for a large proportion of the available oil in the world [
2]. However, this kind of reservoirs has special reservoir characteristics and complex pore structure. Accordingly, oil development under such unconventional conditions encounters problems, such as high injection pressure, low recovery and poor economic benefits [
3]. How to enhance the recovery of ultra-low permeability reservoirs has been a vital task for all the petroleum researchers.
In ultra-low permeability reservoirs, clay minerals are widely present. Clay minerals are layered silicates and these crystal platelets may have multiple octahedral or tetrahedral flakes, connected together by oxygen atoms [
4,
5]. Montmorillonite, which is the main component of bentonite, is composed of two layers of silica tetrahedrons with an alumina octahedron in between [
6]. Moreover, Sodium montmorillonite (Na-Mt) with a cation exchange capacity (CEC) of 90 mmol/100 g, has the highest water sensitivity among many clay minerals [
7]. The hydration and expansion of clay minerals cause them to disperse into fine particles with a diameter of less than 10 µm, which can easily block the mineral voids and reduce permeability. In addition, water molecules can bond closely with each other through hydrogen bonds, forming a dense hydrogen bond network. As a result, water tends to form clusters at nanoscale [
8], which is another key factor of oil displacement in ultra-low permeability reservoirs. Therefore, the development of a functional molecular system that can inhibit the hydration and thickening of clay and enhance the flowability of fluid plays a vital role in the development of ultra-low permeability reservoirs.
There has been a large number of studies carried out to resolve the issue of instability posed by clay swelling. Over the past decades, numerous chemicals have been used as clay inhibitors, including inorganic salts, silicates, polymers, organic amines, ammonium compounds and so on. Recently, as one of the new materials as promising clay inhibitors, ionic liquids (ILs) have been widely investigated. ILs usually refer to an organic salt with a melting point below 100 °C, which is a “green chemical substance” [
9]. Luo et al. [
10] revealed that 1-octyl-3-methylimidazole tetrafluoroborate has better shale inhibition than potassium chloride. Yang et al. [
11] also studied 1-vinyl-3-ethylimidazole bromide monomer and its corresponding homopolymers as clay inhibitors, both of which show good inhibition properties. The type of ionic liquids cation or anion groups affects the size, solubility, melting temperature and hydrophilicity/hydrophobicity of clay and correspondingly affects the anti-swelling performance of clay. Yang et al. [
12] evaluated the effect of cationic components on the inhibitory performance of ionic liquids and discussed how the alkyl chain length of vinyl imidazolium ILs affects their inhibitory effect. Experimental results found that with the shortest chain of ethyl, ILs have the best ability to inhibit hydration. Moreover, as the alkyl chain length increases, the inhibition performance of ILs decreases. Khan et al. [
13] studied four different ILs with the same cationic group 1-allyl-3methylimidazole but different anions (bromide, iodide, chloride and dicyanamide) and studied their influence on clay inhibition. The results were that the ILs with different anions reduce the clay swelling and the clay swelling does not strongly depend on the type of anion in imidazolium-based ILs. Xu et al. [
14] found that the ionic liquid 1-octyl-3-methylimidazolium bromide has good shale hydration inhibition properties and it could effectively reduce surface tension even at low concentrations. Jia et al. [
15] studied the inhibitory ability of 1-hexyl-3-methylimidazole bromide (BMH) and 1,2-bis(3-hexylimidazolium-1-yl) ethane bromide (HMH). They demonstrated both BMH and HMH effectively inhibit the shale hydration and swelling. HMH, as a gemini surface active ionic liquid, compared with BMH, was good in entering the interlayer space of clay, lowering the zeta potential, forming a hydrophobic barrier layer and lowering surface tension, which was the reason for its excellent inhibition performance. On this basis, our group devotes to exploring the potential of imidazole-based tetrafluoroborate ILs in improving oil recovery in ultra-low permeability reservoirs, considering not only the nature of the minerals but also the size of fluid aggregates and the interfacial activity.
Therefore, this paper mainly considers the combined effects of imidazole-based tetrafluoroborate ILs with different lengths of alkyl chains on inhibiting sodium bentonite (Na-bent) swelling, reducing water clusters, flocculation performance and lowering interfacial tension. Different test methods, including 17O NMR, molecular dynamics simulation, anti-swelling rate, XRD, SEM, zeta potential, flocculation experiment and interfacial tension, were conducted. In order to expand the application of ILs in improving oil recovery in ultra-low permeability reservoirs, core flooding experiments were carried out. The results showed that the imidazole-based tetrafluoroborate ILs (C8-OMImBF4) is an excellent candidate for the oilfield practice due to its high performance.
2. Results and Discussion
It can be seen from
Figure 1 that ILs with different alkyl chain lengths have a positive anti-swelling effect on Na-bent at high concentration. After centrifugation, the upper liquid of ILs at high dosage is relatively clear. The anti-swelling rates of ILs are shown in
Table 1 and the decreasing order is: (C
2-EMImBF
4, C
3-PMImBF
4, C
4-BMImBF
4) > (C
5-PnImBF
4, C
6-HMImBF
4, C
8-OMImBF
4) > C
10-DMImBF
4 > C
12-MImBF
4.
When Na-bent is immersed in water, due to the action of hydration and electrostatic repulsion, the interlayer spacing of Na-bent is enlarged microscopically and the particles repel each other, so that the mixture of water and Na-bent form a stable suspension. When ILs with a high positive charge density are added, they insert between the layers of Na-bent particles to supplement the positive charge, weakening the electrostatic repulsion between Na-bent layers and reducing the layer spacing, which results in a reduction in the volume of Na-bent on a macroscopic scale. The short alkyl chain is conducive to the solid–liquid separation, further explained by the following discussion.
In order to explain the role of ILs in the hydration and swelling processes of Na-bent, the interlayer spacing (d-spacing) of Na-bent was measured by XRD. The original d-spacing of Na-bent is 12.53 Å and the d-spacing of the fully hydrated clay particles is 19.81 Å. The d-spacing significantly increases due to hydration expansion. After the addition of ILs, the interlayer spacing of hydrated Na-bent is compressed, as shown in
Figure 2a,b, especially for high ILs dosage. The cationic groups of ILs can be adsorbed on the surface of Na-bent through electrostatic interactions and balance the charge distribution, thereby reducing hydration repulsion and interlayer spacing. It can be seen that the d-spacing almost increases with the increase of the alkyl chain length of ILs, because of the size effect. Moreover, at the concentration of 5000 ppm, the d-spacing of Na-bent decreases significantly under the action of C
8-OMImBF
4, compared with lower concentrations, as shown in
Figure A1.
The interlayer spacing of dry Na-bent samples with different concentrations of ILs is shown in
Figure 2c,d, which can quantitatively describe the degree of intercalation of ILs in the middle layer of Na-bent. The interlayer spacing of Na-bent increases to 12.88–13.29 Å at low concentration and increases a little more at high concentration, indicating that the ILs are successfully inserted into the interlayer space. The results are consistent with a previous report [
15]. Moreover, the surface morphology of sodium bentonite after treatment with deionized water and ILs was analyzed by SEM, as shown in
Figure A2. The original surface of bentonite was dense and smooth. The sodium bentonite contacted with deionized water became swollen, disintegrated and then dispersed. After adding ILs, the surface dispersion of sodium bentonite was reduced.
The half-width of
17O NMR relates to the average relative size of the liquid water cluster structure. The wider the spectrum, the larger the cluster; the narrower the spectrum, the smaller the cluster [
16].
Figure 3 shows the half-peak width of the
17O NMR spectra of H
2O and ILs (C
2-EMImBF
4–C
8-OMImBF
4) at different concentrations. Due to the poor solubility of C
10-DMImBF
4 and C
12-MImBF
4 in water at room temperature, their oxygen spectra are not applied. By comparison, the half-width of each selected IL at both low and high concentrations is lower than that of pure water (peak width = 86.1 Hz), indicating that ILs have the ability to reduce water clusters. As the concentration increases, there is a tendency for the half-peak width to decrease, which might be due to the increase in the concentration of BF
4− and the increased polar effect of F
−, resulting in the formation of stronger hydrogen bonds with water than those between water molecules. There is little change in the measured half-value width of ILs with different alkyl chains at the same concentration, indicating that the length of the alkyl chain has little effect on reducing water clusters. For example, the half-widths of C
3-PMImBF
4, C
5-PnImBF
4 and C
6-HMImBF
4 are 49.9 Hz, 51.0 Hz, 49.7 Hz, respectively, which are a bit better than C
2-EMImBF
4 (51.6 Hz), C
4-BMImBF
4 (51.8 Hz) and C
8-OMImBF
4 (51.6 Hz).
The ionic liquids of C
2-VEImBr are reported to have excellent ability to inhibit hydration in the past study [
11]. In comparison, we also tested the
17O-NMR half-peak width of C
2-VEImBr and they are 102 Hz and 95.5 Hz at low and high concentrations, respectively. The values are much bigger than that of the tetrafluoroborate ILs series, indicating the weak ability of C
2-VEImBr in reducing water molecular clusters. The
17O NMR spectra of H
2O, C
2-EMImBF
4–C
8-OMImBF
4 and C
2-VEImBr are also shown in
Figure A3.
To further reveal the interaction mechanism between ILs and water molecules, the kinetic energy was calculated through MD simulation, as shown in
Figure 4. It is concluded that several hydrogen bonds form between the BF
4− group and water molecules (see inset
Figure 4), resulting in disassembling big water clusters into small ones. In comparison, the C
2-VEImBr molecule hardly forms hydrogen bonds with water molecules, mainly because of the good hydration cooperation between C
2-VEImBr and water molecules and, therefore, the network structure of water cluster cannot be destroyed. The results support the conclusion from
17O NMR results.
As shown in
Figure 5a, when ILs are added to the Na-bent dispersion system, obvious flocculent precipitation is observed compared with pure water. After 30 min, as shown in
Figure 5b, the suspension is clearer, especially for C
2-EMImBF
4–C
8-OMImBF
4 systems at high concentration. The flocculation performance of C
8-OMImBF
4 at high concentration is the best in our study. The reason for the rapid flocculation of ILs comes from the high positive charge density and hydrophobicity.
The surface of Na-bent particles is negatively charged due to isostructural substitution, which means that cations are required to achieve charge balance. When the ILs molecules are adsorbed onto the surface of solid particles, the negative charge on the surface of bentonite particles is neutralized and the electrostatic repulsion force is reduced. It is reported in the literature that an absolute value of zeta potential higher than 30 mV will ensure the stability of the colloidal system [
17]. The zeta potential of Na-bent suspension in deionized water is -33.6 mV, showing good stability. The zeta potential of ILs with different alkyl chains at a high concentration are shown in
Figure A4, which shows that as the alkyl chain length increases the ability of ILs to inhibit the double electron layer of the Na-bent suspension increases, except for C
10-DMImBF
4 and C
12-MImBF
4. The zeta potential almost reaches the isoelectric point (IEP) at high concentrations of C
8-OMImBF
4. As shown in
Figure A5, the zeta potential is lower than the isoelectric point when the concentration is lower than the high concentration of C
8-OMImBF
4. At the isoelectric point (IEP), a relatively fast sedimentation behavior can be observed. On the one hand, after imidazole cations are adsorbed on the surface of particles due to the action of charge, the hydrophobic effect of the extended hydrophobic tail chain is conducive to solid–liquid separation. On the other hand, when ILs with high charge density interact with colloid particles with opposite charge, there will be charge patch effect, so charge patch is also one of the additional gravitational effects. The heterogeneous particle surfaces caused by charge patches generate electrostatic attraction to each other, which intensifies the solid–liquid separation process. In addition, C
10-DMImBF
4 and C
12-MImBF
4 reverse the zeta potential to +15.1 mV and +21.8 mV, respectively. This might be because the IL with positive charge is adsorbed on the surface of bentonite particles and the charge is neutralized, making the potential from negative to positive.
The interfacial tensions of C6-HMImBF4, C8-OMImBF4, C10-DMImBF4, C12-MImBF4 ILs with oil were 14.40, 9.38, 5.40, 0.61 mN/m, respectively. This addition of ILs significantly reduced the interfacial tension of the oil–water interface, which was beneficial to emulsify the crude oil into an emulsion, aggregate and form an oil zone and change the fluidity of the crude oil.
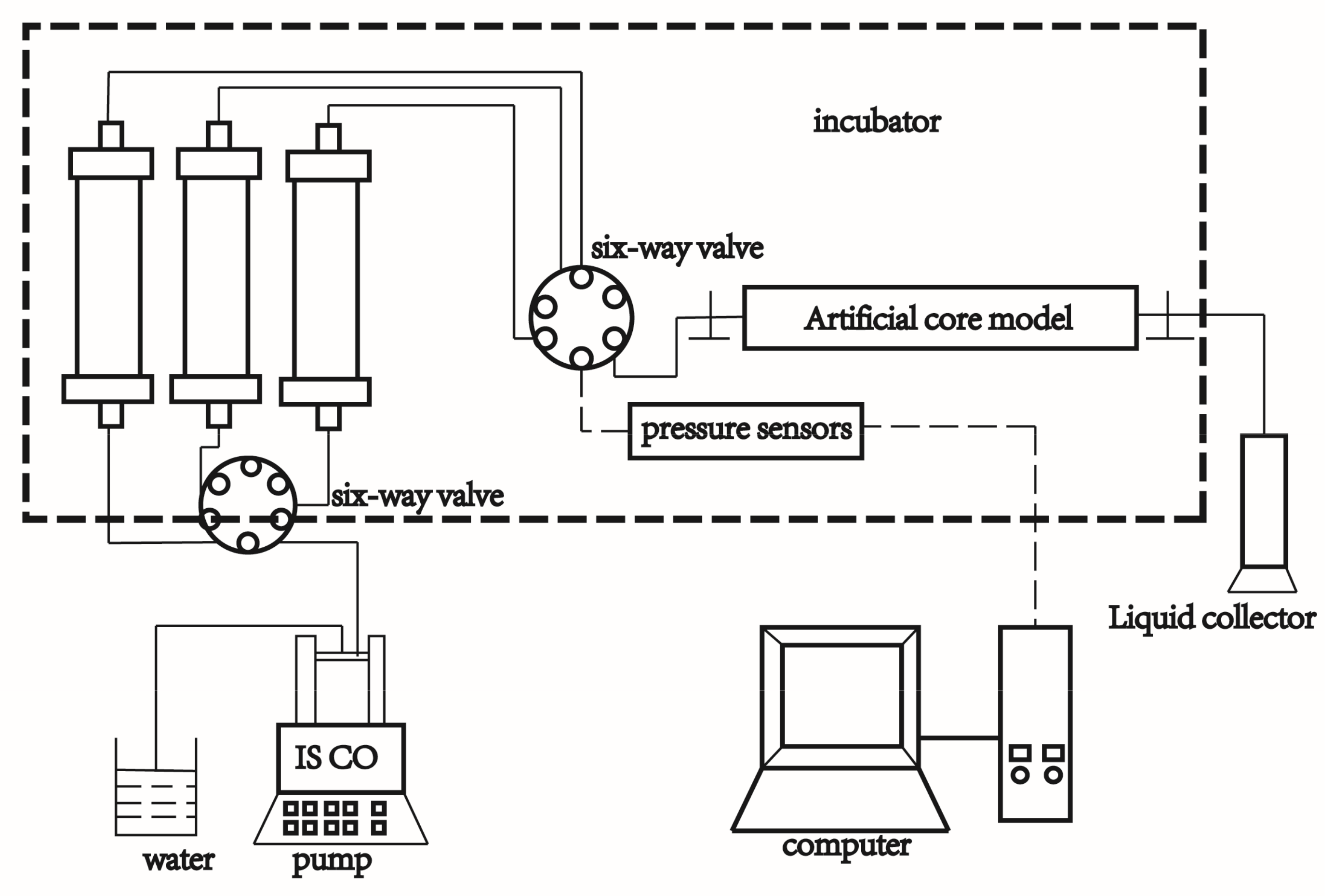
Combining the abilities of decreasing water cluster size, reducing clay swelling and enhancing flocculation, C
8-OMImBF
4 with higher oil–water interfacial activity was selected to perform the oil displacement experiment in the condition of an ultra-low permeability core. The composition and salinity of Xing Hebei Chang-6 simulate formation water is shown in
Appendix A,
Table A1. The parameters of the three cores used in the experiment are shown in
Table A2. The results of injection pressure and recovery ratio are shown in
Figure 6. During the displacement process, the injection pressure first increased, and reached the maximum pressure, and then decreased. Meanwhile, the recovery ratio was recorded until no oil was produced. The enhanced oil recovery is 10.2%, 12.1%, 12.6%, respectively, after injecting 1, 3, 5 PV C
8-OMImBF
4 agents.
C
8-OMImBF
4 with imidazole cation structure is easily adsorbed onto clay through electrostatic interaction, which can compensate the negative sites of Na-bent and compress the electrical double layer. Moreover, ILs can insert into the interlayer space of Na-bent and expel some interlayer water molecules. Hence, the trend of Na-bent in water adsorbing can be reduced. At the same time, ILs are capable of leading the Na-bent to be more hydrophobic, thereby a hydrophobic barrier that restricts water intrusion forms. Because of the special structure and properties of C
8-OMImBF
4, they effectively reduce the clusters of water molecules and favor the fluidity through tiny channels. Moreover, C
8-OMImBF
4 significantly reduces the interfacial tension of the oil–water interface (
Figure A3), which is beneficial when interacting with crude oil and forming an oil disperse system. Altogether, ILs with distinguishing molecular structure and characteristics will help to develop a new guide for practical applications.