Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP)
Abstract
:1. Introduction
2. Results and Discussions
2.1. Physical and Thermal Properties of PCL Formulations and Implant Performance
2.1.1. Pure Non-Blended PCL
2.1.2. PCL MW Blends
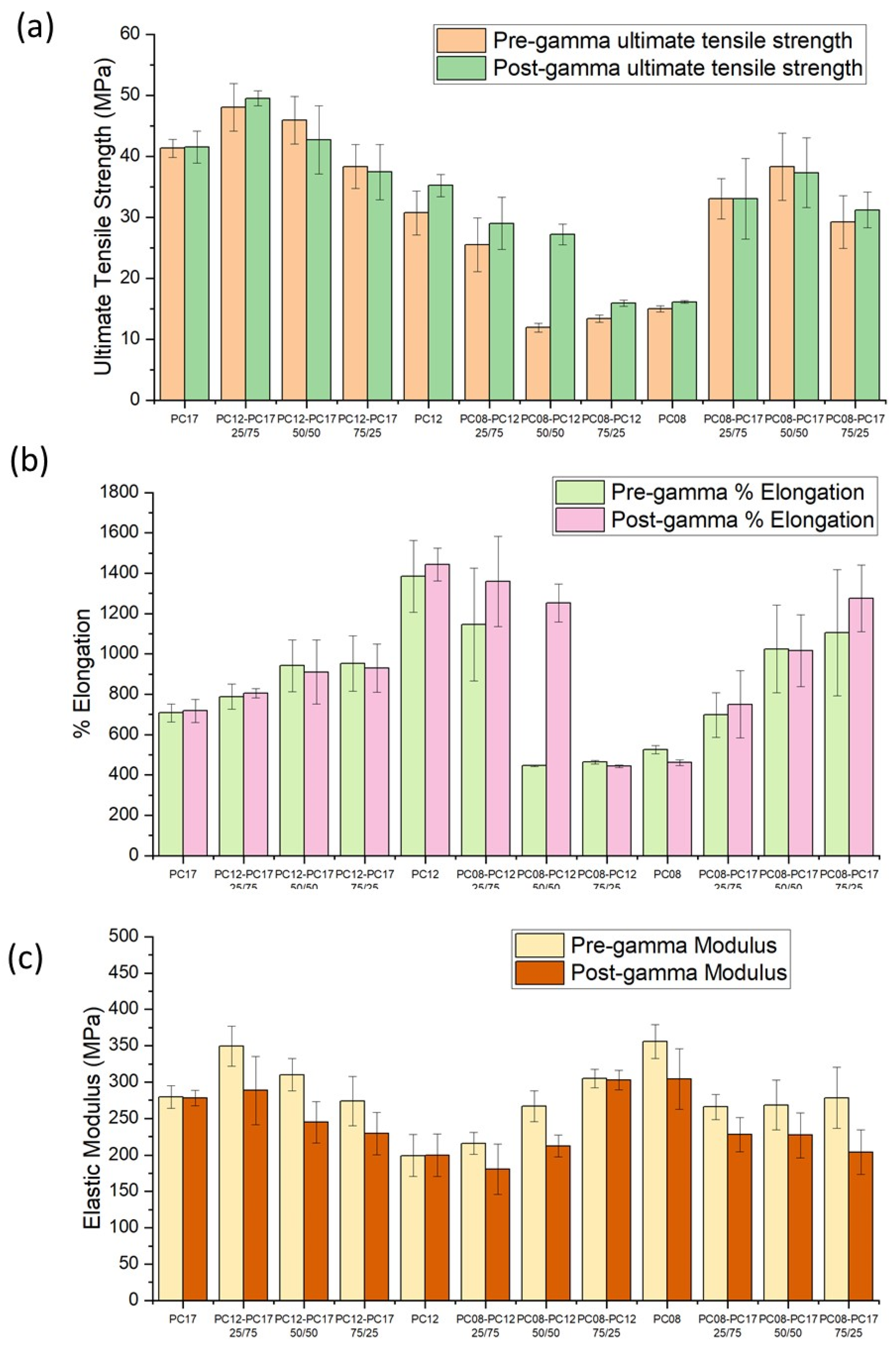
2.2. Effect of PCL MW Blends on the Mechanical Properties of Extruded Tubes
3. Materials and Methods
3.1. Implant Fabrication
3.2. Implant Sterilization
3.3. In Vitro Drug Release Studies
3.4. Stability Analysis of TAF Formulation
3.5. Characterization of PCL Extruded Tubes
3.5.1. Differential Scanning Calorimetry (DSC)
3.5.2. X-ray Diffraction (XRD)
3.5.3. Gel Permeation Chromatography (GPC)
3.5.4. Scanning Electron Microscopy (SEM) and Optical Microscope
3.5.5. Tensile Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Louvrier, A.; Euvrard, E.; Nicod, L.; Rolin, G.; Gindraux, F.; Pazart, L.; Houdayer, C.; Risold, P.Y.; Meyer, F.; Meyer, C. Odontoblastic differentiation of dental pulp stem cells from healthy and carious teeth on an original PCL-based 3D scaffold. Int. Endod. J. 2018, 51 (Suppl. 4), e252–e263. [Google Scholar] [CrossRef] [Green Version]
- Conte, R.; Di Salle, A.; Riccitiello, F.; Petillo, O.; Peluso, G.; Calarco, A. Biodegradable polymers in dental tissue engineering and regeneration. AIMS Mater. Sci. 2018, 5, 1073–1101. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, Y.-G.; Shi, C.; Luo, J.; Zhu, X.X. Block and Random Copolymers Bearing Cholic Acid and Oligo(ethylene glycol) Pendant Groups: Aggregation, Thermosensitivity, and Drug Loading. Biomacromolecules 2014, 15, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, T.; An, J.; Wu, Z.; Zhao, Y.; Dai, X.; Zhang, X.; Li, C. Block versus Random Amphiphilic Glycopolymer Nanopaticles as Glucose-Responsive Vehicles. Biomacromolecules 2015, 16, 3345–3356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Imai, S.; Hirai, Y.; Nagao, C.; Sawamoto, M.; Terashima, T. Programmed Self-Assembly Systems of Amphiphilic Random Copolymers into Size-Controlled and Thermoresponsive Micelles in Water. Macromolecules 2018, 51, 398–409. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Vion, J.M.; Jerome, R.; Teyssie, P.; Aubin, M.; Prudhomme, R.E. Synthesis, characterization, and miscibility of caprolactone random copolymers. Macromolecules 1986, 19, 1828–1838. [Google Scholar] [CrossRef]
- Brode, G.L.; Koleske, J.V. Lactone Polymerization and Polymer Properties. J. Macromol. Sci. Part A—Chem. 1972, 6, 1109–1144. [Google Scholar] [CrossRef]
- Ory, S.J.; Hammond, C.B.; Yancy, S.G.; Hendren, R.W.; Pitt, C.G. The effect of a biodegradable contraceptive capsule (Capronor) containing levonorgestrel on gonadotropin, estrogen, and progesterone levels. Am. J. Obstet. Gynecol. 1983, 145, 600–605. [Google Scholar] [CrossRef]
- Urolon Brochure. June 2018. Available online: http://www.mayumana-healthcare.com/wp-content/uploads/2018/06/MS-250.01_Urolon-Brochure_DEF.pdf (accessed on 14 June 2021).
- Ma, G.; Song, C. PCL/poloxamer 188 blend microsphere for paclitaxel delivery: Influence of poloxamer 188 on morphology and drug release. J. Appl. Polym. Sci. 2007, 104, 1895–1899. [Google Scholar] [CrossRef]
- Tamboli, V.; Mishra, G.P.; Mitra, A.K. Novel pentablock copolymer (PLA-PCL-PEG-PCL-PLA) based nanoparticles for controlled drug delivery: Effect of copolymer compositions on the crystallinity of copolymers and in vitro drug release profile from nanoparticles. Colloid Polym. Sci. 2013, 291, 1235–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravivarapu, H.B.; Moyer, K.L.; Dunn, R.L. Parameters affecting the efficacy of a sustained release polymeric implant of leuprolide. Int. J. Pharm. 2000, 194, 181–191. [Google Scholar] [CrossRef]
- Patel, R.B.; Carlson, A.N.; Solorio, L.; Exner, A.A. Characterization of formulation parameters affecting low molecular weight drug release from in situ forming drug delivery systems. J. Biomed. Mater. Res. Part A 2010, 94, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.B.; Solorio, L.; Wu, H.; Krupka, T.; Exner, A.A. Effect of injection site on in situ implant formation and drug release in vivo. J. Control. Release 2010, 147, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solorio, L.; Olear, A.; Hamilton, J.; Patel, R.; Beiswenger, A.; Wallace, J.; Zhou, H.; Exner, A. Noninvasive Characterization of the Effect of Varying PLGA Molecular Weight Blends on In Situ Forming Implant Behavior Using Ultrasound Imaging. Theranostics 2012, 2, 1064–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons-Faudoa, F.P.; Sizovs, A.; Shelton, K.A.; Momin, Z.; Niles, J.A.; Bushman, L.R.; Xu, J.; Chua, C.Y.X.; Nichols, J.E.; Demaria, S.; et al. Preventive Efficacy of a Tenofovir Alafenamide Fumarate Nanofluidic Implant in SHIV-Challenged Nonhuman Primates. Adv. Ther. 2021, 4, 2000163. [Google Scholar] [CrossRef]
- Simpson, S.M.; Widanapathirana, L.; Su, J.T.; Sung, S.; Watrous, D.; Qiu, J.; Pearson, E.; Evanoff, A.; Karunakaran, D.; Chacon, J.E.; et al. Design of a Drug-Eluting Subcutaneous Implant of the Antiretroviral Tenofovir Alafenamide Fumarate. Pharm. Res. 2020, 37, 83. [Google Scholar] [CrossRef]
- Su, J.T.; Simpson, S.M.; Sung, S.; Tfaily, E.B.; Veazey, R.; Marzinke, M.; Qiu, J.; Watrous, D.; Widanapathirana, L.; Pearson, E.; et al. A Subcutaneous Implant of Tenofovir Alafenamide Fumarate Causes Local Inflammation and Tissue Necrosis in Rabbits and Macaques. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [Green Version]
- Merck Sharp & Dohme Corp. Phase 3, Randomized, Clinical Study in HIV-1-Infected Heavily Treatment-Experienced Participants Evaluating the Antiretroviral Activity of Blinded Islatravir (ISL), Doravirine (DOR), and Doravirine/Islatravir (DOR/ISL), Each Compared to Placebo, and the Antiretroviral Activity, Safety, and Tolerability of Open-Label DOR/ISL. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04233216 (accessed on 18 January 2020).
- Merck Sharp & Dohme Corp. A Phase 2a, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Oral MK-8591 Once-Monthly in Participants at Low-Risk for HIV-1 Infection. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT04003103 (accessed on 1 July 2019).
- Munjal Patel, X.Z.; Cao, Y.; Matthews, R.P.; Plank, R.M.; Sklar, P.; Grobler, J.A.; Robertson, M.N.; Vargo, R. Islatravir PK Threshold & Dose Selection for Monthly Oral HIV-1 PrEP. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Virtual, 3–6 March 2021. [Google Scholar]
- Randolph, P.; Matthews, X.Z.; Barrett, S.; Goodey, A.; Heimbach, T.; Weissler, V.L.; Leyssens, C.; Reynders, T.; Vargo, R.; Liu, Y.; et al. Next- Generation Islatravir Implants Projected to Provide Yearly HIV Prophylaxis. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Virtual, 3–6 March 2021. [Google Scholar]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Johnson, L.M.; Krovi, S.A.; Demkovich, Z.R.; van der Straten, A. Performance and Stability of Tenofovir Alafenamide Formulations within Subcutaneous Biodegradable Implants for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2020, 12, 1057. [Google Scholar] [CrossRef]
- Johnson, L.M.; Krovi, S.A.; Li, L.; Girouard, N.; Demkovich, Z.R.; Myers, D.; Creelman, B.; van der Straten, A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2019, 11, 315. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Krovi, S.A.; Norton, C.; Luecke, E.; Demkovich, Z.; Johnson, P.; Areson, C.; Jimenez, G.; Van Der Straten, A.; Johnson, L.M. Long-Acting Biodegradable Implant for Sustained Delivery of Antiretroviral (ARV) and Hormonal Contraceptive. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, USA, 8–11 March 2020. [Google Scholar]
- Li, L.; Krovi, S.A.; Norton, C.; Johnson, P.; Jimenez, G.; Areson, C.; Van der Straten, A.; Johnson, L.M. Long-Acting Coformulated Biodegradable Implant for HIV Prevention and Contraception. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), Virtual, 3–6 March 2021. [Google Scholar]
- Krogstad, E.A.; Montgomery, E.T.; Atujuna, M.; Minnis, A.M.; O′Rourke, S.; Ahmed, K.; Bekker, L.G.; van der Straten, A. Design of an Implant for Long-Acting HIV Pre-Exposure Prophylaxis: Input from South African Health Care Providers. Aids Patient Care STDS 2019, 33, 157–166. [Google Scholar] [CrossRef]
- Pitt, C.G.; Gratzl, M.M.; Kimmel, G.L.; Surles, J.; Schindler, A. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials 1981, 2, 215–220. [Google Scholar] [CrossRef]
- Speranza, V.; Sorrentino, A.; De Santis, F.; Pantani, R. Characterization of the polycaprolactone melt crystallization: Complementary optical microscopy, DSC, and AFM studies. Sci. World J. 2014, 2014, 720157. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dorset, D.L. Crystal structure of poly(iε-caprolactone). Macromolecules 1990, 23, 4604–4607. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, D.; Xie, H.; Peng, S.; Chen, Y.; Xu, C. Crystallization of poly(ε-caprolactone) in its immiscible blend with polylactide: Insight into the role of annealing histories. Rsc Adv. 2016. [Google Scholar] [CrossRef]
- Miyajima, M.; Koshika, A.; Okada, J.I.; Ikeda, M.; Nishimura, K. Effect of polymer crystallinity on papaverine release from poly (l-lactic acid) matrix. J. Control. Release 1997, 49, 207–215. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Peppas, N.A.; Colombo, P. Crystal dissolution-controlled release systems. II. Metronidazole release from semicrystalline poly(vinyl alcohol) systems. J. Biomed. Mater. Res. 1997, 36, 125–130. [Google Scholar] [CrossRef]
- Pryputniewicz, R.J.; Furlong, C.; Pryputniewicz, E.J. Analytical and experimental characterization of an optical MEMS device. In Proceedings of the International Symposium on Microelectronics, Boston, MA, USA, 18–20 November 2003; pp. 774–779. [Google Scholar]
- Polymer Blends Handbook; Kluwer academic publishers: Amsterdam, The Netherlands, 2014; Volume 1. [CrossRef]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solid Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2012; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119967125 (accessed on 17 October 2012).
- Gupta, B.; Geeta; Ray, A.R. Preparation of poly(ε-caprolactone)/poly(ε-caprolactone-co-lactide) (PCL/PLCL) blend filament by melt spinning. J. Appl. Polym. Sci. 2012, 123, 1944–1950. [Google Scholar] [CrossRef]
- Obregon, N.; Agubra, V.; Pokhrel, M.; Campos, H.; Flores, D.; De la Garza, D.; Mao, Y.; Macossay, J.; Alcoutlabi, M. Effect of Polymer Concentration, Rotational Speed, and Solvent Mixture on Fiber Formation Using Forcespinning®. Fibers 2016, 4, 20. [Google Scholar] [CrossRef]
- Su, H.-H.; Chen, H.-L.; Díaz, A.; Casas, M.T.; Puiggalí, J.; Hoskins, J.N.; Grayson, S.M.; Pérez, R.A.; Müller, A.J. New insights on the crystallization and melting of cyclic PCL chains on the basis of a modified Thomson–Gibbs equation. Polymer 2013, 54, 846–859. [Google Scholar] [CrossRef]
- Núñez, E. Crystallization in Constrained Polymer Structures: Approaching the Unsolved Problems in Polymer Crystallization. Ph.D. Thesis, Ph.D-KTH Royal Institute of Technology, Stockholm, Sweden, 2006. [Google Scholar]
- Tuba, F.; Olah, L.; Nagy, P. Towards the understanding of the molecular weight dependence of essential work of fracture in semi-crystalline polymers: A study on poly(ε-caprolactone). Express Polym. Lett. 2014, 8, 869–879. [Google Scholar] [CrossRef] [Green Version]
- Kosobrodova, E.; Kondyurin, A.; Chrzanowski, W.; Theodoropoulos, C.; Morganti, E.; Hutmacher, D.; Bilek, M.M.M. Effect of plasma immersion ion implantation on polycaprolactone with various molecular weights and crystallinity. J. Mater. Sci. Mater. Med. 2017, 29, 5. [Google Scholar] [CrossRef] [PubMed]
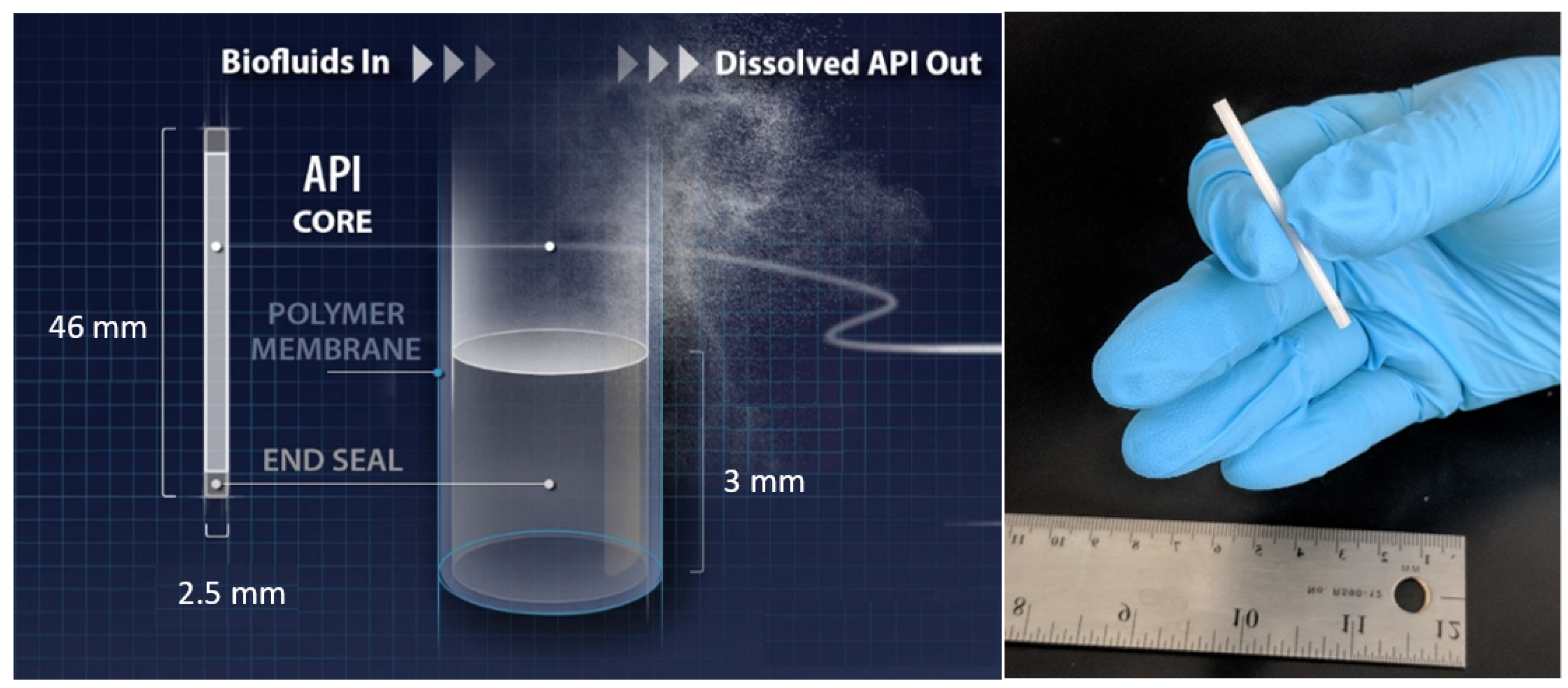
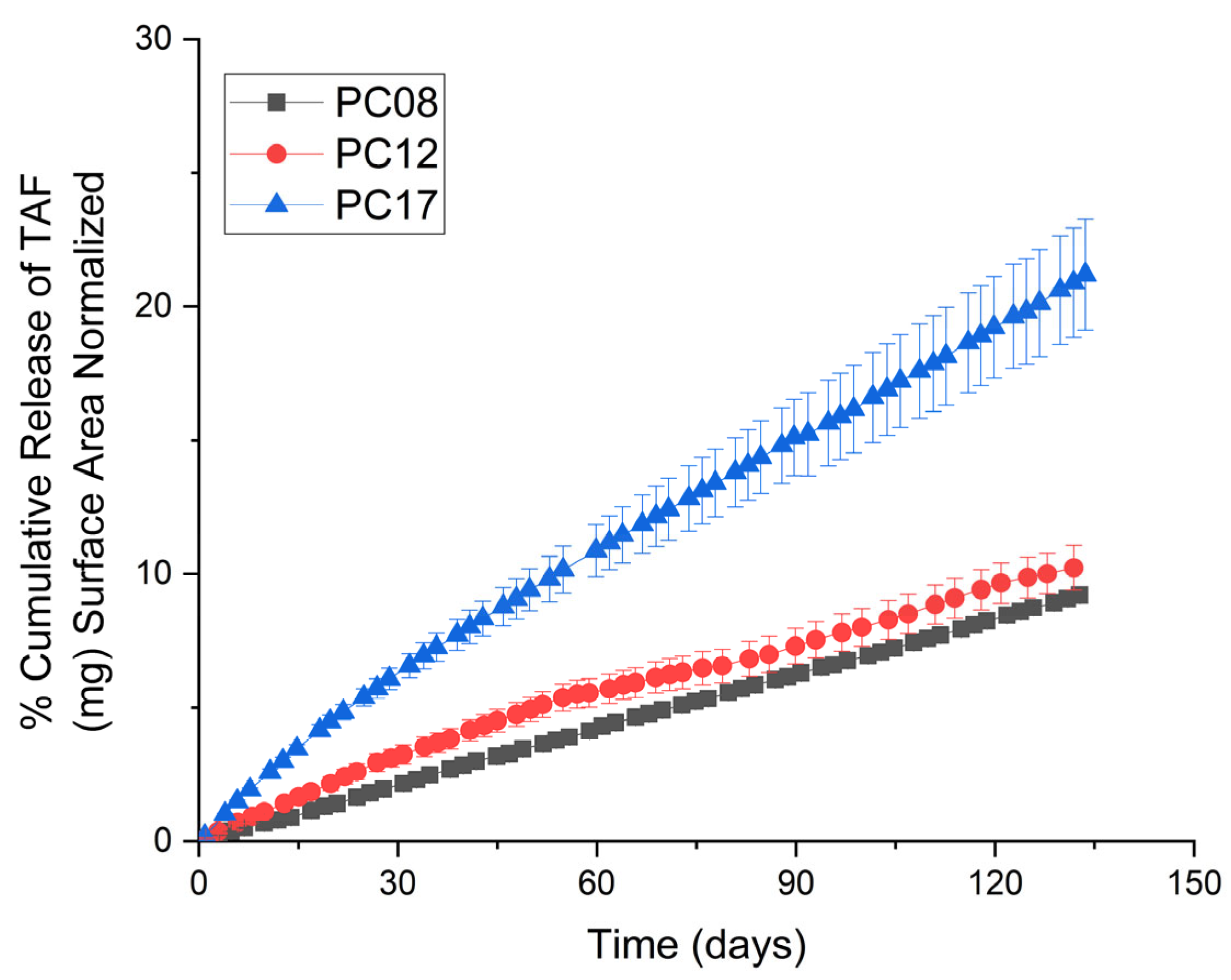
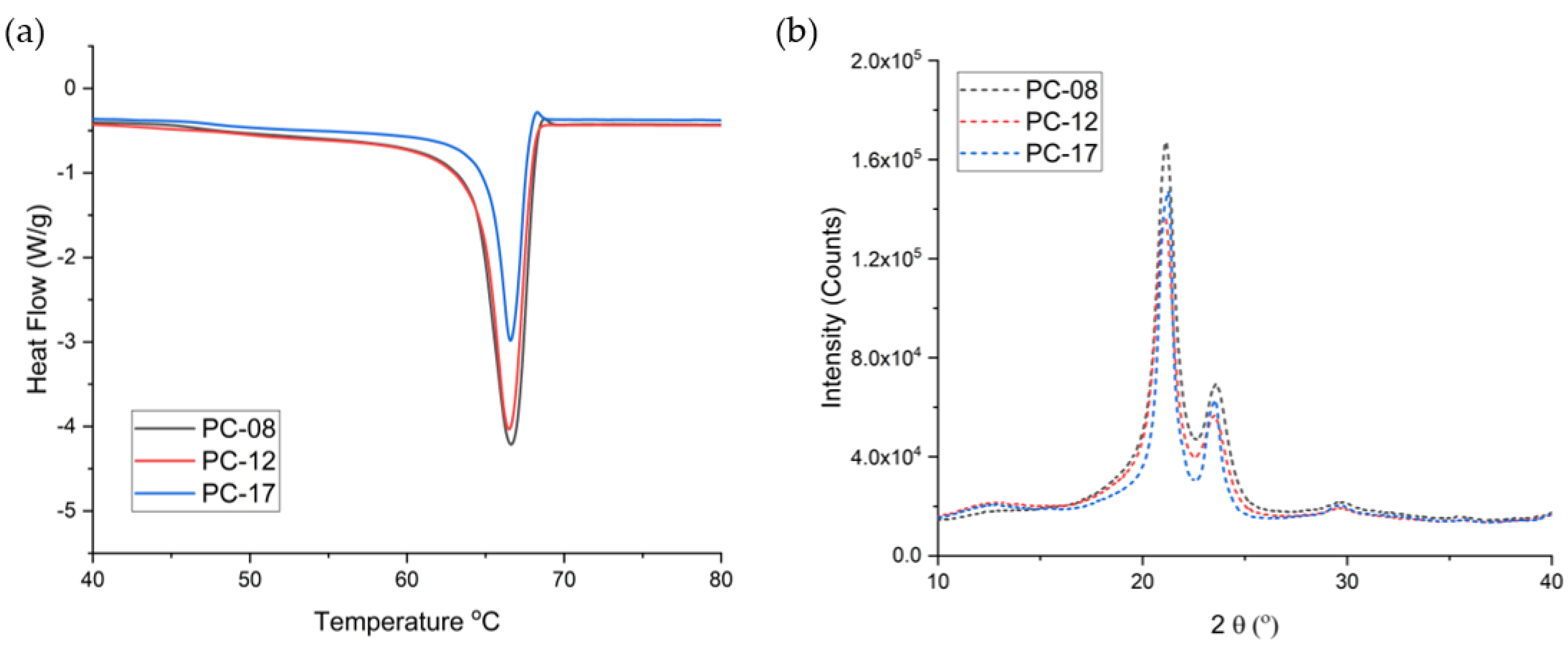
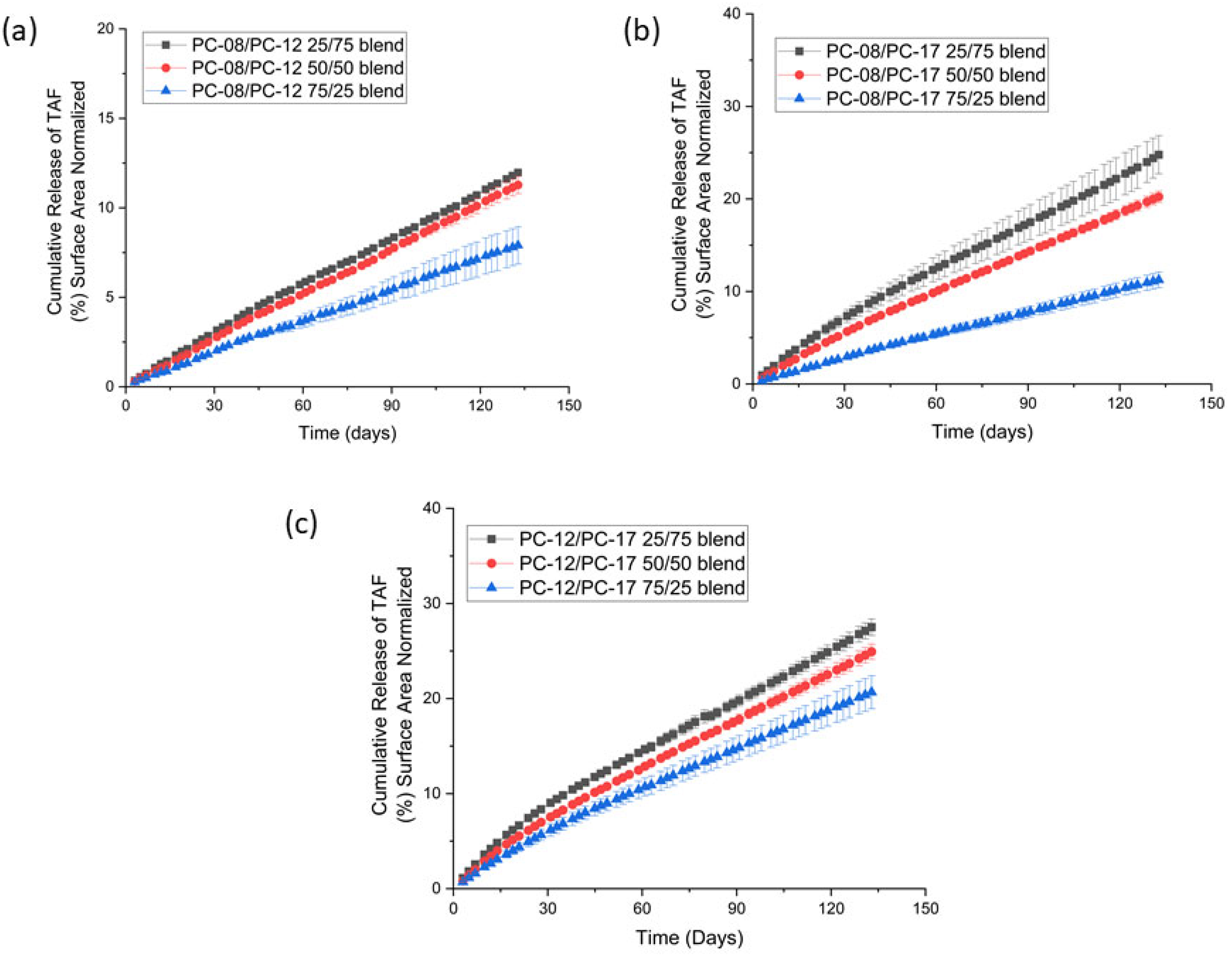
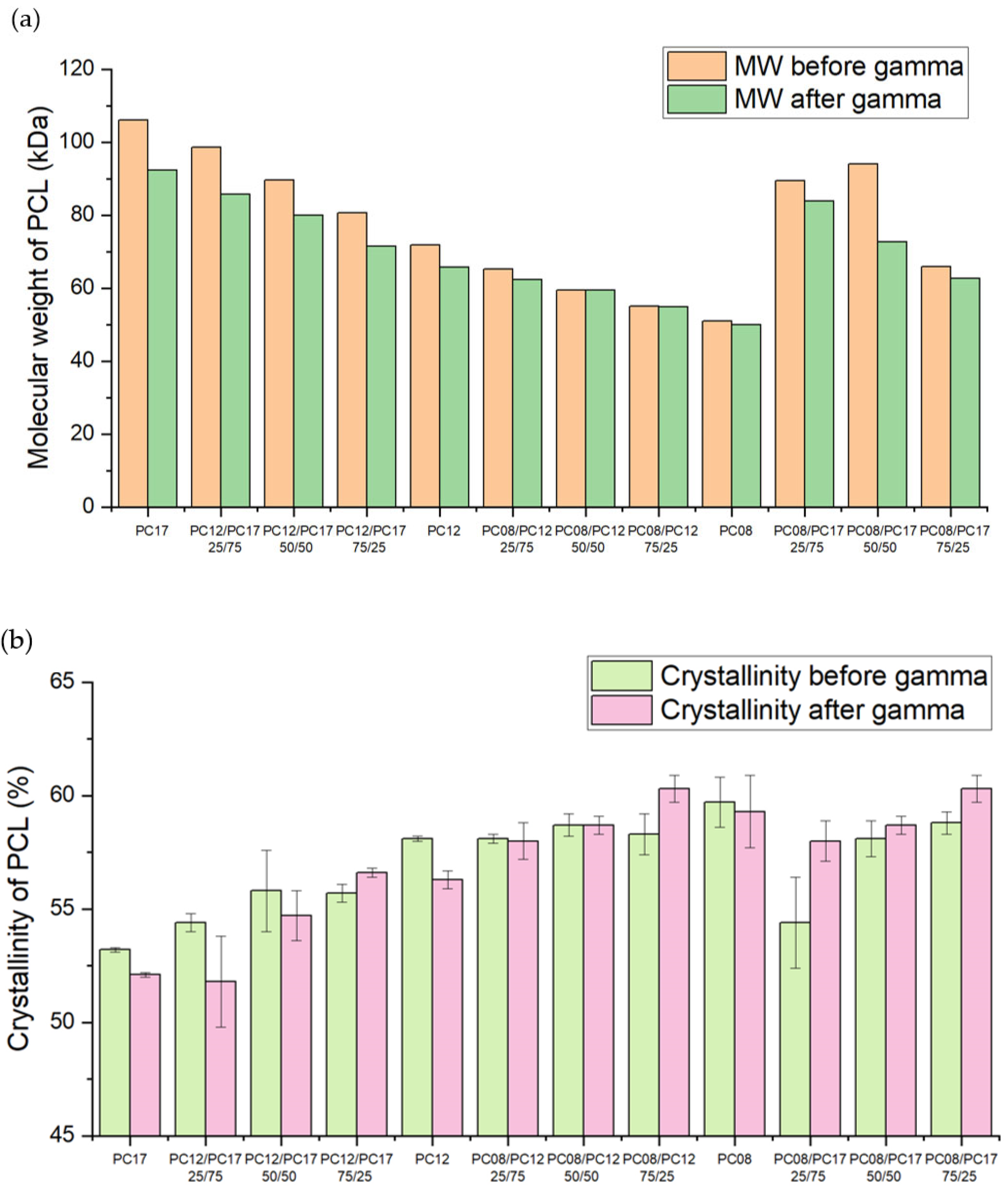
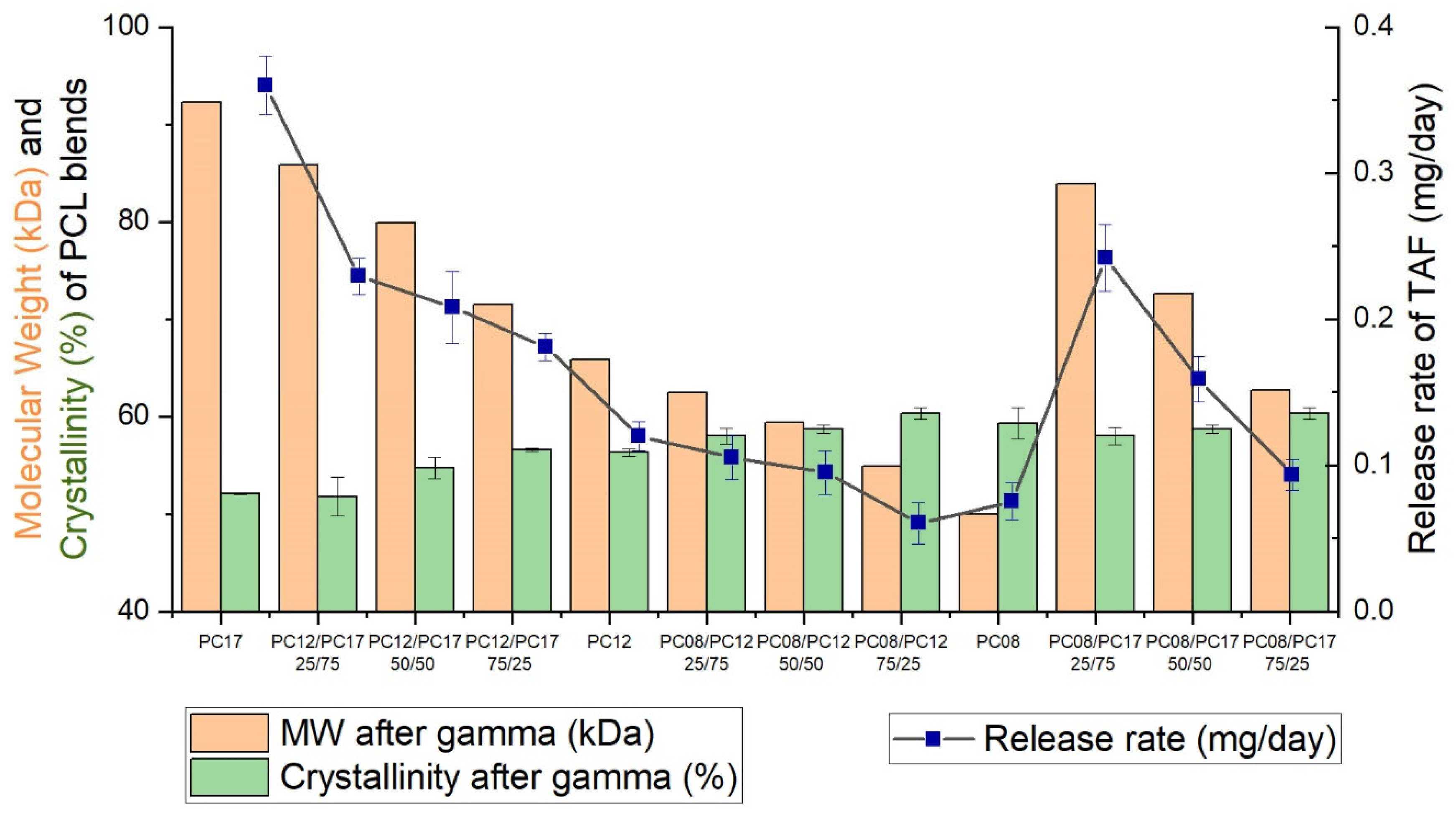
| PCL Type | MW (kDa) before Gamma | MW (kDa) after Gamma | Crystallinity before Gamma (%) | Crystallinity after Gamma (%) | Average Release Rate of the Implant (mg/day) * |
|---|---|---|---|---|---|
| PC-17 | 106.0 | 92.3 | 52.56 ± 0.62 | 53.19 ± 0.12 | 0.36 ± 0.02 |
| PC-12 | 71.9 | 65.8 | 56.27 ± 0.44 | 58.15 ± 0.13 | 0.12 ± 0.01 |
| PC-08 | 50.9 | 50.0 | 59.26 ± 1.93 | 59.69 ± 1.10 | 0.07 ± 0.01 |
| PCL Type | Crystallite Size before Gamma (nm)-DSC | Crystallite Size after Gamma (nm)-DSC | Crystallite Size before Gamma (nm)-XRD | Crystallite Size after Gamma (nm)-XRD | ||
|---|---|---|---|---|---|---|
| L110 | L200 | L110 | L200 | |||
| PC-17 | 27.5 ± 0.2 | 26.9 ± 0.2 | 10.7 | 8.9 | 11.7 | 9.9 |
| PC-12 | 26.9 ± 0.1 | 26.2 ± 0.2 | 10.1 | 8.2 | 10.6 | 8.3 |
| PC-08 | 27.3 ± 0.5 | 27.5 ± 1.5 | 10.6 | 8.5 | 10.7 | 8.4 |
| Formulation | Approximate TAF Payload (mg) | Average Release Rate (mg/day, 40 mm Implant) | % Purity of TAF at Day 136 |
|---|---|---|---|
| PC-12/PC-17 25/75 | 114 ± 5 | 0.33 ± 0.01 | 91.2 ± 3.1 |
| PC-12/PC-17 50/50 | 116 ± 2 | 0.23 ± 0.04 | 96.2 ± 0.8 |
| PC-12/PC-17 75/25 | 116 ± 4 | 0.18 ± 0.01 | 94.8 ± 0.7 |
| PC-08/PC-12 25/75 | 113 ± 2 | 0.10 ± 0.01 | 96.9 ± 0.1 |
| PC-08/PC-12 50/50 | 112 ± 2 | 0.09 ± 0.01 | 96.4 ± 0.4 |
| PC-08/PC-12 75/25 | 112 ± 2 | 0.06 ± 0.01 | 96.7 ± 0.1 |
| PC-08/PC-17 25/75 | 115 ± 4 | 0.24 ± 0.02 | 95.2 ± 0.4 |
| PC-08/PC-17 50/50 | 108 ± 3 | 0.16 ± 0.02 | 93.3 ± 1.1 |
| PC-08/PC-17 75/25 | 119 ± 3 | 0.09 ± 0.01 | 95.1 ± 0.3 |
| PCL Type | Crystallite Size before Gamma (nm)-DSC | Crystallite Size after Gamma (nm)-DSC | CRYSTALLITE Size before Gamma (nm)-XRD | Crystallite Size after Gamma (nm)-XRD | ||
|---|---|---|---|---|---|---|
| L110 | L200 | L110 | L200 | |||
| PC-08/PC-12 25/75 | 26.6 ± 0.3 | 26.2 ± 0.2 | 10.4 | 10.3 | 11.1 | 10.6 |
| PC-08/PC-12 50/50 | 26.5 ± 0.2 | 27.0 ± 0.1 | 10.8 | 8.6 | 10.6 | 8.3 |
| PC-08/PC-12 75/25 | 27.0 ± 0.2 | 26.8 ± 0.1 | 11.2 | 10.6 | 11.0 | 8.3 |
| PC-12/PC-17 25/75 | 27.3 ± 0.1 | 26.9 ± 0.2 | 11.0 | 8.9 | 10.6 | 8.5 |
| PC-12/PC-17 50/50 | 27.2 ± 0.2 | 27.2 ± 0.8 | 10.6 | 10.4 | 10.8 | 8.5 |
| PC-12/PC-17 75/25 | 26.9 ± 0.2 | 27.8 ± 0.3 | 11.0 | 8.6 | 11.0 | 8.7 |
| PC-08/PC-17 25/75 | 27.3 ± 0.4 | 27.3 ± 0.2 | 10.6 | 10.3 | 11.0 | 10.6 |
| PC-08/PC-17 50/50 | 27.2 ± 0.4 | 26.9 ± 0.2 | 11.0 | 10.4 | 10.4 | 8.1 |
| PC-08/PC-17 75/25 | 26.9 ± 0.2 | 26.8 ± 0.4 | 10.7 | 8.5 | 11.1 | 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Areson, C.; van der Straten, A.; Johnson, L.M. Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP). Int. J. Mol. Sci. 2021, 22, 6529. https://doi.org/10.3390/ijms22126529
Li L, Areson C, van der Straten A, Johnson LM. Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP). International Journal of Molecular Sciences. 2021; 22(12):6529. https://doi.org/10.3390/ijms22126529
Chicago/Turabian StyleLi, Linying, Christine Areson, Ariane van der Straten, and Leah M. Johnson. 2021. "Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP)" International Journal of Molecular Sciences 22, no. 12: 6529. https://doi.org/10.3390/ijms22126529
APA StyleLi, L., Areson, C., van der Straten, A., & Johnson, L. M. (2021). Effects of Polymer Blending on the Performance of a Subcutaneous Biodegradable Implant for HIV Pre-Exposure Prophylaxis (PrEP). International Journal of Molecular Sciences, 22(12), 6529. https://doi.org/10.3390/ijms22126529







