Abstract
Globally, cancer is the second (to cardiovascular diseases) leading cause of death. Regardless of various efforts (i.e., finance, research, and workforce) to advance novel cancer theranostics (diagnosis and therapy), there have been few successful attempts towards ongoing clinical treatment options as a result of the complications posed by cancerous tumors. In recent years, the application of magnetic nanomedicine as theranostic devices has garnered enormous attention in cancer treatment research. Magnetic nanoparticles (MNPs) are capable of tuning the magnetic field in their environment, which positively impacts theranostic applications in nanomedicine significantly. MNPs are utilized as contrasting agents for cancer diagnosis, molecular imaging, hyperfusion region visualization, and T cell-based radiotherapy because of their interesting features of small size, high reactive surface area, target ability to cells, and functionalization capability. Radiolabelling of NPs is a powerful diagnostic approach in nuclear medicine imaging and therapy. The use of luminescent radioactive rhenium(I), 188/186Re, tricarbonyl complexes functionalised with magnetite Fe3O4 NPs in nanomedicine has improved the diagnosis and therapy of cancer tumors. This is because the combination of Re(I) with MNPs can improve low distribution and cell penetration into deeper tissues.
1. Introduction
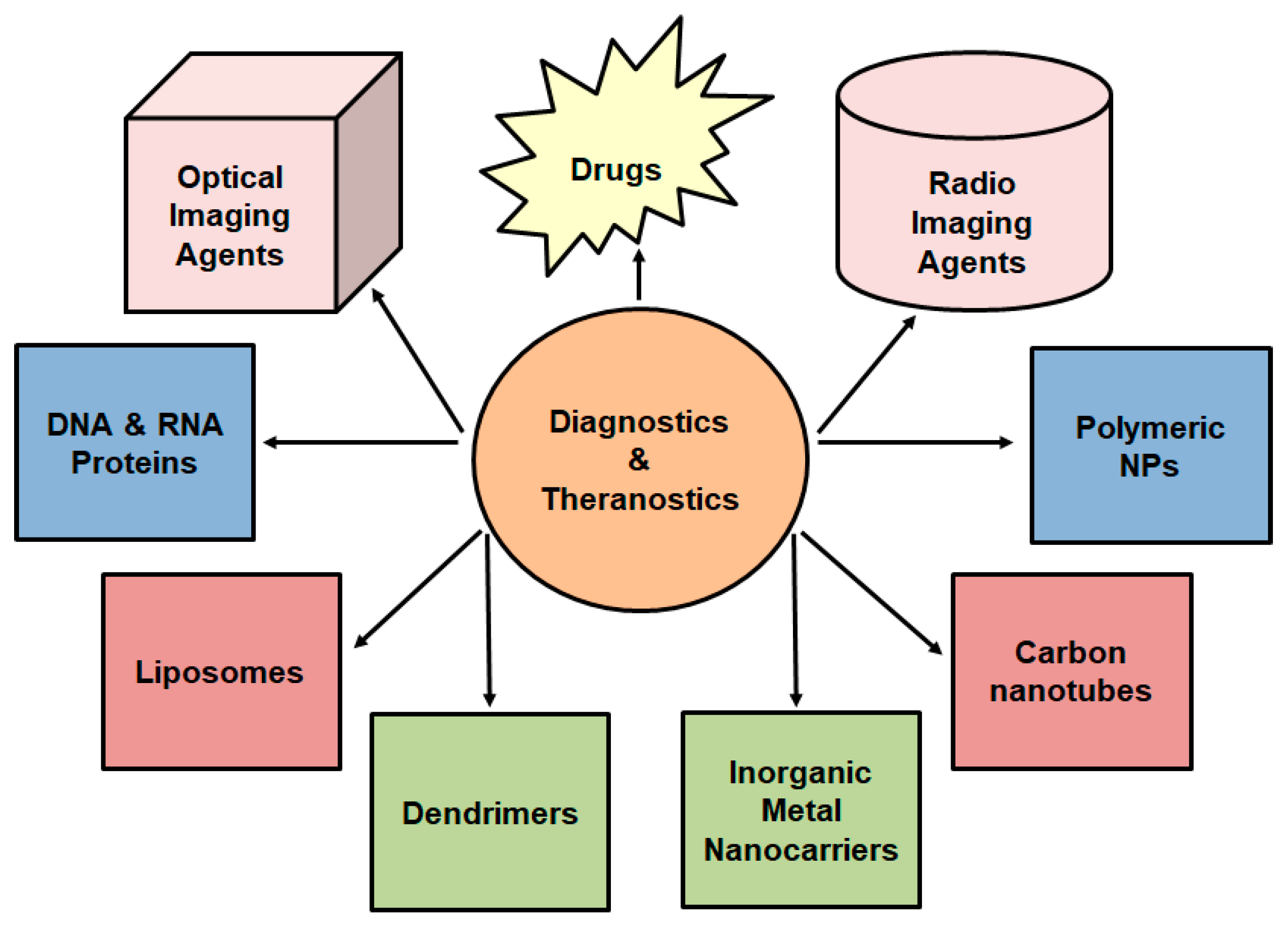
Cancer is a well-known, complicated and multistage disease caused by an uncontrolled division of abnormal cells in the body [1]. Regardless of the continuous progress in cancer diagnosis and therapy, this disease remains the second leading cause of death globally [2]. As much as the conventional cancer treatment approaches (i.e., surgery, radiotherapy, and chemotherapy) have shown positive impacts on cancer mortality rate, there still exist several challenges in cancer management. Amongst the stated treatment approaches, radiotherapy displays an added advantage as patients treated from this approach exhibit an improved long-term survival. Radiotherapy is a cancer treatment approach, falling under the umbrella of nuclear medicine, utilizing high doses of radiation to kill cancer cells and reduce the size of tumors [3]. Nuclear medicine, also known as radiopharmaceuticals, involves the use of radioisotopes bound to biological molecules that are capable of targeting specific organs, tissues, or cells. This field of medicine has been broadly studied as an advanced diagnostic tool where radionuclides are introduced in vivo. This is followed by the detection of the emitted gamma rays and generation of images which give detailed radionuclides distribution as well as physiological characterization of targeted areas [4,5]. An emerging area in nuclear medicine incorporates nano-imaging agents (see Figure 1) with dual behavior as both diagnostic and therapeutic tools [6,7]. However, due to the low distribution and cell penetration of these nanomaterials, their undesired pharmacokinetics had to be improved [8]. Thus, different nanotheranostics based on polymeric NPs have been manufactured and radiolabeled with available radionuclides of choice [9]. Within these polymeric NPs, various techniques are utilized to diagnose and treat cancerous diseases [10,11,12]. Theranostic nanomedicine, also known as nanotheranostics, involves treatments with nanosize particles (<100 nm) and has a large number of capabilities such as targeted delivery, controlled release, greater transport efficiency via endocytosis, stimuli-responsive systems, and the combination of therapeutic approaches such as multimodality diagnosis and therapy [13,14].
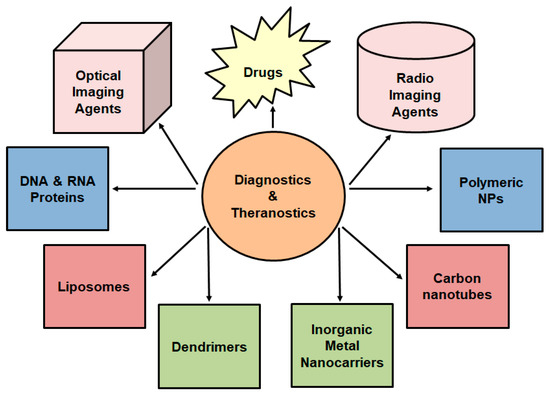
Figure 1.
A schematic representation of nanotheranostics used for simultaneous release and imaging.
Subsequently, nanotheranostics’ stability has been improved by linking molecules such as chelator agents that can bind to radionuclides (i.e., 186Re, 188Re, and 99mTc) and NPs [15]. The radioactive properties of these radionuclides are shown in Table 1.

Table 1.
The categories of radionuclides used as therapeutic and diagnostic agents [5].
Radionuclides which solely emit gamma rays () such as 99mTc possess diagnostic purposes, while radionuclides such as 186Re and 188Re emit beta particles () and gamma rays () for therapeutic and diagnostics purposes, respectively. This ultimately led to the introduction of the Re(I) tricarbonyl core in the theranostic application by Alberto et al. (1999) [16] and Top et al. (1995) [17]. In their studies, they participated in the group VIIB transitional metal chemistry via the synthesis of a facile method to yield the [M(CO)3]+ core, where M = Tc or Re. Most importantly, the low-spin d6 electronic configuration and the stability of the CO ligands make the substitution in the [Tc or Re(CO)3]+ core useful in radiopharmaceutical chemistry [18]. From these two metal cores, the Re(I) tricarbonyl core displays an added advantage, since its chemistry can be studied with this metal being in a natural state as opposed to the radioisotopic state. Moreover, the biological application of the relatively small size Re(I) tricarbonyl moiety as compared to the kinetic stability and inertness, serves as a potential advantage [19,20]. Additionally, the kinetically inert Re(I) tricarbonyl complexes exhibit distinct phosphorescence/luminescence properties, depending on the nature of the ligands. This is another reason they found a huge application as photosensitizers and bio-imaging agents [21]. Conversely, the use of SPION layered material with radionuclides as theranostics provides great potential to improve the delivery processes of radionuclides into the targeted tissues. In this review, the main focus is based on the class of hybrid MRI-OI probes that are made by utilizing ultra SPION and sensitized luminescence compounds with the d-block element, Re. The potential applications of the nanoparticles (i.e., magnetite Fe3O4) functionalised with the Re(I) tricarbonyl complexes as a bimodal contrast agent for MRI and optical imaging of nanoparticles have been demonstrated by Carron et al., 2015 [22].
2. Magnetic Nanoparticles
Magnetic NPs have brought enormous attention to several biomedical applications, due to their intrinsic biocompatibility and interaction with externally applied magnetic fields. This is because magnetic NPs can distort magnetic fields in their surroundings, which establishes the basis for intensified contrast in MRI. In other uses, the applied magnetic fields can create magnetic forces and torques on their magnetic dipoles, leading to particle translation, rotation, and even energy dissipation in the form of heat. This phenomenon results in applications in magnetic biomarker or cell break-up targeted drug delivery, magneto-mechanical actuation of cell surface receptors, magnetic hyperthermia and triggered drug release as well as biomedical imaging. That is why there has been fast-growing research based on the synthesis, characterization, and post-synthesis application-specific to modification of magnetic Fe3O4 and substituted ferrite nanoparticles. This has led to several emerging uses in a broad array of fields such as medical and biomedical applications [23,24,25,26]. Thus, this review focuses on the coordinated Re(I) tricarbonyl complexes, functionalized with the magnetic Fe3O4 NPs as MRI-OI probes.
2.1. Iron Oxide NPs for Biomedical Applications
Several materials and compositions have been utilized to compose magnetic NPs by differentiating magnetic and physical properties necessary for the intended use. Still, in the biomedical arena, the potential biocompatibility and the long-term in vivo fate and clearance of magnetic NPs must be taken into account. These accountabilities prohibit the nanoparticle compositions and formulations that can be applied safely without presenting harm or side effects to living tissue [27,28]. This makes a specific subgroup of ferrite nanoparticles, MxFe3-xO4 (M = Fe, Mn, Ni, or Zn; x = divalent cation) the best candidates for biomedical uses [29,30]. The biocompatibility of magnetic iron oxide NPs is less of a concern because a healthy human body already has mechanisms for handling, storage, and the use of iron [31]. Iron is an essential nutrient to sustain human health and survival. Essentially, it participates in the transport and storage of oxygen throughout the body, DNA synthesis, energy production, and metabolism, and detoxification; thus, it acts as both an antioxidant and pro-oxidant. Generally, the average human body has about 4 g of iron, and smaller contents of the other metals, in the form of two highly significant molecules, ferritin and haemoglobin [32].
Secondly, there has been extensive testing concerning the safety of these nanoparticles in laboratory, preclinical, and clinical settings; this is why ferrite magnetic NPs are preferred over others for biomedical applications. Many formulations of iron oxide have been accepted by regulatory agencies in both the United States and Europe for clinical-stage examination and use. For instance, the treatment of pancreatic and brain tumors [33,34], their applications in imaging and diagnostic settings via magnetic resonance imaging (MRI), and their employment for sentinel lymph node (SLN) mapping [35].
2.2. Magnetic Resonance Imaging (MRI)
MRI is used to investigate the properties of magnetic NPs such as Fe3O4 and Fe2O3. When magnetic NPs are introduced, they generate a local magnetic field, which results in the disturbance of the nuclear relaxation of magnetic nuclei in the environment [36]. These NPs can further stimulate the relaxation process and shorten the relaxation time of neighboring protons, intensifying the signal contrast between the surroundings and distal background in MR images. Unfortunately, MRI applies contrast agents for imaging which can be demanding, because it requires an extra effort to identify and prepare suitable imaging agents for targeted application. However, the use of magnetic Fe3O4 NPs is advantageous because they are bio-compatible for in vivo applications [37].
2.3. Clinical Applications of SPIONs
SPIONs are utilized as iron supplements in anaemic patients due to their non-toxic and bio-compatible nature [38]. They are also being examined for imaging vasculature and tumors [39], gene therapy, drug delivery [40], tracing of labeled cells [41], thermal ablation of tumors via magnetic heating [42], and organ preservation [43]. Within the last decade, the Food and Drug Administration (FDA)’s approval of ferumoxytol (Feraheme) to nurse patients with iron deficiency and chronic kidney disease highlighted the clinical applicability of SPIONs in therapy [44]. It was reported that patients tolerated up to 510 mg Fe/injection, with subsequent growth in haemoglobin level post-injection [45]. No serious adverse events were observed from the study that was reported in 396 US patients who received a total of 570 intravenous (IV) injections of SPION therapy.
3. Multimodal Cancer Theranostics
There are several known molecular imaging modalities such as MRI, single-photon emission computerized tomography (SPECT), and positron emission tomography (PET); however, none of them are perfect and adequate to acquire all the necessary information for a particular question [46]. For instance, it is challenging to quantitatively determine fluorescence signal in vivo, specifically in deep tissues; although the use of MRI would render high resolution, it suffers from low sensitivity, whereas imaging methods relying on radionuclide show very high sensitivity but poor resolution. Therefore, the blend of multiple molecular imaging techniques provides a symbiotic advantage as compared to separate individual modalities. Thus, this review describes the combination of magnetite NPs with rhenium(I) tricarbonyl complexes. Due to the inherently low sensitivity of MRI, exogenous contrast agents such as the magnetic Fe3O4 NPs (induces higher magnetic fields, 4.7–14 T in small animal models) are incorporated to enhance sensitivity and to obtain data for a much longer period. In this instance, a crystalline Fe3O4 core is commonly incorporated into a polymer coating material such as dextran or poly(ethylene glycol) PEG for its use as an MRI contrast agent [47]. As a result, the existence of thousands of iron atoms in each particle will produce a high T2 relaxivity [48].
Additionally, Fe3O4 NPs can be attached to a radionuclide such as 187/188Re to dramatically amplify the signal, enhance receptor-binding affinity, improve the detection sensitivity and quantify imaging, which is only true if the radioisotope remains bound to the NP. 187/188Re isotopes form part of the first radionuclides that were put on trial for NP-based radiotherapy. Amongst them, 188Re has interestingly been examined for magnetically targeted radiotherapy [49,50]. For instance, when the surface of silica-coated Fe3O4 NPs is labeled by 188Re with >90% labeling yield and good in vitro stability, the radioisotope uptake in the tumor is enhanced as a magnetic field is simultaneously applied above the tumor area [51,52]. Liang et al. (2007) reported the successful attachment of amino-functionalized superparamagnetic Fe3O4 NPs with a humanized monoclonal antibody targeted for liver cancer cells. They then radiolabelled with 188Re and consequently, due to their size (between 10 and 15 nm in diameter), these NPs were expected to have high uptake in the reticuloendothelial system (RES), e.g., liver, and to uplift magnetically targeted radiotherapy for the treatment of liver cancer [53].
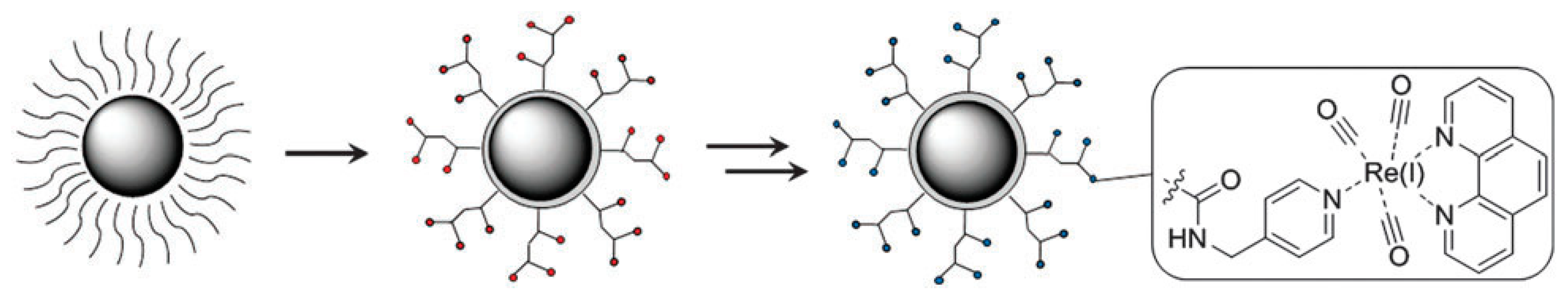
Radiolabelling of magnetic NPs creates a potential bimodal contrast agent for MRI and optical imaging; hence, a few examples attributed to the combination of magnetic Fe3O4 NPs and Re(I) tricarbonyl complexes are illustrated which are in line with the aim of this review. However, other general examples concerning the potential application of Re(I) tricarbonyl complexes functionalized with other types of NPs are also shown. A siloxane luminophore is normally used to functionalize the surface of magnetite to yield water-dispersible Fe3O4-NPs (as illustrated in Scheme 1). This is a convenient way because it produces a biocompatible, inert, and permanent shell that is commonly known for its diverse functionalities. Additionally, it creates a thin layer of functionalized siloxanes around the Fe3O4 NPs which forms an appropriate scaffold for linking Re(I) tricarbonyl complexes [54,55]. In this instance, oleate functionalized Fe3O4 NPs are treated with N-(trimethoxysilylpropyl) ethylenediamine triacetic acid trisodium salt to acquire hydrophilic Fe3O4 NPs with multiple acid functions. This is followed by a multistep preparation with picolylamine, which reacts with the free acid of the NPs to produce a peptide bond with the metal.
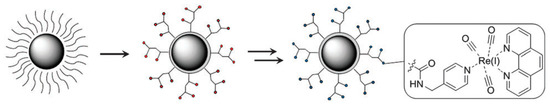
Scheme 1.
Design of the magnetoluminescent iron oxide nanoparticles. Red and blue circles represent hydrophilic Fe3O4 NPs with multiple acid functions and the luminophore, respectively [22].
Interestingly, the Re(I) tricarbonyl complexes (illustrated in Scheme 2) possess potential luminescent properties between the 590 and 620 nm region of the electromagnetic spectrum; hence, they have been identified as the best candidates to be used as OI contrast agents for cancer theranostics [56,57]. This potential of the Re(I) tricarbonyl complex antenna structure has been found useful due to its high affinity towards the pyridine ligands, whilst keeping the Fe3O4 NPs as small as possible so that the benefits of T1 and T2 contributions can be useful for MRI applications [58,59,60].
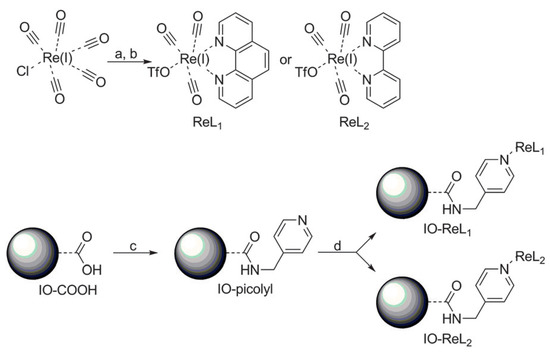
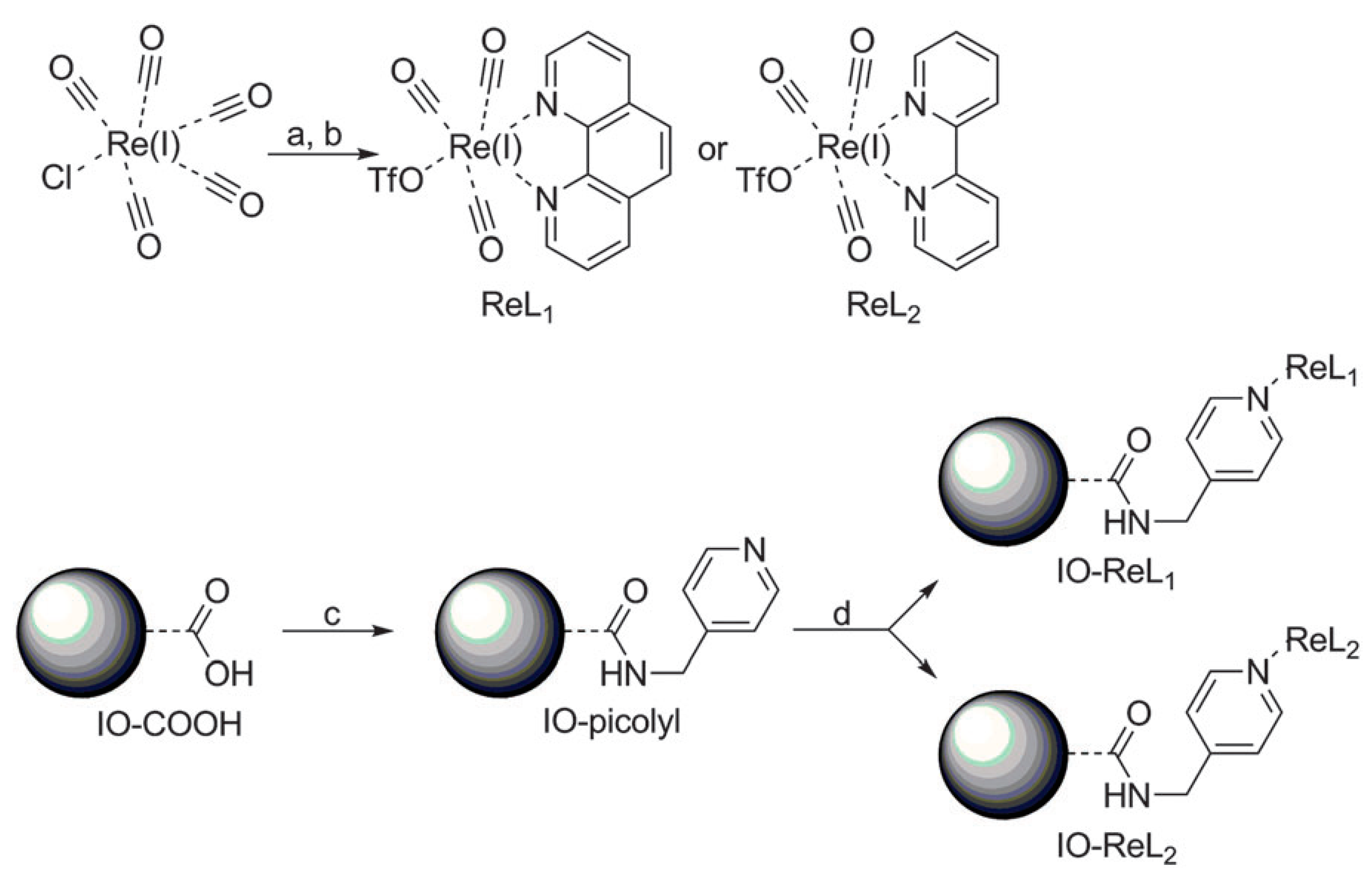
Scheme 2.
Synthetic procedure for the IO-ReL1 and IO-ReL2 molecules. (a) 2 eq. 1,10-Phenanthroline (L1) or 2,2-bipyridine (L2), 1 eq. ClRe(CO)5, benzene, 333 K, 5 h. (b) AgOTf, THF/MeCN, 16 h. (c) H2O/THF, HCl, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), N-hydroxysuccinimide (NHS), sonicate 30 min. (d) H2O/CAN, NaHCO3, 223 K, 17 h [22].
4. Phosphorescence Transition Metal Complexes for Tumor Diagnosis
Several transition metal complexes exhibit different types of excited states depending on the metal centres, the triplet-state energy levels of the ligand, and the local environment. These excited states include metal-to-ligand-charge-transfer (MLCT), intraligand-charge-transfer (ILCT) as well as ligand-to-ligand-charge-transfer (LLCT), and these are mostly found in heavy-metal complexes. However, the MLCT state is commonly seen in transition metal complexes with d6 and d8 configurations; therefore, MLCT is in charge of phosphorescence emission [61].
Phosphorescence is referred to as the process whereby energy is absorbed by a substance and subsequently released slowly in the form of light. Phosphorescent transition metal complexes (PTMCs), such as Ru(II), Re(I), Ir(III), and Au(I) complexes, show potential as phosphorescent imaging agents. Thus, they are versatile and form a dynamic scaffold for the growth of tumor diagnostic probes due to their advantageous photophysical properties such as large Stokes shifts, long luminescent lifetimes, and resistance to photo-bleaching [61,62]. Furthermore, by varying the ligands around these types of complexes (PTMCs), their photo-physical properties can be easily tuned [63]. For instance, the emission spectra will be shifted into the near-infrared radiation when there is an addition of an extensive electronic system in the co-ligands. This is more favored for biological imaging because near-infrared rays penetrate through into deeper tissues within the range of 750–950 nm [64].
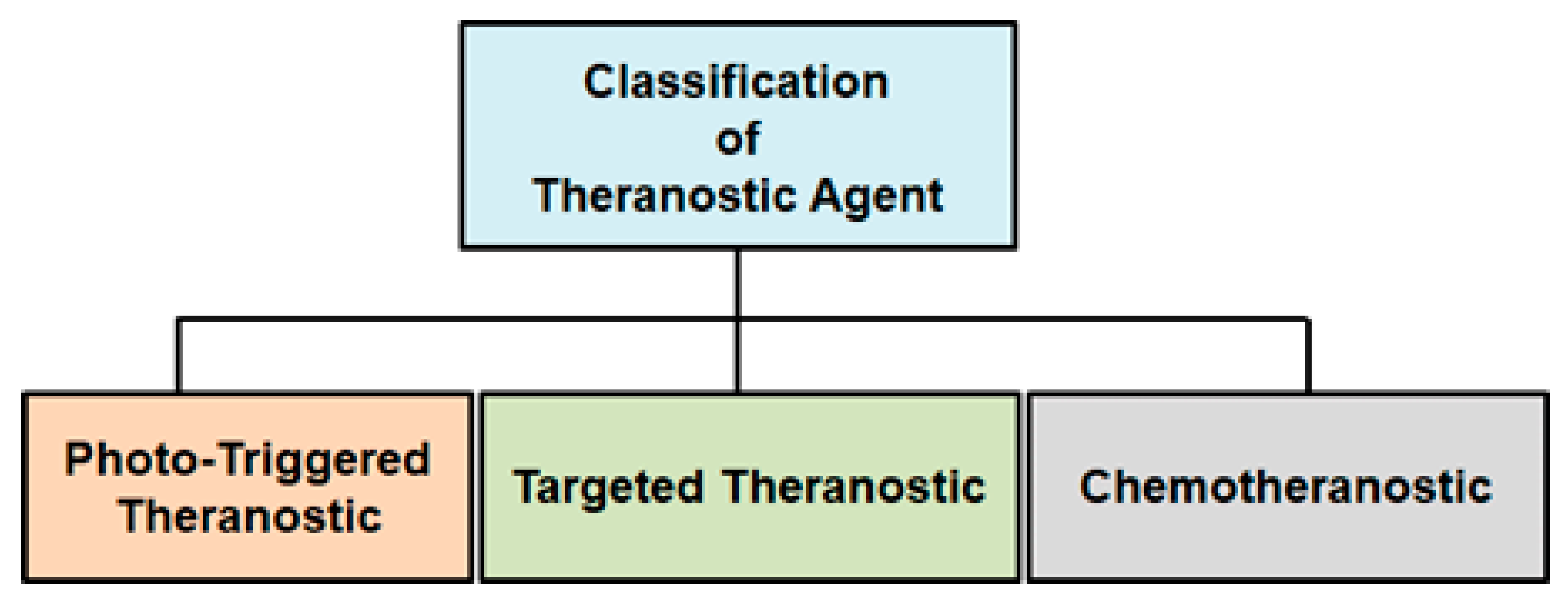
Additionally, the triplet-excited state of PTMCs confers a long-lived phosphorescence (hundreds of nanoseconds (ns) to microseconds (μs), much larger than those of organic fluorophores) with a greater Stokes shift [65,66]. Stokes shift is the distinction between the wavelength at which a molecule emits light and the wavelength at which it was excited. These unique properties permit facile differentiation of the PTMC signal from a highly auto-fluorescent background and also neglect the self-quenching of fluorescence that is displayed by some organic dye molecules [67]. This section outlines the use of phosphorescent Re(I) tricarbonyl complexes for cancer diagnostic applications. The use of -diimine ligand in the fac-[Re(CO)3(X)(-diimine)] (X = halide) structure exerts a powerful influence on the MLCT properties. The application of fac-[Re(CO)3(X)(-diimine)] complexes is advantageous because they allow easy synthesis and give some of the earliest insights into the applications of molecular metal complexes. The vigorous anticancer activity of the existing metal-based chemotherapeutic drugs gives rise to a range of unwanted adverse side effects due to their non-specific distribution throughout the body. Nonetheless, the systematic administration and pharmacokinetics of anticancer drugs as well as the precision of therapeutic drug delivery can be enhanced by the combination of therapeutic and diagnostic approaches into a single ‘‘theranostic” modality. The type of therapeutic modality classifies the anticancer drugs that should be used depending on the kind of therapy, shown in Figure 2.
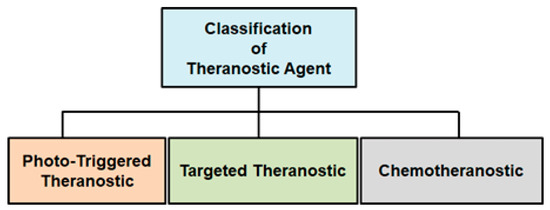
Figure 2.
Design strategies and mechanism of different theranostic approaches.
PTMC-based theranostic agents generally comprise two main constituents, namely: metal complex core and a targeting ligand. On the other hand, theranostic agents based on non-emissive transition metal-based drugs generally need three components, namely: an imaging luminophore, a metal-based pro-drug as well as a targeting ligand. Most importantly, a transition metal complex can be thought to comprise separate modules that each possess different functionalities depending on the type of theranostic method used. For example, the non-emissive platinum pro-drug, such as cis-platin, can be coordinated with extended ligands that behave as the signal transducer and targeting moiety for chemo-theranostic imaging. In contrast, with photodynamic therapy, the complex is emissive for optical imaging. The metal centre reacts as a scaffold for producing reactive radicals for therapeutic aim, while the ligands act as the targeting moiety. Therefore, with the growing interest in organometallic chemistry, several transition metal complexes-based theranostic agents, such as fac-[Re(CO)3(X)(-diimine)] (X = halide), have been synthesized with enhanced selectivity, permeability, efficacy, retention, and cellular uptake efficacy.
4.1. Luminescent Rhenium(I) Tricarbonyl Complexes
The exploitation of facial rhenium(I) tricarbonyl -diimine complexes dates back to the 1970s. Their chemical properties have attracted much attention because of their useful photo-physical attributes. Most recently, they have been widely applied as imaging agents in human cell lines due to their biological stability [56,62,68,69,70]. These types of Re(I) tricarbonyl complexes with the general formula fac-[Re(CO)3(N,N’)X]n+, (where N,N’ = 1,10-phenanthroline (phen) or 2,2 –bipyridine (bpy) X = anionic or neutral monodentate ligand and n = 0 or 1, respectively), have been widely studied due to their distinctive luminescent properties [71]. Additionally, the existence of a single electron-acceptor -diimine ligand, which negates the problem of localization of the excited electron normally occurring for polypyridine ruthenium(II) complexes, makes these complexes extremely interesting also for basic photo-physical studies [72]. The Re(I) tricarbonyl -diimine complexes display d Re → *N,N’ MLCT absorptions, which are similar to other d6 transition metal complexes. These complexes show relatively high molar absorptivity (ε = 104 cm−1.M−1) and moderately long-lived excited states (typically 0.1–1 s in solution at room temperature). During optical excitation most of these species exhibit intense and unstructured emission in solution, centred at approximately 600 nm, which emanates from the MLCT excited states that are mainly of triplet character. According to Villegas et al. (2005) [73], very high photoluminescence quantum yields (up to 0.8°) can be acquired for cationic species, whereas those of neutral species normally do not surpass 0.05 [74].
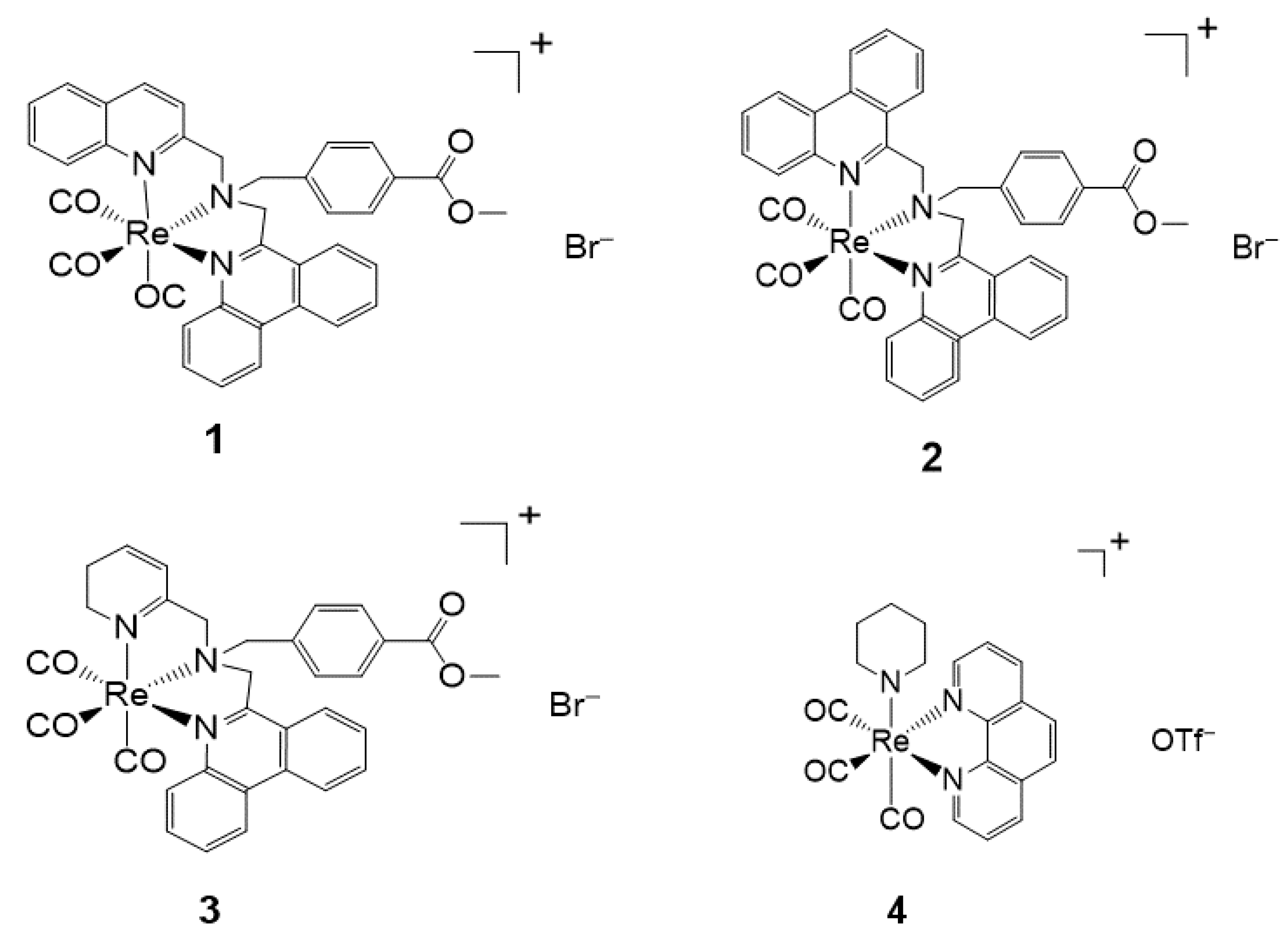
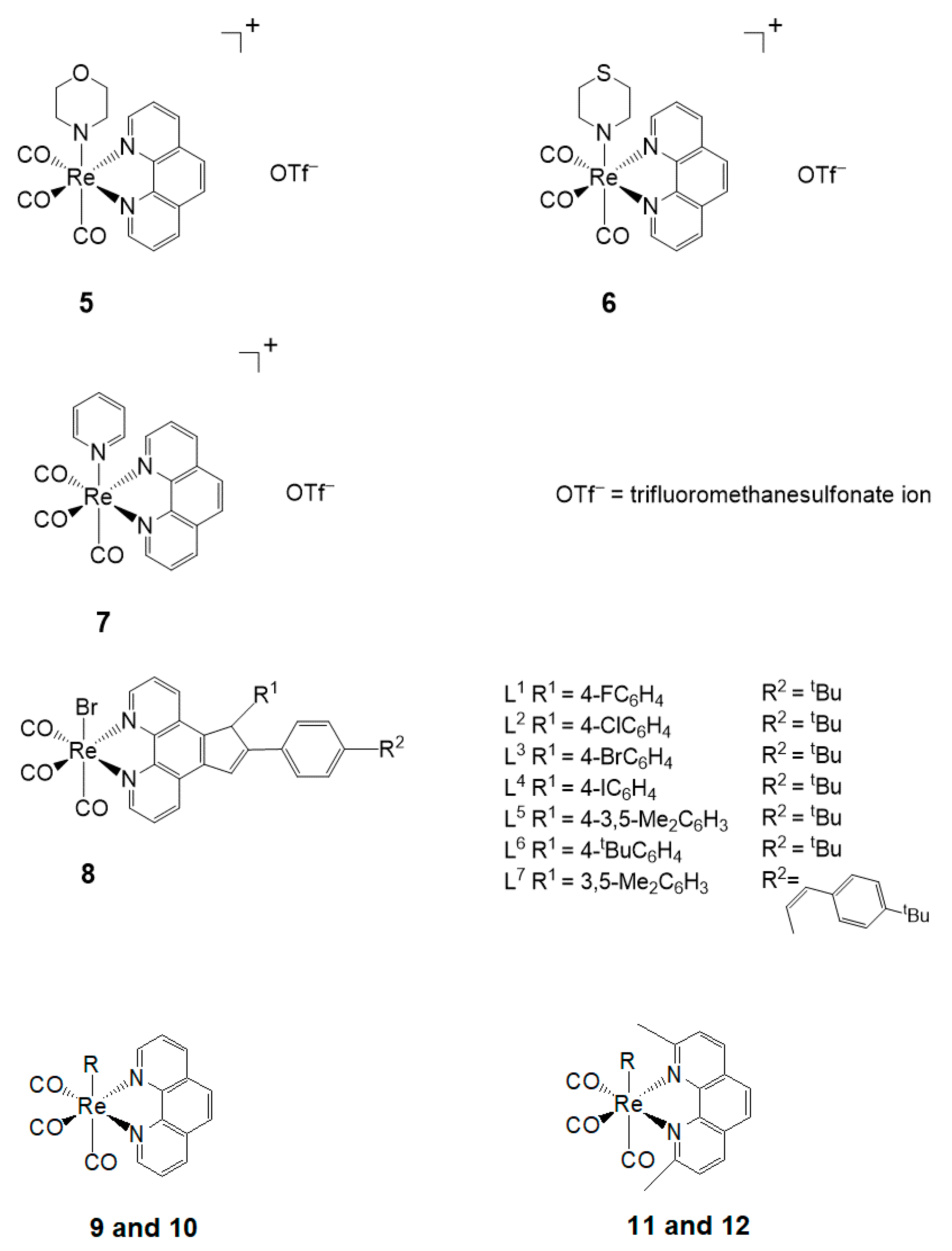
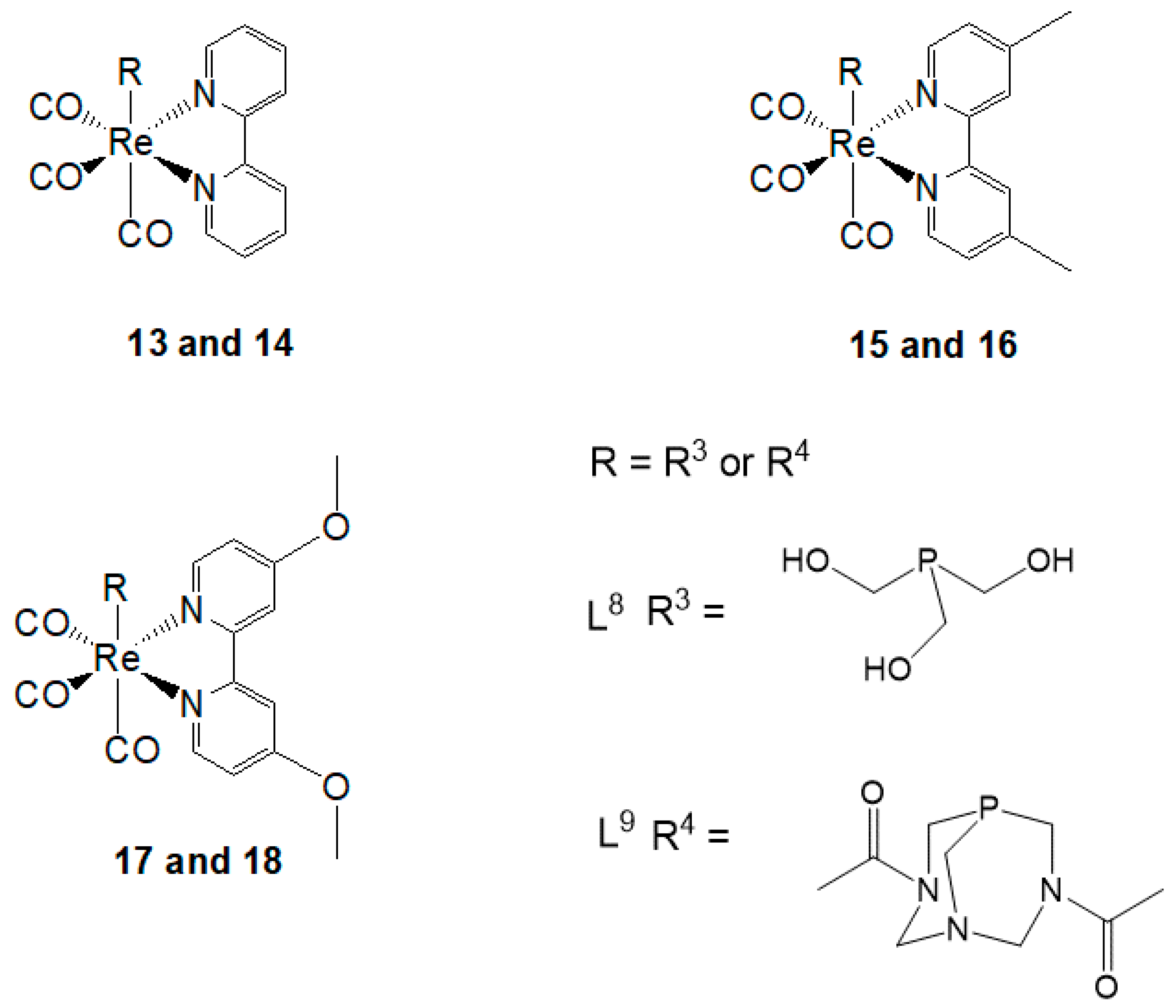
The novel Re(I) tricarbonyl complexes 1–18 (see Figure 3) possess favorable photophysical properties (i.e., emission lifetimes (τ), percentage quantum yields (Φ), emission energy (λmax), as shown in Table 2) at a given maximum wavelength (λmax). Significantly, their favorable luminescence behavior can be displayed in various solutions such as degassed acetonitrile, chloroform, and air-equilibrated water, however small these variations are in the different solvents. These beneficial luminescence properties are further highlighted by the successful application of the reported complexes (see Table 2) as imaging agents.
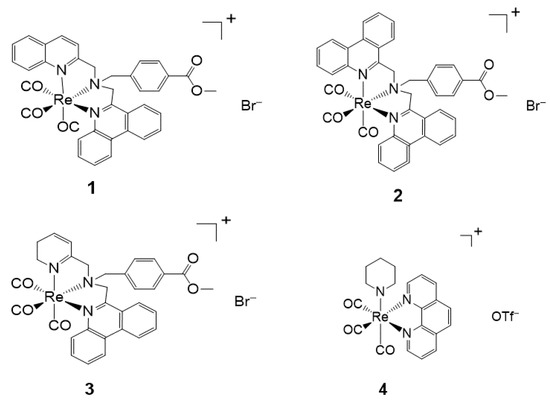
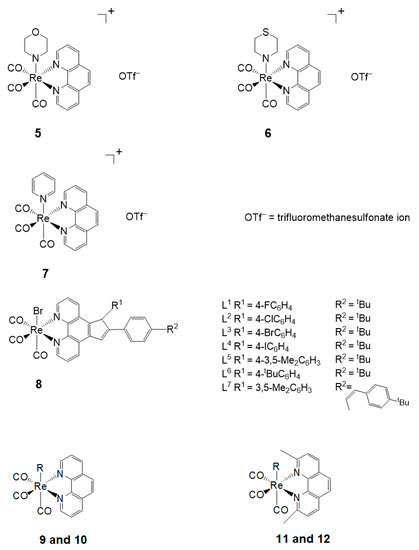
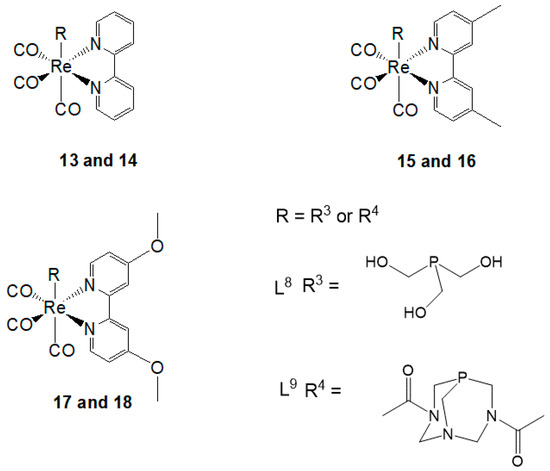
Figure 3.
Re(I) tricarbonyl complexes with favorable photophysical properties. Complexes 4–17 also show biological activities.

Table 2.
Photophysical properties of Re(I) tricarbonyl complexes 1–3 [75], 4–7 [76], 8 (L1–L7) [77] and 9–18 [78].
4.2. Chemo-Theranostic
Chemotherapy is an effective type of cancer treatment utilizing chemotherapeutic agents, which mostly function by impairing mitosis (a division of cells into two daughter cells) in rapidly dividing cancer cells. Transition metal complexes were found to have greater use in the development of chemotherapeutic agents because of their DNA alkylation and/or intercalation abilities. For instance, platinum-based alkylating agents such as cisplatin are exceptionally effective against different types of cancers, for instance, testicular and ovarian cancers. However, their small size and square planar geometry result in them achieving poor site exploitation at the double-helix level. These limitations instigated the growth of new chemotherapeutic techniques with alternative metals and geometries such as Tc and Re [79].
4.3. Cellular Imaging
Many photophysical properties of luminescent transition metal complexes, for example, large Stokes shifts, long luminescent lifetimes, and resistance to photo-bleaching in addition to low toxicity and good uptake, make them better candidates to be used as cell imaging agents [80]. Therefore, several mononuclear rhenium(I) tricarbonyl complexes with a variety of charges and degrees of hydrophobicity have been synthesized and utilized as luminophores in fluorescence cell imaging [81]. On the other hand, chemical groups have been presented in the ligand sphere to interact with or bind to specific biological targets [82]. Additionally, the localization of the excited state of fac-[Re(CO)3(bpy)(X)], (X = halide) complexes on the distinctive bipyridine chromophore [83,84] make easier modifications to permit a response to the environment. The emission from these types of systems is especially sensitive to the local surrounding [85], that involves hydrophobicity of the environments; thus, they can be further used as bio-sensing probes [86].
5. Biological Studies
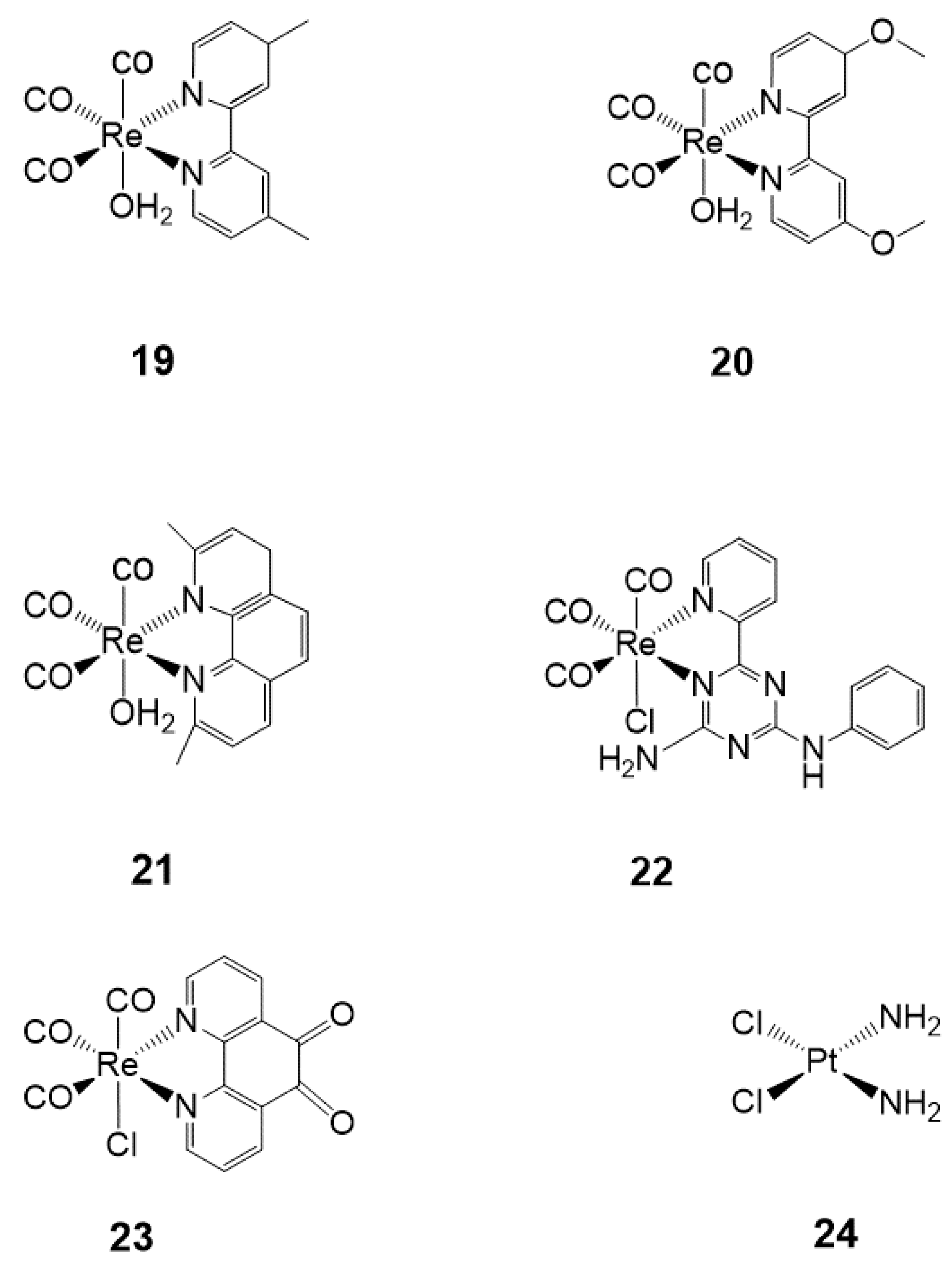
Although cisplatin is a clinically approved drug for cancer therapy, platinum resistance remains the primary concern due to genetic and epigenetic changes of various cellular routes [87]. Hence, several studies currently focus on fighting against resistance and consequently substituting these old drugs. Recently, several studies involving in vitro testing of Re(I) tricarbonyl complexes with the focus on the development of novel and target-specific chemotherapeutic drugs have been reported [87,88]. Herein, the cytotoxicity of a variety of biologically active Re(I) tricarbonyl complexes is explained in different cell cultures. To comprehend the extent of cancer drug cytotoxicity, in vitro applications in different cell lines are performed [89]. Cytotoxicity in cells is described as the inhibitory concentration (IC50) needed for a specimen or complex to kill 50% of the cell population. IC50 values are used to express cytotoxicity, which is determined as the mean percentage increase in comparison to the untreated control. Furthermore, to evaluate the cytotoxic ability of a complex, selectivity index (SI) is applied by measuring the ratio of IC50 of normal cells to the IC50 of cell death population in cancer cells [90,91]. The SI values are indicative of whether a complex is noncytotoxic or not (i.e., the greater the SI value, the more selective a compound is). An SI value of > 2 shows that a compound has selective cytotoxic activity; however, an SI value of < 2 indicates the general cytotoxicity towards cells [89]. Additionally, cellular systems obtained from cancerous tissues are frequently utilized to examine the cytotoxicity of new complexes, which is done by comparing the effect of the compounds on the tissues. Most importantly, the right concentration (µM) of test materials determines whether a particular compound is an active anticancer agent or inactive antiproliferation of cancer cells [92]. Table 3 shows different Re(I) tricarbonyl complexes 19–24 that have been tested and found to possess some cytotoxic activity against their respective cell lines: HeLa, HT-29, PT-45, HepG2, U-2 OS, A2780, CP70, etc. However, according to Knopf et al. (2017), the use of different cell lines may result in inconsistencies in some observed biological properties of complexes [93]. Furthermore, the review by Haase et al. (2019), emphasized that ligands may also play a significant role in the cytotoxicity of Re(I) tricarbonyl complexes [94]. A comparative Table 3 illustrates the active concentrations of the Re(I) tricarbonyl complexes to that of cisplatin (see Figure 4) as the reference active anticancer drug.

Table 3.
IC50 values of Re(I) tricarbonyl complexes 4–7 [76], 8 (L1–L7) [77], 9–18 [78], 19–22 [93], 23 [95], as compared to the cisplatin drug 24 [96,97,98,99,100].
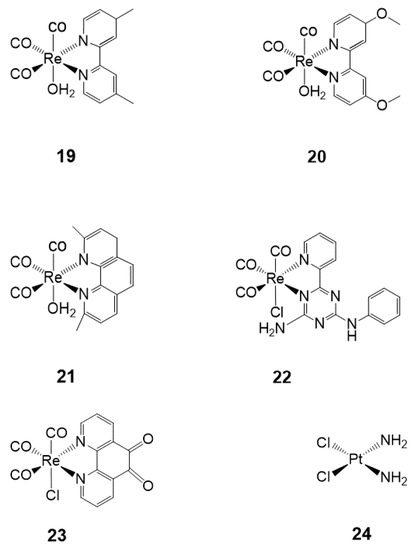
Figure 4.
Biologically active Re(I) tricarbonyl complexes 19–23 as compared to cisplatin 24.
6. Concluding Remarks
Magnetic NPs, particularly SPION crystals, have been a field of active research for pharmaceutical application. The successful clinical translation of these NPs for use in magnetic resonance (MR) contrast imaging, cancer treatment through hyperthermia, and sentinel lymph node (SLN) mapping stand as clear examples of the promise of nanotechnology to modify clinical practice and lead to enhanced patient care. Furthermore, the presence of d-block metal centres, specifically Re(I) tricarbonyl complexes, enables transition metals to set up new electronic states, which result in characteristic photophysical and photochemical properties that are essentially different from those of fluorescent substances such as organic dyes, lanthanide chelates, and quantum dots. Thus, the high photostability, long emission lifetimes, large Stokes shifts, inter/intramolecular energy/electron transfer, and the photogeneration of reactive oxygen species, make Re(I) tricarbonyl complexes promising candidates for the design of specific cell imaging reagents for biological applications. This review outlined the synergistic effect arising from the combination of magnetic NPs with luminescent Re(I) tricarbonyl complexes which results in excellent MRI-OI probes for nanomedicine in cancer theranostics.
Funding
This research was funded by the National Research Foundation, grant numbers 113629 and 117984, and Tshwane University of Technology, Pretoria, South Africa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.-L.E.M. and K.M. would like to thank the National Research Foundation (NRF-Thuthuka) (Grant Nos. 113629 and 117984), Tshwane University of Technology, South Africa, for financial support. M.M. and J.M.M. acknowledge funding from National Research Foundation (NRF) Postgraduate Scholarship, South Africa.
Conflicts of Interest
The authors declare no conflict of interest. Funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| NP(s) | Nanoparticle(s) |
| Fe3O4 | Ferric Oxide or Magnetite |
| Fe2O3 | Ferrous oxide or Hematite |
| DNA | Deoxyribonucleic Acid |
| DMSO | Dimethylsulfoxide |
| MCF-7 | Michigan Cancer Foundation-7 |
| Ag | Silver |
| Ru | Ruthenium |
| Ir | Iridium |
| 99mTc | Technetium-99m |
| MRI | Magnetic Resonance Imaging |
| FM | Fluorescence Microscopy |
| Mm | Mega-meter |
| W | Watt |
| 186Re | Rhenium(I) 186 |
| 188Re | Rhenium(I) 188 |
| α | Alpha particle |
| β | Beta particle |
| γ | Gamma-ray |
| t1/2 | Half-life |
| Emax | Maximum Energy |
| MeV | Mega Electron Volt |
| KeV | Kilo Electron Volt |
| 1O2 | Singlet Oxygen |
| CO | Carbon monoxide |
| HeLa | Henrietta Lacks |
| N | Nitrogen |
| LED | Light Emission Diode |
| O | Oxygen |
| M | Metals |
| XRD | X-ray Diffraction |
| SLN | Sentinel Lymph Node |
| TEM | Transmission Electron Microscopy |
| L | Ligand |
| OI | Optical Imaging |
| T1 | Longitudinal Relation Time |
| T2 | Transverse Relation Time |
| SPION | Superparamagnetic Iron Oxide Nanoparticles |
| OI | Optical Imaging |
| Br− | Bromide |
| Cl− | Chloride |
| H2O | Dihydrogen Monoxide |
| ILCT | Intraligand-Charge-Transfer |
| MLCT | Metal-to-Ligand-Charge-Transfer |
| LLCT | Ligand-to-Ligand-Charge-Transfer |
| H | Hour(s) |
| PTMCs | Phosphorescent Transition Metal Complexes |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Xu, J.; Kochanek, K.D.; Murphy, S.L.; Tejada-Vera, B. Deaths: Final data for 2007. Natl. Vital Stat. Rep. Cent. Dis. Control Prev. Natl. Cent. Health Stat. Natl. Vital Stat. Syst. 2010, 58, 1–19. [Google Scholar]
- Chen, Q.; Chen, J.; Yang, Z.; Xu, J.; Xu, L.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv. Mater. 2019, 31, e1802228–e1802240. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Zhang, Q.; Zeng, J.; Gu, Z.; Gao, M. Radiolabeling nanomaterials for multimodality imaging: New insights into nuclear medicine and cancer diagnosis. Biomaterials 2020, 228, 119553. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, J.R. Theranostic radiopharmaceuticals: Established agents in current use. Br. J. Radiol. 2018, 91, 20170969. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Menon, J.U.; Takahashi, M.; Hsieh, J.T.; Yang, J.; Nguyen, K.T.; Wadajkar, A.S. Thermo-responsive fluorescent nanoparticles for multimodal imaging and treatment of cancers. Nanotheranostics 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Yan Yang, Y.; Wang, S.; Zheng, S.; Fan, W. Polymer-based cancer nanotheranostics: Retrospectives of multi-functionalities and pharmacokinetics. Curr. Drug Metab. 2013, 14, 661–674. [Google Scholar] [CrossRef]
- Jia, H.-R.; Jiang, Y.-W.; Zhu, Y.-X.; Li, Y.-H.; Wang, H.-Y.; Han, X.; Yu, Z.-W.; Gu, N.; Liu, P.; Chen, Z.; et al. Plasma membrane activatable polymeric nanotheranostics with self-enhanced light-triggered photosensitizer cellular influx for photodynamic cancer therapy. J. Control. Release 2017, 255, 231–241. [Google Scholar] [CrossRef]
- Lamb, J.R.; Holland, J.P. Advanced methods for radiolabeling multimodality nanomedicines for SPECT/MRI and PET/MRI. J. Nucl. Med. 2018, 59, 382–389. [Google Scholar] [CrossRef]
- Man, F.; Gawne, P.; de Rosales, R.T. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools. Asian J. Pharm. Sci. 2020, 16, 24–46. [Google Scholar] [CrossRef]
- Edmonds, S.; Volpe, A.; Shmeeda, H.; Parente-Pereira, A.C.; Radia, R.; Baguña-Torres, J.; Szanda, I.; Severin, G.; Livieratos, L.; Blower, P.J.; et al. Exploiting the metal-chelating properties of the drug cargo for in vivo positron emission tomography imaging of liposomal nanomedicines. ACS Nano 2016, 10, 10294–10307. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P.; Abram, U.; Kaden, T.A. A novel organometallic aqua complex of technetium for the labeling of biomolecules: Synthesis of [99mTc(CO)3(H2O)3]+ from [99mTcO4]- in aqueous solution and its reaction with a bifunctional ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Top, S.; El Hafa, H.; Vessieres, A.; Quivy, J.; Vaissermann, J.; Hughes, D.W.; McGlinchey, M.J.; Mornon, J.P.; Thoreau, E.; Jaouen, G. Rhenium Carbonyl Complexes of. beta.-Estradiol Derivatives with High Affinity for the Estradiol Receptor: An Approach to Selective Organometallic Radiopharmaceuticals. J. Am. Chem. Soc. 1995, 117, 8372–8380. [Google Scholar] [CrossRef]
- Schutte, M.; Kemp, G.; Visser, H.G.; Roodt, A. Tuning the reactivity in classic low-spin d6 rhenium (I) tricarbonyl radiopharmaceutical synthon by selective bidentate ligand variation (L, L′-Bid; L, L′ = N, N′, N, O, and O, O′ donor atom sets) in fac-[Re(CO)3(L, L′-Bid)(MeOH)] n complexes. Inorg. Chem. 2011, 50, 12486–12498. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, K.; Ganji, N.; Daravath, S.; Kanneboina, K.; Rangan, K. Crystal structure, DNA interactions, antioxidant and antitumor activity of thermally stable Cu (II), Ni (II) and Co (III) complexes of an N, O donor Schiff base ligand. Polyhedron 2019, 171, 86–97. [Google Scholar] [CrossRef]
- Juergens, S.; Herrmann, W.A.; Kuehn, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Organ. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Yip, A.M.H.; Lo, K.K.W. Luminescent rhenium (I), ruthenium (II), and iridium (III) polypyridine complexes containing a poly (ethylene glycol) pendant or bioorthogonal reaction group as biological probes and photocytotoxic agents. Coord. Chem. Rev. 2018, 361, 138–163. [Google Scholar] [CrossRef]
- Carron, S.; Bloemen, M.; Vander Elst, L.; Laurent, S.; Verbiest, T.; Parac-Vogt, T.N. Potential theranostic and multimodal iron oxide nanoparticles decorated with rhenium–bipyridine and–phenanthroline complexes. J. Mater. Chem. B 2015, 3, 4370–4376. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Del Pino, P.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles–from the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Torres-Díaz, I.; Rinaldi, C. Recent progress in ferrofluids research: Novel applications of magnetically controllable and tunable fluids. Soft Matter 2014, 10, 8584–8602. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef]
- Srinivasan, S.Y.; Paknikar, K.M.; Bodas, D.; Gajbhiye, V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine 2018, 13, 1221–1238. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism, 6th ed.; Cengage Learning: Belmont, MA, USA, 2012; pp. 1–586. [Google Scholar]
- Johannsen, M.; Thiesen, B.; Jordan, A.; Taymoorian, K.; Gneveckow, U.; Waldöfner, N.; Scholz, R.; Koch, M.; Lein, M.; Jung, K.; et al. Magnetic fluid hyperthermia (MFH) reduces prostate cancer growth in the orthotopic Dunning R3327 rat model. Prostate 2005, 64, 283–292. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, H.; Lipowska, M.; Wang, L.; Yu, Q.; Yang, X.; Tiwari, D.; Yang, L.; Mao, H. A dual-modal magnetic nanoparticle probe for preoperative and intraoperative mapping of sentinel lymph nodes by magnetic resonance and near infrared fluorescence imaging. J. Biomater. Appl. 2013, 28, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Gillis, P.; Koenig, S.H. Transverse relaxation of solvent protons induced by magnetized spheres: Application to ferritin, erythrocytes, and magnetite. Magn. Reson. Med. 1987, 5, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358–5374. [Google Scholar] [CrossRef]
- Krishnan, K.M. A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Berman, S.C.; Galpoththawela, C.; Gilad, A.A.; Bulte, J.W.M.; Walczak, P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn. Reson. Med. 2011, 65, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neuro Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdellatif, A.A. Applications of nanopharmaceuticals in delivery and targeting. In Nanopharmaceuticals: Principles and Applications; Springer: Cham, Switzerland, 2021; Volume 1, pp. 73–114. [Google Scholar]
- Lu, M.; Cohen, M.H.; Rieves, D.; Pazdur, R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 2010, 85, 315–319. [Google Scholar] [CrossRef]
- Fishbane, S.; Bolton, W.K.; Winkelmayer, W.C.; Strauss, W.; Li, Z.; Pereira, B.J. Factors affecting response and tolerability to ferumoxytol in nondialysis chronic kidney disease patients. Clin. Nephrol. 2012, 78, 181–188. [Google Scholar] [CrossRef]
- Spencer, S.S.; Theodore, W.H.; Berkovic, S. Clinical applications: MRI, SPECT, and PET. Magn. Reson. Imaging 1995, 13, 1119–1124. [Google Scholar] [CrossRef]
- Thorek, D.L.; Chen, A.K.; Czupryna, J.; Tsourkas, A. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng. 2006, 34, 23–38. [Google Scholar] [CrossRef]
- Bertini, I.; Kowalewski, J.; Luchinat, C.; Parigi, G. Cross Correlation between the Dipole–Dipole Interaction and the Curie Spin Relaxation: The Effect of Anisotropic Magnetic Susceptibility. J. Magn. Reson. 2001, 152, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, J.; Yin, D.; Wang, Y.; Feng, Y.; Tan, J. Preparation and radiolabeling of human serum albumin (HSA)-coated magnetite nanoparticles for magnetically targeted therapy. Appl. Radiat. Isot. 2004, 61, 1255–1259. [Google Scholar] [CrossRef]
- Häfeli, U.O. Magnetically modulated therapeutic systems. Int. J. Pharm. 2004, 277, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, Y.; Yu, J.; Zhang, C.; Xia, J.; Yin, D. Surface modified superparamagnetic iron oxide nanoparticles: As a new carrier for bio-magnetically targeted therapy. J. Mater. Sci. Mater. Electron. 2007, 18, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Bruce, I.J.; Sen, T. Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef]
- Bloemen, M.; Brullot, W.; Luong, T.T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Wrighton, M.; Morse, D.L. Nature of the lowest excited state in tricarbonylchloro-1,10-phenanthrolinerhenium(I) and related complexes. J. Am. Chem. Soc. 1974, 96, 998–1003. [Google Scholar] [CrossRef]
- Ko, C.C.; Ng, C.O.; Yiu, S.M. Luminescent rhenium (I) phenanthroline complexes with a benzoxazol-2-ylidene ligand: Synthesis, characterization, and photophysical study. Organometallics 2012, 31, 7074–7084. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef]
- Kellar, K.E.; Fujii, D.K.; Gunther, W.H.; Briley-Sæbø, K.; Bjørnerud, A.; Spiller, M.; Koenig, S.H. NC100150 injection, a preparation of optimized iron oxide nanoparticles for positive-contrast MR angiography. J. Magn. Reson. Imaging 2000, 11, 488–494. [Google Scholar] [CrossRef]
- Jańczewski, D.; Zhang, Y.; Das, G.K.; Yi, D.K.; Padmanabhan, P.; Bhakoo, K.K.; Tan, T.T.Y.; Selvan, S.T. Bimodal magnetic-fluorescent probes for bioimaging. Microsc. Res. Tech. 2010, 74, 563–576. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef] [PubMed]
- Thorp-Greenwood, F.L.; Balasingham, R.G.; Coogan, M.P. Organometallic complexes of transition metals in luminescent cell imaging applications. J. Organ. Chem. 2012, 714, 12–21. [Google Scholar] [CrossRef]
- Ko, C.-N.; Li, G.; Leung, C.-H.; Ma, D.-L. Dual function luminescent transition metal complexes for cancer theranostics: The combination of diagnosis and therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Wu, C.; Li, G.; Han, Q.-B.; Pei, R.-J.; Liu, J.-B.; Ma, D.-L.; Leung, C.-H. Real-time detection of oxalyl chloride based on a long-lived iridium (III) probe. Dalton Trans. 2017, 46, 17074–17079. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, Z.Z.; Yang, C.; Li, G.; Tse, Y.C.; Leung, C.H.; Ma, D.L. An iridium (III) complex-based chemosensor for the detection of thiourea in living cells. Sens. Actuators B Chem. 2017, 251, 374–379. [Google Scholar] [CrossRef]
- Liu, J.-B.; Yang, C.; Ko, C.-N.; Vellaisamy, K.; Yang, B.; Lee, M.-Y.; Leung, C.-H.; Ma, D.-L. A long lifetime iridium(III) complex as a sensitive luminescent probe for bisulfite detection in living zebrafish. Sens. Actuators B Chem. 2017, 243, 971–976. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Morse, D.L.; Pdungsap, L. Intraligand lowest excited states in tricarbonylhalobis(styrylpyridine)rhenium(I) complexes. J. Am. Chem. Soc. 1975, 97, 2073–2079. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Waibel, R.; Abram, U.; Schubiger, A.P. Basic aqueous chemistry of [M(OH2)3(CO)3]+ (M=Re, Tc) directed towards radiopharmaceutical application. Coord. Chem. Rev. 1999, 190, 901–919. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Rajendran, T.; Murugan, K.S.; Kumar, M.S.; Sivasubramanian, V.K.; Ganesan, M.; Mahesh, A.; Thirunalasundari, T.; Rajagopal, S. Interaction of rhenium(I) complex carrying long alkyl chain with Calf Thymus DNA: Cytotoxic and cell imaging studies. Inorg. Chim. Acta 2015, 434, 51–59. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Jin, J.S.; Liu, B.; Zou, Z.G.; Zuo, J.L.; You, X.Z. Rhenium (I) tricarbonyl complexes with bispyridine ligands attached to sulfur-rich core: Syntheses, structures and properties. J. Organomet. Chem. 2009, 694, 763–770. [Google Scholar] [CrossRef]
- Wallin, S.; Davidsson, J.; Modin, J.; Hammarström, L. Femtosecond transient absorption anisotropy study on [Ru (bpy) 3] 2+ and [Ru (bpy)(py) 4] 2+. Ultrafast interligand randomization of the MLCT state. J. Phys. Chem. A 2005, 109, 4697–4704. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.M.; Stoyanov, S.R.; Huang, W.; Rillema, D.P. Photophysical, spectroscopic, and computational studies of a series of Re (I) tricarbonyl complexes containing 2, 6-dimethylphenylisocyanide and 5-and 6-derivatized phenanthroline ligands. Inorg. Chem. 2005, 44, 2297–2309. [Google Scholar] [CrossRef] [PubMed]
- Striplin, D.R.; Crosby, G.A. Photophysical investigations of rhenium(I)Cl(CO)3(phenanthroline) complexes. Coord. Chem. Rev. 2001, 211, 163–175. [Google Scholar] [CrossRef]
- Raszeja, L.J.; Siegmund, D.; Cordes, A.L.; Güldenhaupt, J.; Gerwert, K.; Hahn, S.; Metzler-Nolte, N. Asymmetric rhenium tricarbonyl complexes show superior luminescence properties in live cell imaging. Chem. Commun. 2016, 53, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.L.; Marker, S.C.; Lambert, V.J.; Woods, J.; MacMillan, S.N.; Wilson, J.J. Synthesis, characterization, and biological properties of rhenium(I) tricarbonyl complexes bearing nitrogen-donor ligands. J. Organomet. Chem. 2020, 907, 121064. [Google Scholar] [CrossRef]
- Bonello, R.O.; Morgan, I.R.; Yeo, B.R.; Jones, L.E.; Kariuki, B.M.; Fallis, I.A.; Pope, S.J. Luminescent rhenium(I) complexes of substituted imidazole[4,5-f]-1,10-phenanthroline derivatives. J. Organomet. Chem. 2014, 749, 150–156. [Google Scholar] [CrossRef]
- Marker, S.C.; MacMillan, S.N.; Zipfel, W.R.; Li, Z.; Ford, P.C.; Wilson, J.J. Photoactivated in vitro anticancer activity of rhenium(i) tricarbonyl complexes bearing water-soluble phosphines. Inorg. Chem. 2018, 57, 1311–1331. [Google Scholar] [CrossRef]
- Gill, M.R.; Thomas, J.A. Ruthenium (II) polypyridyl complexes and DNA—from structural probes to cellular imaging and therapeutics. Chem. Soc. Rev. 2012, 41, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- Balasingham, R.G.; Coogan, M.P.; Thorp-Greenwood, F.L. Complexes in context: Attempting to control the cellular uptake and localisation of rhenium fac-tricarbonyl polypyridyl complexes. Dalton Trans. 2011, 40, 11663–11674. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Arthur, R.J.; Coogan, M.P.; Fernández-Moreira, V.; Hayes, A.J.; Lloyd, D.; Millet, C.; Pope, S.J. 3-Chloromethylpyridyl bipyridine fac-tricarbonyl rhenium: A thiol-reactive luminophore for fluorescence microscopy accumulates in mitochondria. New J. Chem. 2008, 32, 1097–1102. [Google Scholar] [CrossRef]
- Lo, K.K.W.; Hui, W.K.; Chung, C.K.; Tsang, K.H.K.; Ng, D.C.M.; Zhu, N.; Cheung, K.K. Biological labelling reagents and probes derived from luminescent transition metal polypyridine complexes. Coord. Chem. Rev. 2005, 249, 1434–1450. [Google Scholar] [CrossRef]
- Záliš, S.; Consani, C.; El Nahhas, A.; Cannizzo, A.; Chergui, M.; Hartl, F.; Vlček, A., Jr. Origin of electronic absorption spectra of MLCT-excited and one-electron reduced 2, 2′-bipyridine and 1, 10-phenanthroline complexes. Inorg. Chim. Acta 2011, 374, 578–585. [Google Scholar] [CrossRef]
- El Nahhas, A.; Cannizzo, A.; van Mourik, F.; Blanco-Rodríguez, A.M.; Záliš, S.; Vlček, A., Jr.; Chergui, M. Ultrafast excited-state dynamics of [Re (L)(CO) 3 (bpy)] n complexes: Involvement of the solvent. J. Phys. Chem. A 2010, 114, 6361–6369. [Google Scholar] [CrossRef] [PubMed]
- Caspar, J.V.; Sullivan, B.P.; Meyer, T.J. Synthetic routes to luminescent 2, 2′-bipyridyl complexes of rhenium: Preparation and spectral and redox properties of mono (bipyridyl) complexes of rhenium (III) and rhenium (I). Inorg. Chem. 1984, 23, 2104–2109. [Google Scholar] [CrossRef]
- Mari, C.; Panigati, M.; D’Alfonso, L.; Zanoni, I.; Donghi, D.; Sironi, L.; Collini, M.; Maiorana, S.; Baldoli, C.; D’Alfonso, G.; et al. Luminescent conjugates between dinuclear rhenium complexes and peptide nucleic acids (PNA): Synthesis, photophysical characterization, and cell uptake. Organometallics 2012, 31, 5918–5928. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef]
- Corrie, P.G. Cytotoxic chemotherapy: Clinical aspects. Medicine 2008, 36, 24–28. [Google Scholar] [CrossRef]
- Shafiee, M.A.M.; Asri, M.A.M.; Alwi, S.S.S. Review on the In Vitro Cytotoxicity Assessment in Accordance to the International Organization for Standardization (ISO). Malays. J. Med. Health Sci. 2012, 17, 261–269. [Google Scholar]
- He, Y.; Zhu, Q.; Chen, M.; Huang, Q.; Wang, W.; Li, Q.; Huang, Y.; Di, W. The changing 50% inhibitory concentration (IC50) of cisplatin: A pilot study on the artifacts of the MTT assay and the precise measurement of density-dependent chemoresistance in ovarian cancer. Oncotarget 2016, 7, 70803–70821. [Google Scholar] [CrossRef] [PubMed]
- Artun, F.T.; Karagoz, A.; Ozcan, G.; Melikoglu, G.; Anil, S.; Kultur, S.; Sutlupinar, N. In vitro anticancer and cytotoxic activities of some plant extracts on HeLa and Vero cell lines. J. Balk. Union Oncol. 2016, 21, 720–725. [Google Scholar] [CrossRef]
- Pastan, I.; FitzGerald, D. Recombinant toxins for cancer treatment. Science 1991, 254, 1173–1177. [Google Scholar] [CrossRef]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In vitro anticancer activity and in vivo biodistribution of rhenium (I) tricarbonyl aqua complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- Haase, A.A.; Bauer, E.B.; Kühn, F.E.; Crans, D.C. Speciation and toxicity of rhenium salts, organometallics and coordination complexes. Coord. Chem. Rev. 2019, 394, 135–161. [Google Scholar] [CrossRef]
- Kaplanis, M.; Stamatakis, G.; Papakonstantinou, V.D.; Paravatou-Petsotas, M.; Demopoulos, C.A.; Mitsopoulou, C.A. Re (I) tricarbonyl complex of 1, 10-phenanthroline-5, 6-dione: DNA binding, cytotoxicity, anti-inflammatory and anti-coagulant effects towards platelet activating factor. J. Inorg. Biochem. 2014, 135, 1–9. [Google Scholar] [CrossRef]
- Sengupta, S.; Panda, B.K. Development of rhenium radiopharmaceuticals from coordination chemistry view point. Asian J. Res. Chem. 2017, 10, 369. [Google Scholar] [CrossRef]
- Uccelli, L.; Martini, P.; Pasquali, M.; Boschi, A. Monoclonal antibodies radiolabeling with Rhenium-188 for radioimmunotherapy. Biomed. Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-L.; Che, C.-M.; Siu, F.-M.; Yang, M.; Wong, K.-Y. DNA Binding and cytotoxicity of ruthenium(II) and rhenium(I) complexes of 2-amino-4-phenylamino-6-(2-pyridyl)-1,3,5-triazine. Inorg. Chem. 2007, 46, 740–749. [Google Scholar] [CrossRef]
- Muñoz-Osses, M.; Godoy, F.; Fierro, A.; Gómez, A.; Metzler-Nolte, N. New organometallic imines of rhenium(i) as potential ligands of GSK-3β: Synthesis, characterization and biological studies. Dalton Trans. 2018, 47, 1233–1242. [Google Scholar] [CrossRef]
- Kitanovic, I.; Can, S.; Alborzinia, H.; Kitanovic, A.; Pierroz, V.; Leonidova, A.; Pinto, A.; Spingler, B.; Ferrari, S.; Molteni, R.; et al. Anticancer activity of a ReI bisquinoline complex. Chemistry 2014, 20, 2496–2507. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).