Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases
Abstract
:1. Introduction
2. Extracellular Vesicles
2.1. The Molecular Content of EVs
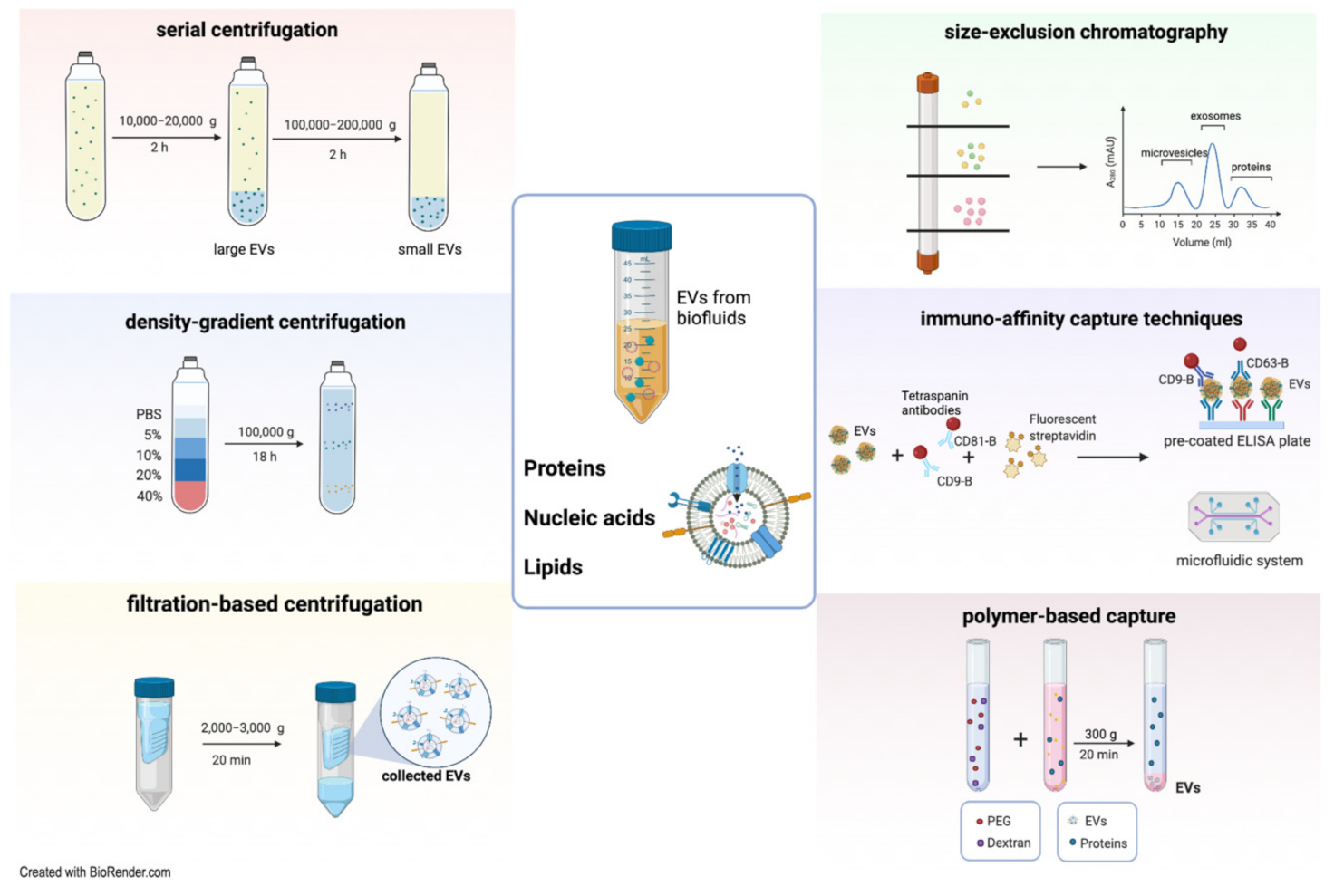
2.2. Methods of EV Isolation
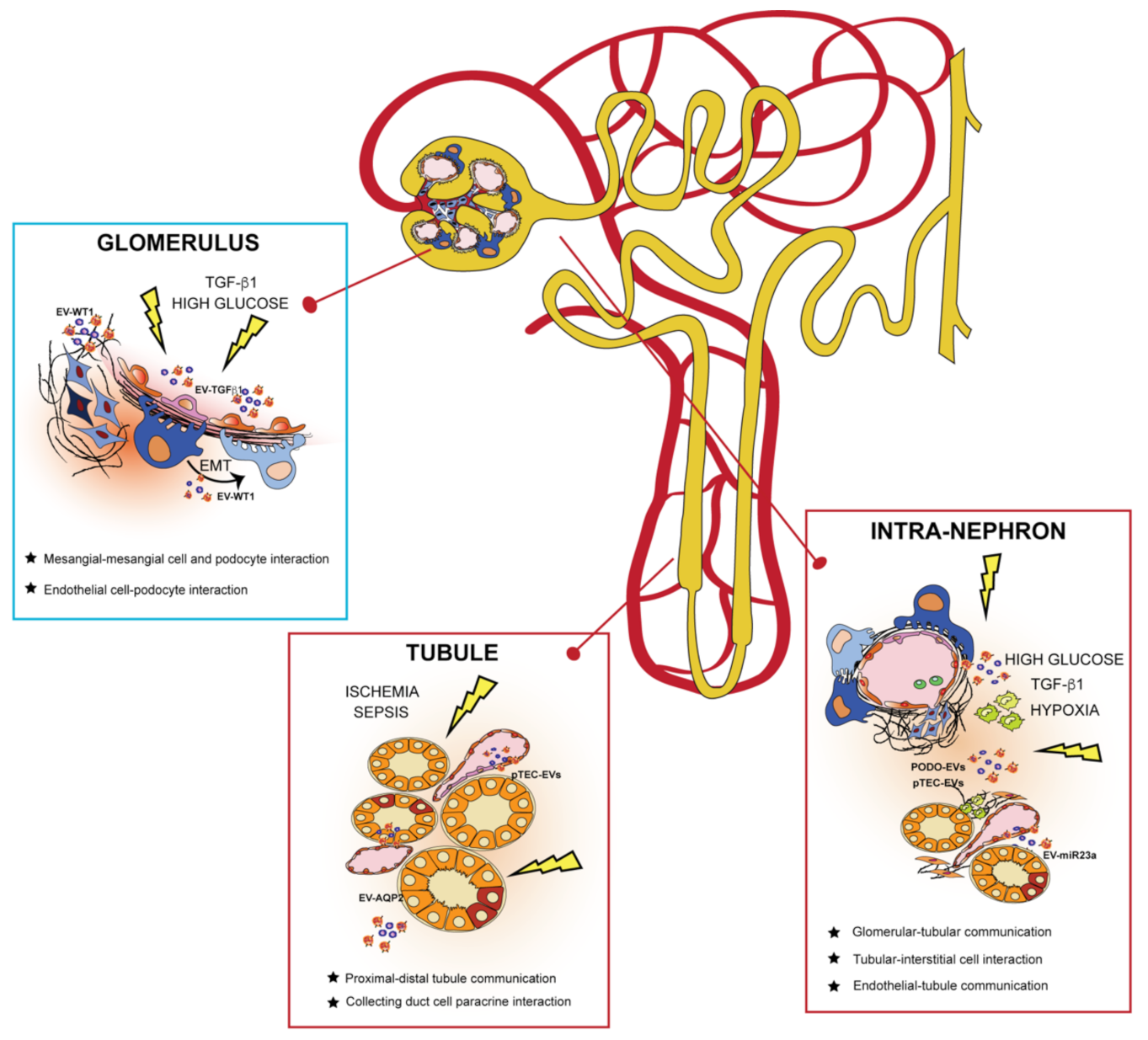
3. EVs in the Kidney: Mechanism of Cell-to-Cell Communication
3.1. EVs and Glomerulus
3.2. EVs and Kidney Tubules
3.3. EVs in Communication from Different Nephron Sections
3.4. Non-Kidney Cell EVs in Intra-Renal Communication
4. uEVs as Biomarkers for Personalized Medicine
5. uEVs as a Liquid Biopsy in Primary Kidney Diseases
5.1. Nephrotic Syndrome
5.2. Nephritic Syndrome
5.3. Ciliopathies
5.4. Tubulopathies
6. uEVs as a Liquid Biopsy in Secondary Kidney Diseases
6.1. Diabetic Nephropathy
6.2. Obstructive Nephropathy
7. EVs in Clinics: Perspectives and Limitations
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Taal, M.; Chertow, G.; Marsden, P.; Skorecki, K.; Yu, A.; Brenner, B. Brenner and Rector’s The Kidney, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gounden, V.; Vashisht, R.; Jialal, I. Hypoalbuminemia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sirolli, V.; Pieroni, L.; Di Liberato, L.; Urbani, A.; Bonomini, M. Urinary Peptidomic Biomarkers in Kidney Diseases. Int. J. Mol. Sci. 2019, 21, 96. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.O.; Lerman, L.O. Urinary microRNA in kidney disease: Utility and roles. Am. J. Physiol. Ren. Physiol. 2019, 316, F785–F793. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [CrossRef]
- Campion, C.G.; Sanchez-Ferras, O.; Batchu, S.N. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can. J. Kidney Health Dis. 2017, 4. [Google Scholar] [CrossRef]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzás, E.I.; Toth, E.A.; Sodar, B.W.; Szabo-Taylor, K.E. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 2018, 40, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ståhl, A.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.R.; Devarajan, P. Proteomic analysis of acute kidney injury: Biomarkers to mechanisms. Proteom. Clin. Appl. 2011, 5, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Lázaro, D.; Hernández, J.L.G.; García, A.C.; Martínez, A.C.; Mielgo-Ayuso, J.; Cruz-Hernández, J.J. Liquid biopsy as novel tool in precision medicine: Origins, properties, identification and clinical perspective of cancer’s biomarkers. Diagnostics 2020, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdbrügger, U.; Le, T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, T.; Myette, R.L.; Almeida, J.R.; Silva, A.A.; Burger, D. Extracellular vesicles: Cell-derived biomarkers of glomerular and tubular injury. Cell. Physiol. Biochem. 2020, 54, 88–109. [Google Scholar] [CrossRef] [Green Version]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Sapet, C.; Simoncini, S.; Loriod, B.; Puthier, D.; Sampol, J.; Nguyen, C.; Dignat-George, F.; Anfosso, F. Thrombin-induced endothelial microparticle generation: Identification of a novel pathway involving ROCK-II activation by caspase-2. Blood 2006, 108, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Combes, V.; Simon, A.C.; Grau, G.E.; Arnoux, D.; Camoin, L.; Sabatier, F.; Mutin, M.; Sanmarco, M.; Sampol, J.; Dignat-George, F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J. Clin. Invest. 1999, 104, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.E.; Tan, H.S.; Datta, A.; Lai, R.C.; Zhang, H.; Meng, W.; Lim, S.K.; Sze, S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteom. 2010, 9, 1085–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tati, R.; Kristoffersson, A.-C.; Ståhl, A.-L.; Rebetz, J.; Wang, L.; Licht, C.; Motto, D.; Karpman, D. Complement activation associated with ADAMTS13 deficiency in human and murine thrombotic microangiopathy. J. Immunol. 2013, 191, 2184–2193. [Google Scholar] [CrossRef] [Green Version]
- Collino, F.; Lopes, J.A.; Corrêa, S.; Abdelhay, E.; Takiya, C.M.; Wendt, C.H.C.; de Miranda, K.R.; Vieyra, A.; Lindoso, R.S. Adipose-Derived Mesenchymal Stromal Cells Under Hypoxia: Changes in Extracellular Vesicles Secretion and Improvement of Renal Recovery after Ischemic Injury. Cell. Physiol. Biochem. 2019, 52, 1463–1483. [Google Scholar] [CrossRef] [PubMed]
- Hyland, M.; Mennan, C.; Wilson, E.; Clayton, A.; Kehoe, O. Pro-Inflammatory Priming of Umbilical Cord Mesenchymal Stromal Cells Alters the Protein Cargo of Their Extracellular Vesicles. Cells 2020, 9, 726. [Google Scholar] [CrossRef] [Green Version]
- Borges, F.T.; Melo, S.A.; Ozdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H.; LeBleu, V.S.; Kalluri, R. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trino, S.; Lamorte, D.; Caivano, A.; De Luca, L.; Sgambato, A.; Laurenzana, I. Clinical relevance of extracellular vesicles in hematological neoplasms: From liquid biopsy to cell biopsy. Leukemia 2021, 35, 661–678. [Google Scholar] [CrossRef]
- Brenner, A.W.; Su, G.H.; Momen-Heravi, F. Isolation of Extracellular Vesicles for Cancer Diagnosis and Functional Studies. Methods Mol. Biol. 2019, 1882, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLOS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef] [PubMed]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Agrahari, V.; Agrahari, V.; Burnouf, P.-A.; Chew, C.H.; Burnouf, T. Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol. 2019, 37, 707–729. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Gangoda, L.; Liem, M.; Fonseka, P.; Atukorala, I.; Ozcitti, C.; Mechler, A.; Adda, C.G.; Ang, C.-S.; Mathivanan, S. Proteogenomic analysis reveals exosomes are more oncogenic than ectosomes. Oncotarget 2015, 6, 15375–15396. [Google Scholar] [CrossRef] [Green Version]
- Dear, J.W.; Street, J.M.; Bailey, M.A. Urinary exosomes: A reservoir for biomarker discovery and potential mediators of intrarenal signalling. Proteomics 2013, 13, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.; Zietse, R.; Hoorn, E.J. Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am. J. Physiol. Ren. Physiol. 2014, 306, 1251–1259. [Google Scholar] [CrossRef] [Green Version]
- Burger, D.; Thibodeau, J.F.; Holterman, C.E.; Burns, K.D.; Touyz, R.M.; Kennedy, C.R.J. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J. Am. Soc. Nephrol. 2014, 25, 1401–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Kajiyama, H.; Tsuji, T.; Hu, X.; Leelahavanichkul, A.; Vento, S.; Frank, R.; Kopp, J.B.; Trachtman, H.; Star, R.A.; et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am. J. Physiol. Ren. Physiol. 2013, 305, F553–F559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.K.; Han, K.H.; Lee, S.E.; Kim, S.H.; Kang, H.G.; Cheong, H. Il Urinary exosomal WT1 in childhood nephrotic syndrome. Pediatr. Nephrol. 2012, 27, 317–320. [Google Scholar] [CrossRef] [PubMed]
- De Laval, P.; Mobarrez, F.; Almquist, T.; Vassil, L.; Fellström, B.; Soveri, I. Acute effects of haemodialysis on circulating microparticles. Clin. Kidney J. 2019, 12, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.C.; Kuro-o, M.; Moe, O.W. The emerging role of Klotho in clinical nephrology. Nephrol. Dial. Transplant. 2012, 27, 2650–2657. [Google Scholar] [CrossRef]
- Pefanis, E.; Wang, J.; Rothschild, G.; Lim, J.; Kazadi, D.; Sun, J.; Federation, A.; Chao, J.; Elliott, O.; Liu, Z.-P.; et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell 2015, 161, 774–789. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.L.; Cao, Y.H.; Pan, M.M.; Liu, H.; Tang, R.N.; Ma, K.L.; Chen, P.S.; Liu, B.C. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clin. Chim. Acta 2014, 428, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary exosomal miRNA signature in type II diabetic nephropathy patients. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.-L.; Cao, Y.-H.; Ni, H.-F.; Xu, M.; Liu, D.; Liu, H.; Chen, P.-S.; Liu, B.-C. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Ren. Physiol. 2013, 305, F1220–F1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichii, O.; Otsuka-Kanazawa, S.; Horino, T.; Kimura, J.; Nakamura, T.; Matsumoto, M.; Toi, M.; Kon, Y. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS ONE 2014, 9, 1–12. [Google Scholar] [CrossRef]
- Malkin, E.Z.; Bratman, S. V Bioactive DNA from extracellular vesicles and particles. Cell Death Dis. 2020, 11, 584. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [Green Version]
- Junker, K.; Heinzelmann, J.; Beckham, C.; Ochiya, T.; Jenster, G. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur. Urol. 2016, 70, 323–331. [Google Scholar] [CrossRef]
- Wang, D.; Sun, W. Urinary extracellular microvesicles: Isolation methods and prospects for urinary proteome. Proteomics 2014, 14, 1922–1932. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of extracellular vesicles: Determining the correct approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Rood, I.M.; Deegens, J.K.J.; Merchant, M.L.; Tamboer, W.P.M.; Wilkey, D.W.; Wetzels, J.F.M.; Klein, J.B. Comparison of three methods for isolation of urinary microvesicles to identify biomarkers of nephrotic syndrome. Kidney Int. 2010, 78, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Cantin, R.; Diou, J.; Bélanger, D.; Tremblay, A.M.; Gilbert, C. Discrimination between exosomes and HIV-1: Purification of both vesicles from cell-free supernatants. J. Immunol. Methods 2008, 338, 21–30. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.-J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef] [PubMed]
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Molecular Biotechnology 2021, 63, 249–266. [Google Scholar] [CrossRef]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.B.; Knepper, M.A.; Yuen, P.S.T.; Star, R.A. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liga, A.; Vliegenthart, A.D.B.; Oosthuyzen, W.; Dear, J.W.; Kersaudy-Kerhoas, M. Exosome isolation: A microfluidic road-map. Lab. Chip 2015, 15, 2388–2394. [Google Scholar] [CrossRef] [Green Version]
- Zeringer, E.; Barta, T.; Li, M.; Vlassov, A. V Strategies for isolation of exosomes. Cold Spring Harb. Protoc. 2015, 2015, 319–323. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and Technologies for Exosome Isolation and Characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteom. 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Morales-Kastresana, A.; Musich, T.A.; Welsh, J.A.; Telford, W.; Demberg, T.; Wood, J.C.S.; Bigos, M.; Ross, C.D.; Kachynski, A.; Dean, A.; et al. High-fidelity detection and sorting of nanoscale vesicles in viral disease and cancer. J. Extracell. Vesicles 2019, 8, 1597603. [Google Scholar] [CrossRef] [Green Version]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheinani, A.H.; Vögeli, M.; Baumgartner, U.; Vassella, E.; Draeger, A.; Burkhard, F.C.; Monastyrskaya, K. Improved isolation strategies to increase the yield and purity of human urinary exosomes for biomarker discovery. Sci. Rep. 2018, 8, 3945. [Google Scholar] [CrossRef] [PubMed]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; la Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, B.; Zhu, Y.; Ni, J.; Ruan, J.; Thompson, J.; Malouf, D.; Bucci, J.; Graham, P.; Li, Y. Quality Assessment and Comparison of Plasma-Derived Extracellular Vesicles Separated by Three Commercial Kits for Prostate Cancer Diagnosis. Int. J. Nanomed. 2020, 15, 10241–10256. [Google Scholar] [CrossRef]
- Onodi, Z.; Pelyhe, C.; Terezia Nagy, C.; Brenner, G.B.; Almasi, L.; Kittel, A.; Mancek-Keber, M.; Ferdinandy, P.; Buzas, E.I.; Giricz, Z. Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography from Blood Plasma. Front. Physiol. 2018, 9, 1479. [Google Scholar] [CrossRef] [Green Version]
- Mussack, V.; Wittmann, G.; Pfaffl, M.W. Comparing small urinary extracellular vesicle purification methods with a view to RNA sequencing-Enabling robust and non-invasive biomarker research. Biomol. Detect. Quantif. 2019, 17, 100089. [Google Scholar] [CrossRef]
- Alvarez, M.L.; Khosroheidari, M.; Kanchi Ravi, R.; di Stefano, J.K. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012, 82, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Pisitkun, T.; Shen, R.-F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 13368–13373. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.S.; Li, Y.; Dai, C.; Kiss, L.P.; Wu, C.; Liu, Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010, 78, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.-L.; Feng, Y.; Tang, T.-T.; Liu, B.-C. New insight into the role of extracellular vesicles in kidney disease. J. Cell. Mol. Med. 2019, 23, 731–739. [Google Scholar] [CrossRef]
- Hill, N.; Michell, D.L.; Sheng, Q.; Pusey, C.; Vickers, K.C.; Woollard, K.J. Glomerular endothelial derived vesicles mediate podocyte dysfunction: A potential role for miRNA. PLoS ONE 2020, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prunotto, M.; Farina, A.; Lane, L.; Pernin, A.; Schifferli, J.; Hochstrasser, D.F.; Lescuyer, P.; Moll, S. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J. Proteom. 2013, 82, 193–229. [Google Scholar] [CrossRef]
- da Silva Novaes, A.; Borges, F.T.; Maquigussa, E.; Varela, V.A.; Dias, M.V.S.; Boim, M.A. Influence of high glucose on mesangial cell-derived exosome composition, secretion and cell communication. Sci. Rep. 2019, 9, 6270. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Y.; Xu, L.; Dang, W.; Yan, H.; Zou, D.; Zhu, Z.; Luo, L.; Tian, N.; Wang, X.; et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci. Rep. 2017, 7, 9371. [Google Scholar] [CrossRef] [Green Version]
- Sun, I.O.; Lerman, L.O. Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics 2020, 10, 311. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-C.; Tang, T.-T.; Lv, L.-L.; Lan, H.-Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Rigalli, J.P.; Barros, E.R.; Sommers, V.; Bindels, R.J.M.; Hoenderop, J.G.J. Novel Aspects of Extracellular Vesicles in the Regulation of Renal Physiological and Pathophysiological Processes. Front. Cell Dev. Biol. 2020, 8, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gildea, J.J.; Seaton, J.E.; Victor, K.G.; Reyes, C.M.; Bigler Wang, D.; Pettigrew, A.C.; Courtner, C.E.; Shah, N.; Tran, H.T.; Van Sciver, R.E.; et al. Exosomal transfer from human renal proximal tubule cells to distal tubule and collecting duct cells. Clin. Biochem. 2014, 47, 89–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Street, J.M.; Birkhoff, W.; Menzies, R.I.; Webb, D.J.; Bailey, M.A.; Dear, J.W. Exosomal transmission of functional aquaporin 2 in kidney cortical collecting duct cells. J. Physiol. 2011, 589, 6119–6127. [Google Scholar] [CrossRef]
- Gracia, T.; Wang, X.; Su, Y.; Norgett, E.E.; Williams, T.L.; Moreno, P.; Micklem, G.; Frankl, F.E.K. Urinary exosomes contain microRNAs capable of paracrine modulation of tubular transporters in kidney. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Panich, T.; Chancharoenthana, W.; Somparn, P.; Issara-Amphorn, J.; Hirankarn, N.; Leelahavanichkul, A. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrol. 2017, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, S.; Pasha, M.; Agouni, A.; Munusamy, S. Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury. Biomolecules 2019, 9, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkonda, M.N.; Akbari, S.; Landry, C.; Sun, S.; Xiao, F.; Turner, M.; Holterman, C.E.; Nasrallah, R.; Hébert, R.L.; Kennedy, C.R.J.; et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J. Extracell. Vesicles 2018, 7, 1432206. [Google Scholar] [CrossRef] [PubMed]
- Okada, H. A new look at tubulointerstitial communication with exosomes. J. Am. Soc. Nephrol. 2013, 24, 330–332. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-L.; Lv, L.-L.; Tang, T.-T.; Wang, B.; Feng, Y.; Zhou, L.-T.; Cao, J.-Y.; Tang, R.-N.; Wu, M.; Liu, H.; et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019, 95, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kim, E.; Bae, Y.-U.; Yang, W.M.; Lee, H.; Kim, H.; Noh, H.; Han, D.C.; Ryu, S.; Kwon, S.H. microRNA in Extracellular Vesicles Released by Damaged Podocytes Promote Apoptosis of Renal Tubular Epithelial Cells. Cells 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martínez, A.B.; Torija, A.V.; Carracedo, J.; Ramirez, R.; de Lucio-Cazaña, F.J. Microparticles released by vascular endothelial cells increase hypoxia inducible factor expression in human proximal tubular HK-2 cells. Int. J. Biochem. Cell Biol. 2014, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Mitra, A.K.; Lengyel, E.; Peter, M.E. MicroRNAs as mediators and communicators between cancer cells and the tumor microenvironment. Oncogene 2015, 34, 5857–5868. [Google Scholar] [CrossRef] [Green Version]
- Eyre, J.; Burton, J.O.; Saleem, M.A.; Mathieson, P.W.; Topham, P.S.; Brunskill, N.J. Monocyte- and endothelial-derived microparticles induce an inflammatory phenotype in human podocytes. Nephron. Exp. Nephrol. 2011, 119, e58–e66. [Google Scholar] [CrossRef]
- Singhto, N.; Thongboonkerd, V. Exosomes derived from calcium oxalate-exposed macrophages enhance IL-8 production from renal cells, neutrophil migration and crystal invasion through extracellular matrix. J. Proteom. 2018, 185, 64–76. [Google Scholar] [CrossRef]
- Masaoutis, C.; Al Besher, S.; Koutroulis, I.; Theocharis, S. Exosomes in Nephropathies: A Rich Source of Novel Biomarkers. Dis. Markers 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, N.S.; Salajegheh, A.; Gaspar, H.; Brett, P.O. Improving early OSV design robustness by applying ‘Multivariate Big Data Analytics’ on a ship’s life cycle. J. Ind. Inf. Integr. 2018, 10, 29–38. [Google Scholar] [CrossRef]
- Svenningsen, P.; Sabaratnam, R.; Jensen, B.L. Urinary extracellular vesicles: Origin, role as intercellular messengers and biomarkers; efficient sorting and potential treatment options. Acta Physiol. 2020, 228, e13346. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zhou, J.; Zhang, Y. Urinary exosomal MIR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Wang, C.; Yu, H.; Ding, M.; Zhang, C.; Lu, X.; Zhang, C.Y.; Zhang, C. Increased urinary exosomal microRNAs in children with idiopathic nephrotic syndrome. EBioMedicine 2019, 39, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, C.D.; Park, S.H.; Hwang, D.; et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2011, 11, 2459–2475. [Google Scholar] [CrossRef]
- Feng, Y.; Lv, L.-L.; Wu, W.-J.; Li, Z.-L.; Chen, J.; Ni, H.-F.; Zhou, L.-T.; Tang, T.-T.; Wang, F.-M.; Wang, B.; et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. Am. J. Pathol. 2018, 188, 2542–2552. [Google Scholar] [CrossRef] [Green Version]
- Perez-Hernandez, J.; Martinez-Arroyo, O.; Ortega, A.; Galera, M.; Solis-Salguero, M.A.; Chaves, F.J.; Redon, J.; Forner, M.J.; Cortes, R. Urinary exosomal miR-146a as a marker of albuminuria, activity changes and disease flares in lupus nephritis. J. Nephrol. 2020. [Google Scholar] [CrossRef]
- Sole, C.; Cortes-Hernandez, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. MIR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Tangtanatakul, P.; Klinchanhom, S.; Sodsai, P.; Sutichet, T.; Promjeen, C.; Avihingsanon, Y.; Hirankarn, N. Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac. J. Allergy Immunol. 2019, 37, 189–197. [Google Scholar] [CrossRef]
- Stokman, M.F.; Bijnsdorp, I.V.; Schelfhorst, T.; Pham, T.V.; Piersma, S.R.; Knol, J.C.; Giles, R.H.; Bongers, E.M.H.F.; Knoers, N.V.A.M.; Lilien, M.R.; et al. Changes in the urinary extracellular vesicle proteome are associated with nephronophthisis-related ciliopathies. J. Proteom. 2019, 192, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.C.; Manganelli, L.; Woollard, J.R.; Masyuk, A.I.; Masyuk, T.V.; Tammachote, R.; Huang, B.Q.; Leontovich, A.A.; Beito, T.G.; Madden, B.J.; et al. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 2009, 20, 278–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalani, A.; Mohan, A.; Godbole, M.M.; Bhatia, E.; Gupta, A.; Sharma, R.K.; Tiwari, S. Wilm’s Tumor-1 Protein Levels in Urinary Exosomes from Diabetic Patients with or without Proteinuria. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Kong, Q.; Guo, X.; Guo, Z.; Su, T. Urinary exosome miR-424 and miR-218 as biomarkers for type 1 diabetes in children. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef]
- Ghai, V.; Wu, X.; Bheda-Malge, A.; Argyropoulos, C.P.; Bernardo, J.F.; Orchard, T.; Galas, D.; Wang, K. Genome-wide Profiling of Urinary Extracellular Vesicle microRNAs Associated With Diabetic Nephropathy in Type 1 Diabetes. Kidney Int. Rep. 2018, 3, 555–572. [Google Scholar] [CrossRef] [Green Version]
- Trnka, P.; Ivanova, L.; Hiatt, M.J.; Matsell, D.G. Article Urinary Biomarkers in Obstructive Nephropathy. Clin. J. Am. Soc. Nephrol. 2012, 7, 1567–1575. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zhang, W.; Luo, M.; Qin, X.; Yang, F.; Wei, Q. iTRAQ-based proteomics and in vitro experiments reveals essential roles of ACE and AP-N in the renin–angiotensin system-mediated congenital ureteropelvic junction obstruction. Exp. Cell Res. 2020, 393, 112086. [Google Scholar] [CrossRef]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Santorelli, L.; Morello, W.; Barigazzi, E.; Capitoli, G.; Tamburello, C.; Ghio, L.; Crapella, B.; Galimberti, S.; Montini, G.; Pitto, M.; et al. Urinary Extracellular Vesicle Protein Profiles Discriminate Different Clinical Subgroups of Children with Idiopathic Nephrotic Syndrome. Diagnostics 2021, 11, 456. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Liu, M.L. Extracellular vesicles and lupus nephritis—New insights into pathophysiology and clinical implications. J. Autoimmun. 2020, 115, 102540. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.T.; Ostergaard, O.; Rekvig, O.P.; Sturfelt, G.; Jacobsen, S.; Heegaard, N.H.H. Galectin-3 binding protein links circulating microparticles with electron dense glomerular deposits in lupus nephritis. Lupus 2015, 24, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Perez-Hernandez, J.; Cortes, R. Extracellular vesicles as biomarkers of systemic lupus erythematosus. Dis. Markers 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef]
- Zuo, X.; Lobo, G.; Fulmer, D.; Guo, L.; Dang, Y.; Su, Y.; Ilatovskaya, D.V.; Nihalani, D.; Rohrer, B.; Body, S.C.; et al. The exocyst acting through the primary cilium is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis. J. Biol. Chem. 2019, 294, 6710–6718. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 2002, 13, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Barr, M.M. Cell–cell communication via ciliary extracellular vesicles: Clues from model systems. Essays Biochem. 2018, 62, 205–213. [Google Scholar] [CrossRef]
- Hogan, M.C.; Bakeberg, J.L.; Gainullin, V.G.; Irazabal, M.V.; Harmon, A.J.; Lieske, J.C.; Charlesworth, M.C.; Johnson, K.L.; Madden, B.J.; Zenka, R.M.; et al. Identification of biomarkers for PKD1 using urinary exosomes. J. Am. Soc. Nephrol. 2015, 26, 1661–1670. [Google Scholar] [CrossRef] [Green Version]
- Gerlach, J.Q.; Krüger, A.; Gallogly, S.; Hanley, S.A.; Hogan, M.C.; Ward, C.J.; Joshi, L.; Griffin, M.D. Surface Glycosylation Profiles of Urine Extracellular Vesicles. PLoS ONE 2013, 8, e74801. [Google Scholar] [CrossRef]
- Besouw, M.T.P.; Kleta, R.; Bockenhauer, D. Bartter and Gitelman syndromes: Questions of class. Pediatr. Nephrol. 2020, 35, 1815–1824. [Google Scholar] [CrossRef] [Green Version]
- Koulouridis, E.; Koulouridis, I. Molecular pathophysiology of Bartter’s and Gitelman’s syndromes. World J. Pediatr. 2015, 11, 113–125. [Google Scholar] [CrossRef]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary Excretion of Aquaporin-2 in Patients with Diabetes Insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef]
- Kotnik, P.; Battelino, T.; Debeljak, M.; Podkrajsek, K.T. Correlation Between AVPR2 Mutations and Urinary AQP2 Excretion in Patients with Nephrogenic Diabetes Insipidus. Artic. J. Pediatr. Endocrinol. Metab. 2007. [Google Scholar] [CrossRef]
- Abe, H.; Sakurai, A.; Ono, H.; Hayashi, S.; Yoshimoto, S.; Ochi, A.; Ueda, S.; Nishimura, K.; Shibata, E.; Tamaki, M.; et al. Urinary Exosomal mRNA of WT1 as Diagnostic and Prognostic Biomarker for Diabetic Nephropathy. J. Med. Invest. 2018, 65, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, A.; De Souza, A.; Konijeti, R.; Baskin, L.S. The anatomy and embryology of posterior urethral valves. J. Urol. 2006, 175, 1214–1220. [Google Scholar] [CrossRef]
- Chevalier, R.L. Congenital Urinary Tract Obstruction: The Long View. Adv. Chronic Kidney Dis. 2015, 22, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Cully, M. Exosome-based candidates move into the clinic. Nat. Rev. Drug Discov. 2020, 20, 6–7. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Sallustio, F.; Schena, F.P. Clinical Application of Human Urinary Extracellular Vesicles in Kidney and Urologic Diseases. Int. J. Mol. Sci. 2016, 17, 1043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuen, P.S.T.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Lin, G.; Zhu, Y.; Duan, W.; Jin, D. Emerging technologies for profiling extracellular vesicle heterogeneity. Lab. Chip 2020, 20, 2423–2437. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; He, S.; Chen, C.; Yan, X. Flow Cytometric Analysis of Nanoscale Biological Particles and Organelles. Annu. Rev. Anal. Chem. 2019, 12, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Gandham, S.; Su, X.; Wood, J.; Nocera, A.L.; Alli, S.C.; Milane, L.; Zimmerman, A.; Amiji, M.; Ivanov, A.R. Technologies and Standardization in Research on Extracellular Vesicles. Trends Biotechnol. 2020, 38, 1066–1098. [Google Scholar] [CrossRef] [PubMed]
| Exosomes | Microvesicles | |
|---|---|---|
| Size (nm) | 20–150 | 100–1000 |
| Origin | MVB fusion with plasma membrane | Outward blebbing of plasma membrane |
| Release process | Constitutive/Cellular Activation | Constitutive/Cellular Activation |
| Pathways | Tetraspanins-dependent | Cytoskeleton reorganization- and Ca2+-dependent |
| Composition | ||
| Proteins | CD9, CD63, CD81, Alix, TSG101, Rabs, annexins, MHC-II, CD86, signaling-, oncogenic-, integrin-, adhesion molecules, flotillins, Hsp70 and Hsp90 | Annexins, flotillins, Alix, TSG101, CD40, ARF-6, selectins, phosphatidylserine, Rho family members, MMP2, ADAM, GAPDH, pyruvate kinase, proteins localized to centrosome, nucleolus, cytoplasm and mitochondria |
| Nucleic Acids | mRNAs, miRNAs, lncRNAs, mtDNA, gDNA, nDNA, dsDNA | mRNAs, miRNAs, gDNA |
| Isolation Method | UC, DG-UC, filtration, immune- affinity, PEG precipitation, SEC | UC, DG-UC, filtration, SEC |
| Disease | No. of Patient | Target | uEV Isolation | Detection Method | Main Results | Ref. |
|---|---|---|---|---|---|---|
| 25 patients and 5 HD | WT1 | Differential UC | WB | -WT1 was increased FSGS patients compared with healthy volunteers or SSNS patients. -Urinary exosomal WT1 was decreased in patients in remission for either FSGS or SSNS or following steroid treatment in six SSNS subjects. | H. Zhou, 2013 [36] | |
| Nephrotic syndrome (NS) | 40 | WT1 | Differential UC | WB | -WT1 was detected in 25 patients (62.5%). -No significant correlation between WT1 amount and steroid responsiveness or renal pathological condition was found. | H. Lee, 2012 [37] |
| 13 | miR-193a | -ExoQuick exosome precipitation (System Biosciences) | -qRT-PCR -ROC analysis -WB | -miR-193a was higher in children with primary FSGS than those in children with MCNS. | Z. Huang, 2017 [98] | |
| 129 NS children and 126 age-/sex-matched HD. | miR-194-5p, miR-146b-5p, miR-378a-3p, miR-23b-3p and miR-30a-5p | UC | -High-throughput Illumina sequencing -qRT-PCR | -These 5 miRNAs were increased in NS and markedly reduced during the clinical remission period. -The concentrations of miR-194-5p and miR-23b-3p were positively correlated with the urine protein content and were markedly higher in the high urine protein group than in the low urine protein group. | T. Chen, 2019 [99] | |
| Glomerulonephritis (GN) | 12 IgAN, 12 TBMN patients and 6 HD | ANPEP, VASN, A1AT and CP | Differential UC | Label-free LC-MS/MS | -These four proteins are biomarkers to distinguish between early IgAN from TBMN. | P. G. Moon, 2011 [100] |
| 55 IgAN patients and 24 HD | CCL2 mRNA | Differential UC | qRT-PCR | - CCL2 was specifically expressed in uEVs of patients with GN compared to healthy controls. - Exosomal CCL2 was correlated with tubulointerstitial inflammation and C3 deposition in GN patients. | Y. Feng, 2018 [101] | |
| Lupus nephritis (LN) | 13 LN patients and 8 HD | miR-26a | Differential UC | qRT-PCR | -miR-26a levels in urinary exosomes were higher compared with healthy control. | O. Ichii, 2014 [46] |
| 41 SLE patients, 27 LN and 20 HD | miR-146a | UC | qRT-PCR | -Compared to controls, urinary level of miR-146a was higher in SLE patients. | J. Perez-Hernandez, 2020 [102] | |
| 32 s patients with biopsy-proven LN, 15 non-lupus CKD and 20 HD | miR-29-c | Differential UC | qRT-PCR | -miR-29c correlated with the degree of renal chronicity but not with renal function. -MiR-29c expression levels could predict the degree of chronicity in patients with LN. | C. Solé, 2015 [103] | |
| 31 patients | let-7a and miR-21 | UC | qRT-PCR | -Urinary exosome-associated miRNA, let-7a and miR-21, could be used to guide the clinical stage of LN patients and possibly plays a role in epigenetic regulation of the kidney during the disease. | P. Tangtanatakul, 2019 [104] | |
| Ciliopathies | 12 ciliopathy patients and 12 age- and gender-matched HD | 156 differentially expressed proteins | Differential UC | -Electro-phoresis -In-depth label-free LC-MS/MS proteomics analysis | -The most overexpressed or downregulated proteins in uEVs (VCAN, DPEP1 and FAT4) correlated with nephronophthisis-related ciliopathies. | M.F. Stokman, 2019 [105] |
| 1 ADPKD patient and multiple HD | PC1, PC2, TMEM2 | -Differential UC - DG-UC | Gel electro-phoresis | In patients with PKD1 mutations, levels of PC1 and PC2 were reduced. -TMEM2 was higher in individuals with PKD1 mutations. -The PC1/TMEM2 ratio correlated inversely with height-adjusted total kidney volume in the discovery cohort. -The ratio of PC1/TMEM2 or PC2/TMEM2 could be used to distinguish individuals with PKD1 mutations from controls in a confirmation cohort. | M.C. Hogan, 2009 [106] | |
| Diabetic nephropathy (DN) | 48 type-1 diabetes mellitus patients and 25 HD | WT1 | UC | WB | -WT1 expression in in uEVs was higher than in controls. -WT1 levels were associated with an increase in urine protein-to-creatinine ratio, albumin-to-creatinine ratio, and serum creatinine as well as a decline in eGFR. | A. Kalani, 2013 [107] |
| 30 HD and 30 T1D | miR-424 and miR-218 | combined centrifugation | RT-qPCR | Association of urinary exosomal level of miR-424 and miR-218 with renal damage in T1D patients. | Q. Kong, 2019 [108] | |
| 48 | uEV miRNAs | qEV size exclusion columns (Izon Science) | NGS | Identification of a set of miRNAs with concentration changes associated with DN occurrence, microalbuminuria status, and other variables. | V. Ghai, 2018 [109] | |
| Obstructive nephropathy | 27 patients and 20 HD | AQP2 TGFβ-1 L1CAM | Centrifugation | Immuno-blotting | In boys with PUV, AQP2, TGFβ-1 and L1CAM correlated with eGFR. | P. Trnka, 2012 [110] |
| 3 UPJO and 3 healthy fetusis | 633 differentially expressed proteins were identified in the amniotic fluid-derived exosomes from patients with UPJO. | Exosome Extraction Kit | iTRAQ quantitative proteomic profiles | - ACE and AP-N were significantly decreased in the amniotic fluid exosomes of women with a fetus diagnosed UPJO. - They correlate with suppressed cell proliferation, elevated ROS production, and increased pro-inflammatory cytokine levels in tubular cells. | R. Liu, 2020 [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cricrì, G.; Bellucci, L.; Montini, G.; Collino, F. Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases. Int. J. Mol. Sci. 2021, 22, 6507. https://doi.org/10.3390/ijms22126507
Cricrì G, Bellucci L, Montini G, Collino F. Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases. International Journal of Molecular Sciences. 2021; 22(12):6507. https://doi.org/10.3390/ijms22126507
Chicago/Turabian StyleCricrì, Giulia, Linda Bellucci, Giovanni Montini, and Federica Collino. 2021. "Urinary Extracellular Vesicles: Uncovering the Basis of the Pathological Processes in Kidney-Related Diseases" International Journal of Molecular Sciences 22, no. 12: 6507. https://doi.org/10.3390/ijms22126507






