Dysregulated Expression of Arterial MicroRNAs and Their Target Gene Networks in Temporal Arteries of Treatment-Naïve Patients with Giant Cell Arteritis
Abstract
:1. Introduction
2. Results
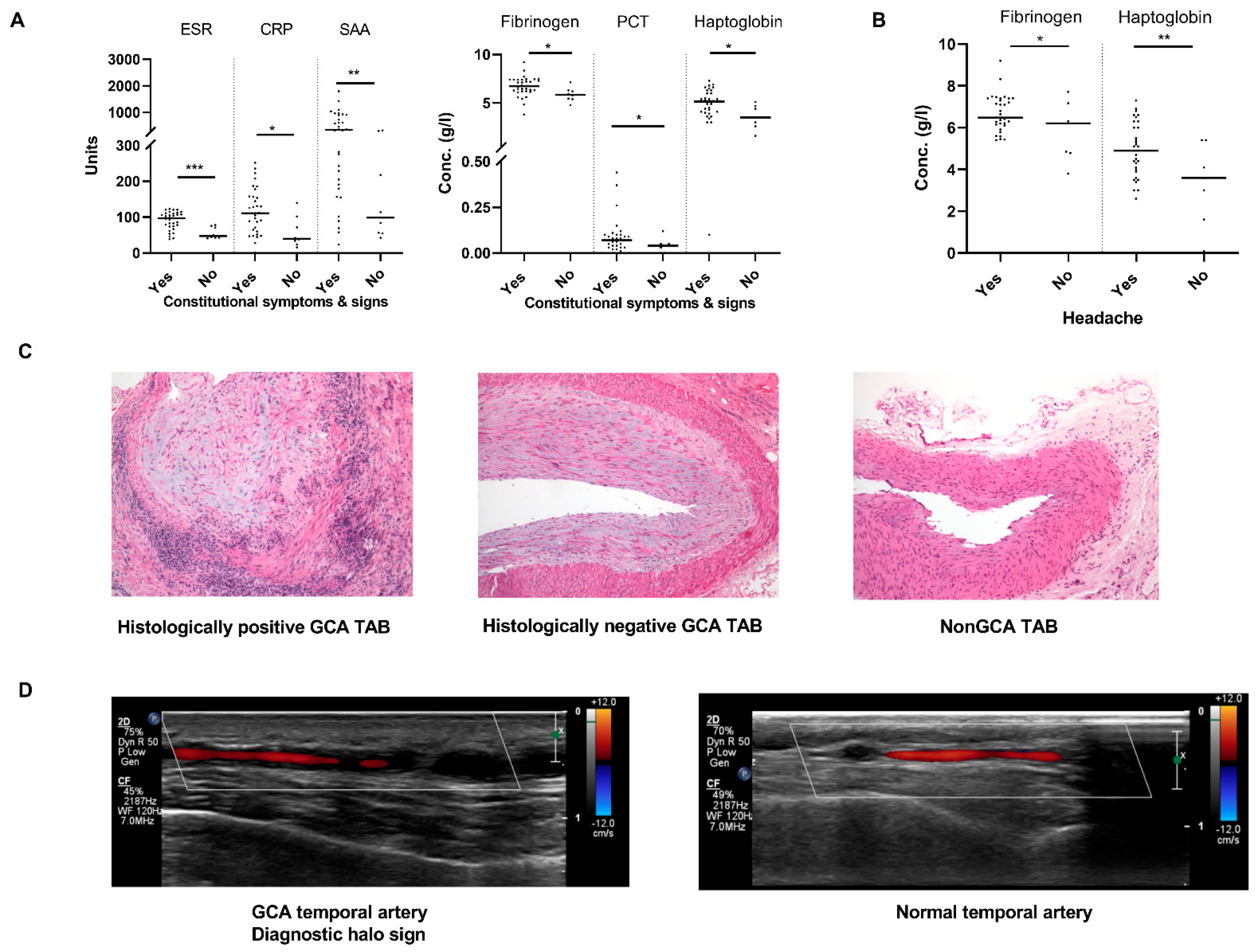
2.1. Patient Characteristics and Establishment of GCA Diagnosis
2.2. Sonographic Pattern of GCA Affected Arteries Associates with Ischaemic Complications
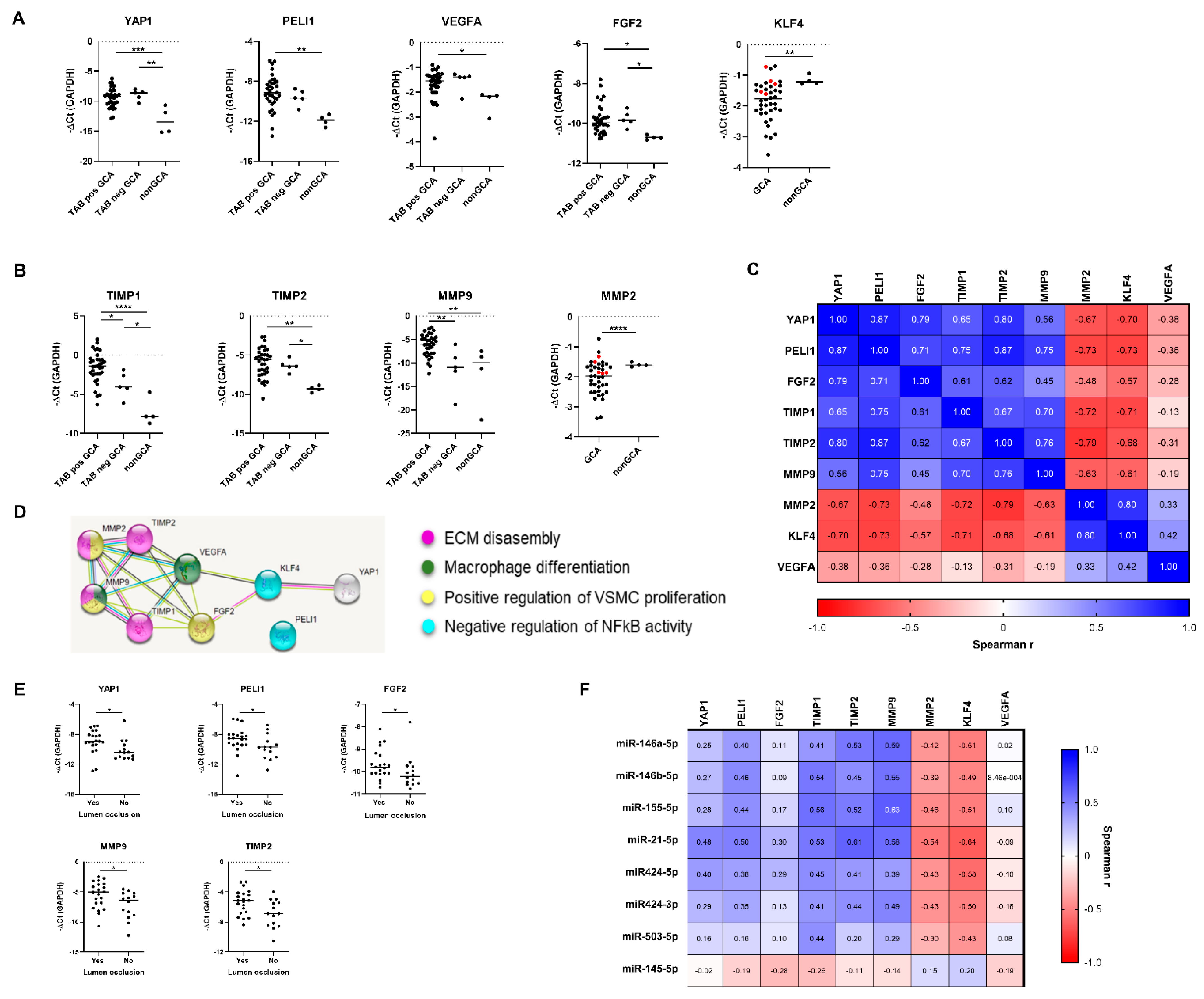
2.3. MiR-424-3p and miR-503-5p Are Novel Deregulated miRs in GCA TABs
2.4. Deregulated miRs Exhibit a Highly Interrelated Expression Signature in GCA TABs
2.5. Analysis of miR-Target Genes Identifies PELI1, YAP1 and KLF4 as New Deregulated Genes in GCA TABs
2.6. Deregulated miR-Target Genes and Matrix Remodelling Genes Constitute an Interrelated Gene Network in GCA Arteries
2.7. Correlative Analysis of miR-mRNA Expression Identifies KLF4 as Candidate Target Gene of Deregulated miR Network in GCA TABs
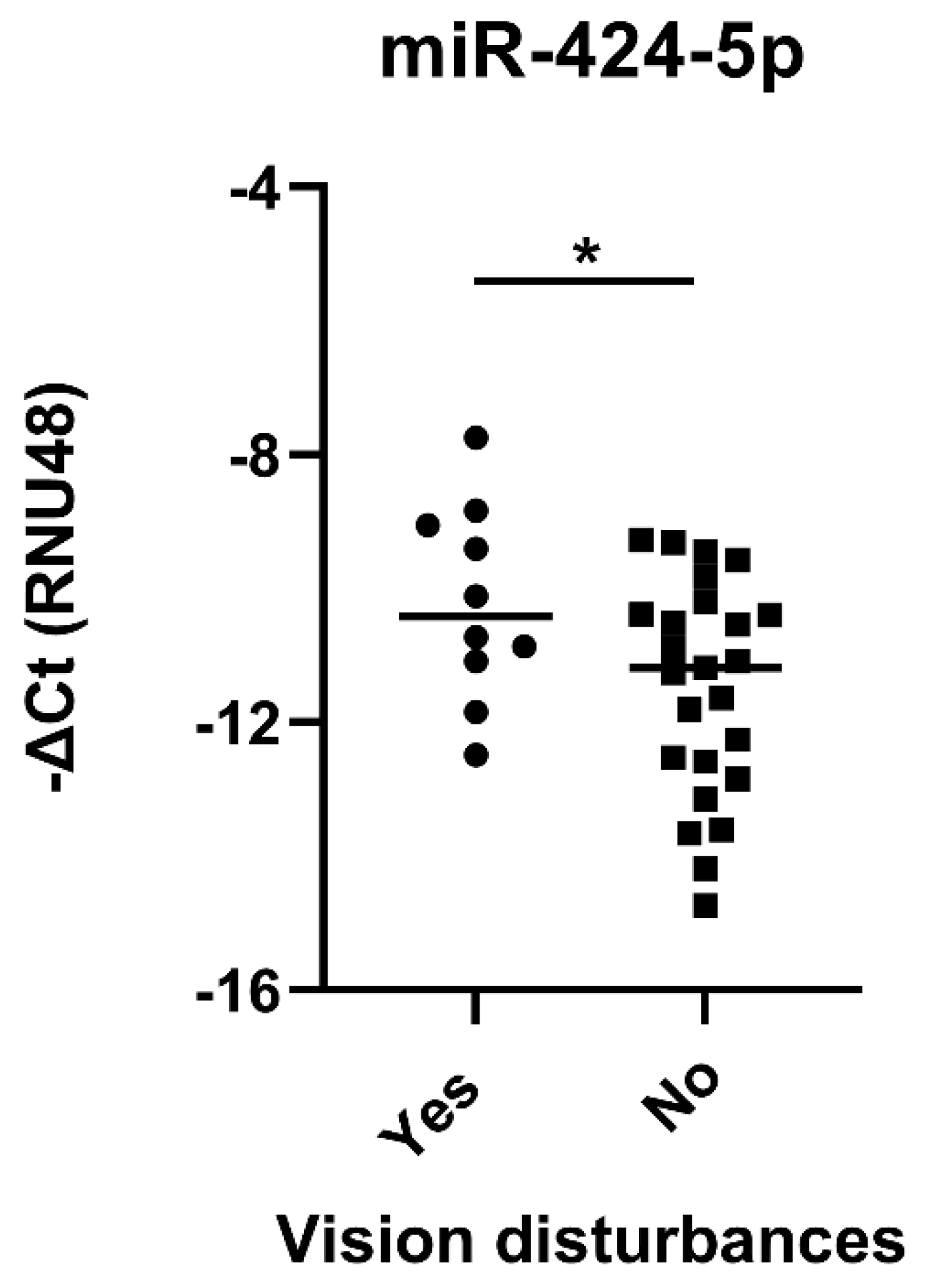
2.8. Temporal Artery Expression of miR-424-5p Is Higher in GCA Patients with Visual Impairment
3. Discussion
4. Materials and Methods
4.1. Study Design, Study Subjects and Clinical Data Collection
4.2. Laboratory Parameters
4.3. Temporal Artery Biopsy
4.4. Arterial Ultrasound Imaging
4.5. RNA Isolation
4.6. MiR Expression Analysis
4.7. MiR-Target Prediction and Selection of Target Genes
4.8. MRNA Expression Analysis
4.9. STRING Protein-Protein Network Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GCA | Giant cell arteritis |
| GC | Glucocorticoids |
| TAB | Temporal artery biopsy |
| TA | Temporal artery |
| miR | MicroRNA |
| qPCR | Quantitative polymerase chain reaction |
| PELI1 | Pellino E3 ubiquitin protein ligase 1 |
| YAP1 | Yes-associated protein 1 |
| KLF4 | Kruppel-like factor 4 |
| MYOCD | Myocardin |
| PDCD4 | Programmed cell death protein 4 |
| SMURF2 | SMAD specific E3 ubiquitin protein ligase 2 |
| MMP | Matrix metalloproteinase |
| TIMP | Tissue inhibitor of matrix metalloproteinase |
| FGF2 | Fibroblast growth factor 2 |
| VEGFA | Vascular endothelial growth factor A |
| CDS | Colour Doppler ultrasound examination |
| ESR | Erythrocyte sedimentation rate |
| CRP | C-reactive protein |
| SAA | Serum amyloid A |
| VSMC | Vascular smooth muscle cell |
| Ct | Threshold cycle |
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Salvarani, C.; Pipitone, N.; Versari, A.; Hunder, G.G. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat. Rev. Rheumatol. 2012, 8, 509–521. [Google Scholar] [CrossRef]
- Soriano, A.; Muratore, F.; Pipitone, N.; Boiardi, L.; Cimino, L.; Salvarani, C. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat. Rev. Rheumatol. 2017, 13, 476–484. [Google Scholar] [CrossRef]
- Burja, B.; Feichtinger, J.; Lakota, K.; Thallinger, G.G.; Sodin-Semrl, S.; Kuret, T.; Rotar, Z.; Jese, R.; Zigon, P.; Cucnik, S.; et al. Utility of serological biomarkers for giant cell arteritis in a large cohort of treatment-naive patients. Clin. Rheumatol. 2019, 38, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Oh, L.J.; Wong, E.; Gill, A.J.; McCluskey, P.; Smith, J.E.H. Value of temporal artery biopsy length in diagnosing giant cell arteritis. ANZ J. Surg. 2018, 88, 191–195. [Google Scholar] [CrossRef]
- Poller, D.N.; van Wyk, Q.; Jeffrey, M.J. The importance of skip lesions in temporal arteritis. J. Clin. Pathol. 2000, 53, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowling, K.; Rait, J.; Atkinson, J.; Srinivas, G. Temporal artery biopsy in the diagnosis of giant cell arteritis: Does the end justify the means? Ann. Med. Surg. 2017, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Monti, S.; Floris, A.; Ponte, C.; Schmidt, W.A.; Diamantopoulos, A.P.; Pereira, C.; Piper, J.; Luqmani, R. The use of ultrasound to assess giant cell arteritis: Review of the current evidence and practical guide for the rheumatologist. Rheumatology 2018, 57, 227–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyand, C.M.; Goronzy, J.J. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N. Engl. J. Med. 2014, 371, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Younge, B.R.; Olshen, R.A.; Goronzy, J.J.; Weyand, C.M. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation 2010, 121, 906–915. [Google Scholar] [CrossRef] [Green Version]
- Weyand, C.M.; Tetzlaff, N.; Bjornsson, J.; Brack, A.; Younge, B.; Goronzy, J.J. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997, 40, 19–26. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, Y.; Berry, G.; Shahram, F.; Li, Y.; Watanabe, R.; Liao, Y.J.; Goronzy, J.J.; Weyand, C.M. The microvascular niche instructs T cells in large vessel vasculitis via the VEGF-Jagged1-Notch pathway. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Goronzy, J.J.; Berry, G.; Liao, Y.J.; Weyand, C.M. Giant Cell Arteritis: From Pathogenesis to Therapeutic Management. Curr. Treatm. Opt. Rheumatol. 2016, 2, 126–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piggott, K.; Biousse, V.; Newman, N.J.; Goronzy, J.J.; Weyand, C.M. Vascular damage in giant cell arteritis. Autoimmunity 2009, 42, 596–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samson, M.; Corbera-Bellalta, M.; Audia, S.; Planas-Rigol, E.; Martin, L.; Cid, M.C.; Bonnotte, B. Recent advances in our understanding of giant cell arteritis pathogenesis. Autoimmun. Rev. 2017, 16, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pla, A.; Bosch-Gil, J.A.; Rossello-Urgell, J.; Huguet-Redecilla, P.; Stone, J.H.; Vilardell-Tarres, M. Metalloproteinase-2 and -9 in giant cell arteritis: Involvement in vascular remodeling. Circulation 2005, 112, 264–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciccia, F.; Rizzo, A.; Ferrante, A.; Guggino, G.; Croci, S.; Cavazza, A.; Salvarani, C.; Triolo, G. New insights into the pathogenesis of giant cell arteritis. Autoimmun. Rev. 2017, 16, 675–683. [Google Scholar] [CrossRef]
- Garo, L.P.; Murugaiyan, G. Contribution of MicroRNAs to autoimmune diseases. Cell. Mol. Life Sci. 2016, 73, 2041–2051. [Google Scholar] [CrossRef]
- Sato, F.; Tsuchiya, S.; Meltzer, S.J.; Shimizu, K. MicroRNAs and epigenetics. FEBS J. 2011, 278, 1598–1609. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Q.; Yu, J.; Rao, Y.; Kou, Z.; Fang, G.; Shi, X.; Liu, W.; Han, H. A Systematic Way to Infer the Regulation Relations of miRNAs on Target Genes and Critical miRNAs in Cancers. Front Genet. 2020, 11, 278. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A brief review on the mechanisms of miRNA regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Coit, P.; Direskeneli, H.; Sawalha, A.H. An update on the role of epigenetics in systemic vasculitis. Curr. Opin. Rheumatol. 2018, 30, 4–15. [Google Scholar] [CrossRef]
- Ouboussad, L.; Hunt, L.; Hensor, E.M.A.; Nam, J.L.; Barnes, N.A.; Emery, P.; McDermott, M.F.; Buch, M.H. Profiling microRNAs in individuals at risk of progression to rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croci, S.; Zerbini, A.; Boiardi, L.; Muratore, F.; Bisagni, A.; Nicoli, D.; Farnetti, E.; Pazzola, G.; Cimino, L.; Moramarco, A.; et al. MicroRNA markers of inflammation and remodelling in temporal arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1527–1533. [Google Scholar] [CrossRef]
- Bolha, L.; Pizem, J.; Frank-Bertoncelj, M.; Hocevar, A.; Tomsic, M.; Jurcic, V. Identification of microRNAs and their target gene networks implicated in arterial wall remodelling in giant cell arteritis. Rheumatology 2020, 59, 3540–3552. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G. microRNAs Distinctively Regulate Vascular Smooth Muscle and Endothelial Cells: Functional Implications in Angiogenesis, Atherosclerosis, and In-Stent Restenosis. Adv. Exp. Med. Biol. 2015, 887, 53–77. [Google Scholar] [CrossRef] [Green Version]
- Nejad, C.; Stunden, H.J.; Gantier, M.P. A guide to miRNAs in inflammation and innate immune responses. FEBS J. 2018, 285, 3695–3716. [Google Scholar] [CrossRef]
- Sun, L.L.; Li, W.D.; Lei, F.R.; Li, X.Q. The regulatory role of microRNAs in angiogenesis-related diseases. J. Cell. Mol. Med. 2018, 22, 4568–4587. [Google Scholar] [CrossRef] [Green Version]
- Sonkoly, E.; Stahle, M.; Pivarcsi, A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Semin. Cancer Biol. 2008, 18, 131–140. [Google Scholar] [CrossRef]
- Wang, F.; Liang, R.; Tandon, N.; Matthews, E.R.; Shrestha, S.; Yang, J.; Soibam, B.; Yang, J.; Liu, Y. H19X-encoded miR-424(322)/-503 cluster: Emerging roles in cell differentiation, proliferation, plasticity and metabolism. Cell. Mol. Life Sci. 2019, 76, 903–920. [Google Scholar] [CrossRef]
- Friedman, Y.; Naamati, G.; Linial, M. MiRror: A combinatorial analysis web tool for ensembles of microRNAs and their targets. Bioinformatics 2010, 26, 1920–1921. [Google Scholar] [CrossRef]
- Kuret, T.; Burja, B.; Feichtinger, J.; Thallinger, G.G.; Frank-Bertoncelj, M.; Lakota, K.; Zigon, P.; Sodin-Semrl, S.; Cucnik, S.; Tomsic, M.; et al. Gene and miRNA expression in giant cell arteritis-a concise systematic review of significantly modified studies. Clin. Rheumatol. 2019, 38, 307–316. [Google Scholar] [CrossRef]
- Rodriguez-Pla, A.; Martinez-Murillo, F.; Savino, P.J.; Eagle, R.C., Jr.; Seo, P.; Soloski, M.J. MMP-12, a novel matrix metalloproteinase associated with giant cell arteritis. Rheumatology 2009, 48, 1460–1461. [Google Scholar] [CrossRef] [Green Version]
- Segarra, M.; Garcia-Martinez, A.; Sanchez, M.; Hernandez-Rodriguez, J.; Lozano, E.; Grau, J.M.; Cid, M.C. Gelatinase expression and proteolytic activity in giant-cell arteritis. Ann. Rheum. Dis. 2007, 66, 1429–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, G.; Zamboni, F.; Tice, A.; Farci, P. Integrated ordination of miRNA and mRNA expression profiles. BMC Genomics 2015, 16, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Zhao, Y.; Yang, S.; Zhang, H.; Chen, F. Integrative analysis of miRNA-mRNA and miRNA-miRNA interactions. Biomed. Res. Int. 2014, 2014, 907420. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Wendlandt, E.; Galle, C.S.; Keck, K.; McCaffrey, A.P. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 298, G535–G541. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Xiong, S.; Jiang, P.; Liu, R.; Liu, X.; Qian, J.; Zheng, X.; Chu, Y. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: A novel anti-inflammation mechanism. Free Radic. Biol. Med. 2012, 52, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Walker, E.A.; Kissane, S.; Khan, I.; Murray, P.I.; Rauz, S.; Wallace, G.R. Gene expression and miR profiles of human corneal fibroblasts in response to dexamethasone. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7282–7288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, T.E.; Kis-Toth, K.; Szanto, A.; Tsokos, G.C. Glucocorticoids suppress T cell function by up-regulating microRNA-98. Arthritis Rheum. 2013, 65, 1882–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hocevar, A.; Jese, R.; Tomsic, M.; Rotar, Z. Risk factors for severe cranial ischaemic complications in giant cell arteritis. Rheumatology 2020, 59, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Chean, C.S.; Prior, J.A.; Helliwell, T.; Belcher, J.; Mackie, S.L.; Hider, S.L.; Liddle, J.; Mallen, C.D. Characteristics of patients with giant cell arteritis who experience visual symptoms. Rheumatol. Int. 2019, 39, 1789–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czihal, M.; Tschaidse, J.; Bernau, C.; Lottspeich, C.; Kohler, A.; Dechant, C.; Schulze-Koops, H.; Hoffmann, U.; Mackert, M.J.; Thurau, S. Ocular ischaemic complications in giant cell arteritis: CHADS2-score predicts risk of permanent visual impairment. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S117), 61–64. [Google Scholar] [PubMed]
- Xie, C.; Guo, Y.; Zhu, T.; Zhang, J.; Ma, P.X.; Chen, Y.E. Yap1 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J. Biol. Chem. 2012, 287, 14598–14605. [Google Scholar] [CrossRef] [Green Version]
- Kimura, T.E.; Duggirala, A.; Smith, M.C.; White, S.; Sala-Newby, G.B.; Newby, A.C.; Bond, M. The Hippo pathway mediates inhibition of vascular smooth muscle cell proliferation by cAMP. J. Mol. Cell. Cardiol. 2016, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhou, L.; Ling, L.; Meng, X.; Chu, F.; Zhang, S.; Zhou, F. The Crosstalk Between Hippo-YAP Pathway and Innate Immunity. Front Immunol. 2020, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.; Han, M.; Wen, J.K. Role of Kruppel-like factor 4 in phenotypic switching and proliferation of vascular smooth muscle cells. IUBMB Life 2010, 62, 132–139. [Google Scholar] [CrossRef]
- Sweet, D.R.; Fan, L.; Hsieh, P.N.; Jain, M.K. Kruppel-Like Factors in Vascular Inflammation: Mechanistic Insights and Therapeutic Potential. Front Cardiovasc. Med. 2018, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.; Molloy, E.S. The role of toll like receptors in giant cell arteritis. Rheumatology 2016, 55, 1921–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanczyk, J.; Pedrioli, D.M.; Brentano, F.; Sanchez-Pernaute, O.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; Kyburz, D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Miyaki, S.; Okubo, A.; Hashimoto, M.; Nishida, K.; Ochi, M.; Asahara, H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008, 58, 1284–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonkoly, E.; Wei, T.; Janson, P.C.; Saaf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007, 2, e610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, L.A.; Sheedy, F.J.; McCoy, C.E. MicroRNAs: The fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 2011, 11, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [Green Version]
- Sheedy, F.J.; Palsson-McDermott, E.; Hennessy, E.J.; Martin, C.; O’Leary, J.J.; Ruan, Q.; Johnson, D.S.; Chen, Y.; O’Neill, L.A. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Murphy, M.; Zhou, H.; Li, X. E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses. Immunol. Rev. 2015, 266, 109–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moynagh, P.N. The roles of Pellino E3 ubiquitin ligases in immunity. Nat. Rev. Immunol. 2014, 14, 122–131. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.Y.; Li, B.; Tian, X.Y.; Chen, L.J.; Huang, Y.; Liu, J.; Deng, D.; Lau, C.W.; Wan, S.; et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 2016, 540, 579–582. [Google Scholar] [CrossRef]
- Bertero, T.; Oldham, W.M.; Cottrill, K.A.; Pisano, S.; Vanderpool, R.R.; Yu, Q.; Zhao, J.; Tai, Y.; Tang, Y.; Zhang, Y.Y.; et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016, 126, 3313–3335. [Google Scholar] [CrossRef] [Green Version]
- Bertero, T.; Cottrill, K.A.; Lu, Y.; Haeger, C.M.; Dieffenbach, P.; Annis, S.; Hale, A.; Bhat, B.; Kaimal, V.; Zhang, Y.Y.; et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015, 13, 1016–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Liu, P.; Zhou, X.; Li, M.T.; Li, F.L.; Wang, Z.; Meng, Z.; Sun, Y.P.; Yu, Y.; Xiong, Y.; et al. Thromboxane A2 Activates YAP/TAZ Protein to Induce Vascular Smooth Muscle Cell Proliferation and Migration. J. Biol. Chem. 2016, 291, 18947–18958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Pachera, E.; Assassi, S.; Salazar, G.A.; Stellato, M.; Renoux, F.; Wunderlin, A.; Blyszczuk, P.; Lafyatis, R.; Kurreeman, F.; de Vries-Bouwstra, J.; et al. Long noncoding RNA H19X is a key mediator of TGF-beta-driven fibrosis. J. Clin. Investig. 2020, 130, 4888–4905. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Xu, S.; Xu, Y.; Little, P.J.; Jin, Z.G. Targeting Mechanosensitive Transcription Factors in Atherosclerosis. Trends Pharmacol. Sci. 2019, 40, 253–266. [Google Scholar] [CrossRef]
- Fang, Y.; Davies, P.F. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Mochizuki, N. Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell Cycle 2017, 16, 1893–1901. [Google Scholar] [CrossRef]
- Yuan, Y.; Park, J.; Feng, A.; Awasthi, P.; Wang, Z.; Chen, Q.; Iglesias-Bartolome, R. YAP1/TAZ-TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting KLF4 activity. Nat. Commun. 2020, 11, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Wei, J.; Wang, Y.; Qu, D.; Zhang, J. miR-21 regulates immunosuppression mediated by myeloid-derived suppressor cells by impairing RUNX1-YAP interaction in lung cancer. Cancer Cell Int. 2020, 20, 495. [Google Scholar] [CrossRef]
- Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Stevens, M.B.; Arend, W.P.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990, 33, 1122–1128. [Google Scholar] [CrossRef]
- Chrysidis, S.; Duftner, C.; Dejaco, C.; Schafer, V.S.; Ramiro, S.; Carrara, G.; Scire, C.A.; Hocevar, A.; Diamantopoulos, A.P.; Iagnocco, A.; et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: A study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018, 4, e000598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | GCA (n = 41) |
|---|---|
| Clinical symptoms/signs, n (%) | |
| Symptom duration (days), median (Q25–Q75) | 30 (21–60) |
| Constitutive symptoms/signs | 33/41 (81) |
| Fatigue | 21/41 (51) |
| Fever | 16/41 (39) |
| Weight loss | 24/41 (59) |
| Sweating | 13/41 (32) |
| Myalgia | 5/41 (12) |
| Arthralgia | 1/41 (2) |
| New onset headache | 34/41 (83) |
| Jaw claudication | 26/41 (63) |
| Visual disturbances | 11/41 (27) |
| Permanent vision loss | 7/41 (17) |
| Polymyalgia rheumatica | 6/41 (15) |
| GCA relapse | 10/41 (24) |
| Temporal artery biopsy, n (%) | |
| Transmural inflammation | 35/41 (85) |
| Lumen occlusion | 21/41 (51) |
| Arterial ultrasound, n (%) | |
| Temporal artery—halo sign | 39/41 (95) |
| Temporal artery wall thickness (cm), median (Q25–Q75) | 0.069 (0.055–0.090) |
| Occipital artery | 19/41 (46) |
| Vertebral artery | 4/41 (10) |
| Extended cranial arteritis (≥2 arteries) | 28/41 (68) |
| Large vessel vasculitis | 8/41 (20) |
| Carotid artery | 6/41 (15) |
| Subclavian artery | 6/41 (15) |
| Axillary artery | 5/41 (12) |
| Systemic inflammation markers, median (Q25–Q75) | |
| ESR (mm/h) | 83 (69–108) |
| CRP (g/L) | 102 (48–150) |
| SAA (mg/L) | 294 (124–699) |
| Ferritin (g/L) | 281 (196–525) |
| Fibrinogen (g/L) | 6.4 (6.0–7.4) |
| Haptoglobin (g/L) | 4.8 (3.6–5.9) |
| Procalcitonin (g/L) | 0.07 (0.03–0.10) |
| Leukocytes (×109/L) | 9.2 (7.8–11.5) |
| Thrombocytes (×109/L) | 378 (325–468) |
| Haemoglobin (g/L) | 120 (105–129) |
| Headache | Jaw Claudication | Vision Disturbances | Vision Loss | |
|---|---|---|---|---|
| Extended cranial arteritis | n.s. | 0.015 | n.s. | n.s. |
| Vertebral artery | n.s. | n.s. | n.s. | n.s. |
| Facial artery | n.s. | 0.060 | 0.038 | n.s. |
| Occipital artery | n.s. | n.s. | n.s. | n.s. |
| Large vessel vasculitis | n.s. | n.s. | n.s. | n.s. |
| Carotid artery | 0.051 | n.s. | 0.035 | 0.051 |
| Subclavian artery | 0.051 | n.s. | n.s. | n.s. |
| Axillary artery | 0.028 | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuret, T.; Lakota, K.; Čučnik, S.; Jurčič, V.; Distler, O.; Rotar, Ž.; Hočevar, A.; Sodin-Šemrl, S.; Frank-Bertoncelj, M. Dysregulated Expression of Arterial MicroRNAs and Their Target Gene Networks in Temporal Arteries of Treatment-Naïve Patients with Giant Cell Arteritis. Int. J. Mol. Sci. 2021, 22, 6520. https://doi.org/10.3390/ijms22126520
Kuret T, Lakota K, Čučnik S, Jurčič V, Distler O, Rotar Ž, Hočevar A, Sodin-Šemrl S, Frank-Bertoncelj M. Dysregulated Expression of Arterial MicroRNAs and Their Target Gene Networks in Temporal Arteries of Treatment-Naïve Patients with Giant Cell Arteritis. International Journal of Molecular Sciences. 2021; 22(12):6520. https://doi.org/10.3390/ijms22126520
Chicago/Turabian StyleKuret, Tadeja, Katja Lakota, Saša Čučnik, Vesna Jurčič, Oliver Distler, Žiga Rotar, Alojzija Hočevar, Snežna Sodin-Šemrl, and Mojca Frank-Bertoncelj. 2021. "Dysregulated Expression of Arterial MicroRNAs and Their Target Gene Networks in Temporal Arteries of Treatment-Naïve Patients with Giant Cell Arteritis" International Journal of Molecular Sciences 22, no. 12: 6520. https://doi.org/10.3390/ijms22126520






