Abstract
The amyloid β peptide (Aβ) is a central player in the neuropathology of Alzheimer’s disease (AD). The alteration of Aβ homeostasis may impact the fine-tuning of cell signaling from the very beginning of the disease, when amyloid plaque is not deposited yet. For this reason, primary culture of rat cortical neurons was exposed to Aβ25-35, a non-oligomerizable form of Aβ. Cell viability, metabotropic glutamate receptors (mGluR) and adenosine receptors (AR) expression and signalling were assessed. Aβ25-35 increased mGluR density and affinity, mainly due to a higher gene expression and protein presence of Group I mGluR (mGluR1 and mGluR5) in the membrane of cortical neurons. Intriguingly, the main effector of group I mGluR, the phospholipase C β1 isoform, was less responsive. Also, the inhibitory action of group II and group III mGluR on adenylate cyclase (AC) activity was unaltered or increased, respectively. Interestingly, pre-treatment of cortical neurons with an antagonist of group I mGluR reduced the Aβ25-35-induced cell death. Besides, Aβ25-35 increased the density of A1R and A2AR, along with an increase in their gene expression. However, while A1R-mediated AC inhibition was increased, the A2AR-mediated stimulation of AC remained unchanged. Therefore, one of the early events that takes place after Aβ25-35 exposure is the up-regulation of adenosine A1R, A2AR, and group I mGluR, and the different impacts on their corresponding signaling pathways. These results emphasize the importance of deciphering the early events and the possible involvement of metabotropic glutamate and adenosine receptors in AD physiopathology.
1. Introduction
Alzheimer’s disease (AD) is characterized by a progressive decline in cognitive functions, and the accumulation of amyloid β (Aβ) peptide (such as Aβ1-42) in senile plaques is one of the main hallmarks in AD []. It has been controversial the enigmatic roles of the different forms of Aβ peptide, derived from the metabolism of amyloid precursor protein (APP), in physiological and pathological conditions. It has been assumed for decades that APP has a prominent role in memory acquisition that could be mediated by Aβ peptide [], but it has also been believed that Aβ1-42 peptide is synaptotoxic per se in the absence of plaque burdens []. In this sense, it has been known for a long time that the physiological production of soluble Aβ1-40 peptide is also a crucial factor for the maintenance of neurons viability []; even some authors propose that soluble purified monomers Aβ1-42 peptide may have a neuroprotective role [,]. It has been reported that Aβ monomers can protect primary cortical neurons against trophic deprivation and excitotoxicity by means of a mechanism involving the activation of the phosphatidyl-inositol-3-kinase (PI-3-K) pathway []. The protective effects of Aβ seems to be Aβ size-form specific, with the Aβ1-42 size form affording limited protection and the Aβ25-35 size form having very little protective effect []. Therefore, the question of the protective function of Aβ monomers seems to be underexplored [,]. While Aβ1-42 peptide is the Aβ form most prone to aggregation [], Aβ25-35 form, a truncated form of Aβ1-42, represents the minimum functional domain of the Aβ peptide required for both neurotrophic and neurotoxic effects [,]. In this sense, Aβ25-35 has often been chosen as a model in structural and functional studies, which has contributed to the understanding of the effects of Aβ-mediated toxicity and has also facilitated the study of the modulation of its toxicity [].
Furthermore, although Aβ accumulation into senile plaques is considered as an AD diagnostic tool, nowadays there is strong evidence that there are increased levels of soluble oligomer forms of Aβ1-42 peptide, the main cytotoxic form that is also responsible for electrophysiological, anatomical, and behavioural abnormalities reported in AD brains [,,]. Despite all this knowledge supporting the “amyloid hypothesis”, researchers have not been able to develop an effective therapy based on the blockade of the increase of Aβ peptide. Therefore, some authors suggest that the role of Aβ in AD should be thoroughly challenged, and new ideas and explanations sought [].
Glutamate is the main excitatory neurotransmitter in the mammalian nervous system, playing a key role in cognitive and motor functions. Glutamate-elicited responses are mainly mediated by specific receptors that have been classified into ionotropic and metabotropic glutamate receptors (mGluR). The latter belong to the G protein-coupled receptors (GPCR) superfamily and are the main modulators of glutamate action. Based on sequence homology, signal transduction mechanisms and agonist selectivity mGluR have been divided into three groups []. The mGluR1 and mGluR5 subtypes are included in Group I mGluR, whose canonical transduction pathway is the stimulation of the phospholipase C (PLC) system through Gq/11 proteins, and they are the most studied receptors among the mGluR family. Different neurodegenerative diseases have been linked to changes in mGluR-mediated signalling []. Several years ago, the possible role of mGluR ligands, especially mGluR5, as neuroprotective agents in AD, was proposed []. Moreover, there is a growing body of evidence supporting the relationship between molecular changes in the glutamatergic system and the neurodegenerative processes triggered by Aβ in AD and other neurodegenerative diseases [], even more since it was reported that Aβ interacts with mGluR5 []. In this sense, it has been described that Aβ1-42 oligomers promote the clustering and mobilization of mGluR5 on hippocampal cultured neurons, which suggests that mGluR5 might have a prominent role in the early-synaptic failure induced by Aβ []. Furthermore, the intracerebral administration of soluble Aβ1-42 induced a predominant loss of glutamatergic terminals, which correlates with the period where memory impairment appears with no affection of other terminals than glutamatergic [] and that the long term depression (LTD) induced by soluble Aβ oligomers was mediated by NMDA or mGluR activity [].
Adenosine is a widely distributed neuromodulator involved in a variety of physiological functions. Many of these actions are mediated by adenosine receptors (AR) that, like mGluR, are included in the GPCR superfamily. AR are subdivided into adenosine A1, A2A, A2B, and A3 receptors (A1R, A2AR, A2BR, and A3R, respectively), A1R and A2AR being the main mediators of adenosine action in the CNS []. The canonical transduction pathway of AR includes the interaction with the adenylyl cyclase (AC) system, A1R and A3R being able to inhibit AC through Gi/o proteins and A2AR and A2BR able to stimulate AC through Gs proteins []. Besides, AR alteration in human disease has been extensively studied. The levels and functionality of AR have been reported to be altered in several neurodegenerative diseases, including AD []. In this regard, while A1R activation may confer neuroprotection against neurotoxic stimuli, A2AR activation may contribute to neuronal injury through the enhancement of glutamate release [].
Despite the solid evidence of the effect of soluble Aβ oligomers on neurons and/or other systems, little is known about the molecular changes into the glutamatergic and adenosinergic systems that takes place when the concentration of Aβ starts raising. We have previously reported the existence of cross-interaction between mGluR and AR [], the modulation of AR [] and mGluR [] in the cerebral cortex from AD patients, and the modulation of these receptors in a mouse model of accelerated senescence [,]. Thus, the present work aimed to study the effect of the Aβ25-35 form in the functionality and expression of metabotropic glutamate receptors, mainly group I, and adenosine receptors, mainly A1R and A2AR, in rat primary cortical neurons.
2. Results
2.1. Characterization of Aβ-Induced Toxicity on Cortical Neurons
Cortical neurons were exposed to increasing concentrations (1, 10, 25, and 50 µM) of Aβ25-35 for 24 h or maintained under control conditions, and cell viability was measured using MTT reduction assay. A concentration-dependent decrease in cell viability was detected (Figure 1, panel A). Using the highest concentrations of Aβ25-35 (25 µM and 50 µM), a significant (p < 0.05 and p < 0.01, respectively) decrease in cell viability of almost 40% was observed. Nevertheless, the difference between those treatments was not statistically significant.
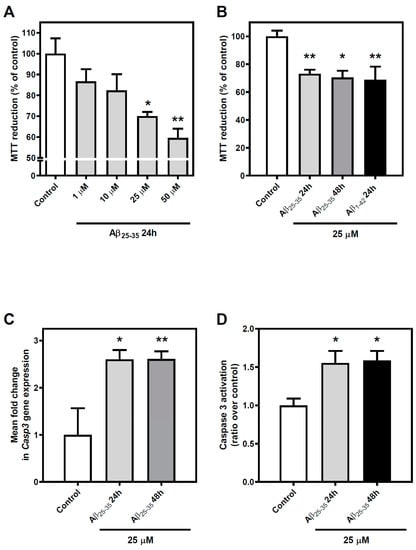
Figure 1.
Effect of Aβ25-35 and Aβ1-42 exposure on cell viability of cortical neurons. (A) Cortical neurons were exposed to different concentrations of the soluble form of Aβ (Aβ25–35) (1–50 µM) for 24 h and MTT reduction was used as a measure of cell viability. Only high concentrations of Aβ25–35 (≥25 μM) significantly reduced cell viability under these conditions. (B) Cortical neurons were subjected to 25 μM Aβ25–35 for 24 and 48 h or to 25 μM Aβ1–42 for 24 h and cell viability was determined as described in Methods. No differences were reported when the exposition time was prolonged using Aβ25-35 or when Aβ1–42 was used instead Aβ25-35 in the same conditions. (C) Expression levels of Caspase 3 (Casp3) were determined under experimental conditions. Gene expression was incremented when cortical neurons were exposed to Aβ25–35. (D) Caspase 3 activity was increased by Aβ25-35 exposure. Mean ± SEM values obtained are represented; each point was measured in triplicate employing, at least four different cultures. * p < 0.05, ** p < 0.01 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test.
To check whether the effect of Aβ25-35 exposure was acute or not, we performed further experiments in which we tested the effect of exposure time in the reported decrease of cell viability induced by Aβ25-35. In these experiments, cortical neurons were exposed to 25 µM for 24 or 48 h, and again MTT was used to measure cell viability. We introduced Aβ1-42 (25 µM, 24 h) as a positive control to compare with the effects of the whole peptide form of Aβ (Figure 1, panel B). A decrease in cell viability was reported in all cases (at least p < 0.05), but no statistical differences were observed when the different conditions were compared to each other.
Finally, to complete the validation of this experimental model, we wanted to check that Aβ25-35 exposure maintained the Aβ1-42-mediated induction of apoptosis reported in the scientific literature. To this end, we studied the gene expression of the caspase 3 coding gene and its activity in cortical neurons exposed to Aβ25-35 or maintained under control conditions. On the one hand, exposure to 25 µM Aβ25-35 produces a significant increase in the gene expression of the caspase 3 coding gene (at least p < 0.05) at both 24 and 48 h of exposure (Figure 1, panel C). On the other hand, under these same experimental conditions, an increase (p < 0.05) in Aβ25-35-induced caspase 3 activity was observed (Figure 1, panel D).
These results confirm that Aβ25-35 exposure induces cell death in rat cortical neurons in a concentration-dependent manner and settle the conditions to establish our experimental model.
2.2. Aβ25-35 Exposure Effect on the Density and Affinity of Metabotropic Glutamate Receptors
To investigate the effect of Aβ25-35 exposure on the total number and affinity of mGluR, we performed a radioligand binding assay using L-[3H]glutamic acid as radioligand under conditions in which specific binding to other glutamate receptors was blocked, as described in Materials and Methods Section 4. Rat cortical neurons were exposed to Aβ25-35 (25 µM, 24 or 48 h) or maintained under control conditions and total mGluR levels were assessed (Table 1). Total mGluR density (Bmax) was markedly increased after Aβ25-35 exposure (61% over control value at 24 h, p < 0.01; 99% over control value at 48 h, p < 0.001) and mGluR affinity (1/KD) was significantly decreased (almost 50% of the control value, p < 0.01). These data suggest that Aβ25-35 exposure modulates mGluR, inducing an increase in total receptors density and in their affinity for glutamate.

Table 1.
Aβ25-35 exposure effect on receptors density and affinity in cortical neurons.
2.3. Aβ25-35 Effect on Group I Metabotropic Glutamate Receptors’ Gene Expression and Protein Levels
As the previous results indicate an important increase in total mGluR density and affinity, we tried to determine the specific modulation of Group I mGluR after Aβ25-35 exposure. To assess that possible modulation, we studied mGluR1 and mGluR5 gene expression and protein levels under control and treated conditions in cortical neurons. To analyse the possible modulation of genes coding for Group I mGluR proteins, namely GRM1 and GRM5, we performed real-time PCR with total RNA isolated from control and Aβ25-35 (25 µM, 24 or 48 h) treated cortical neurons. After Aβ25-35 exposure, cortical neurons showed an increase in mGluR1 gene expression (at least p < 0.05) but did not present any significant variation in mGluR5 gene expression when compared with control neurons (Figure 2, panel A).
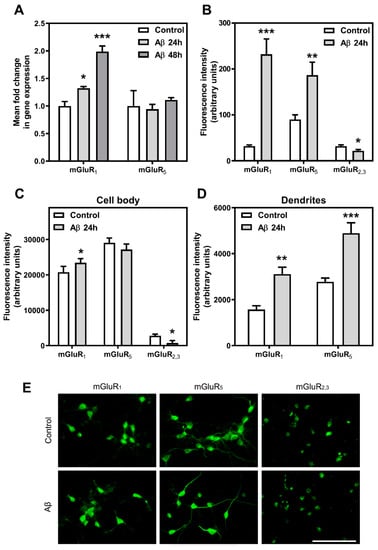
Figure 2.
Aβ25-35 effect on mGluR gene expression and protein level. (A) Quantitative real time PCR assays after Aβ25-35 treatment were performed as described in Methods. Histograms represent mean ± SEM values obtained from, at least, three independent experiments performed in duplicate using different preparations. ** p < 0.01, *** p < 0.001 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test. The effect of Aβ25-35 treatment on mGluR1, mGluR5, and mGluR2,3 membrane protein level was assayed using image analysis of immunostaining in the whole-cell (B), the cell bodies (C), and the dendrites (D). Histograms represent mean ± SEM values obtained from, at least, three independent experiments performed in duplicate using different preparations. * p < 0.05, ** p < 0.01, *** p < 0.001 significantly different from untreated condition using two-tailed Student’s t-test. (E) Representative photomicrographs of mGluR1, mGluR5, and mGluR2,3 immunochemistry in control and Aβ25-35-treated cortical neurons. Bar represents 100 μm.
Nevertheless, as gene expression levels do not always correlate with protein levels (30,36), we measured the protein levels of mGluR1, mGluR5, and mGluR2,3 at the membrane surface of cortical neurons exposed to Aβ25-35 (25 µM, 24 h) or maintained under control conditions by immunostaining in non-permeabilized cells employing extracellular specific antibodies (Figure 2, panel B). An increase in the density of mGluR1 and mGluR5 was detected at the membrane level after Aβ25-35 exposure, especially significant in the case of mGluR1 (mGluR1: more than 7-fold increase over control value, p < 0.001; mGluR5: twice over control value, p < 0.01). Interestingly, a further analysis by differentiating cell body and dendrite regions revealed that such increase was mainly observed in dendrites (Figure 2, panels C and D). Nevertheless, a decrease in the density of mGluR2,3 was observed at the membrane surface of cell bodies after Aβ25-35 exposure. Quantitation of each condition and representative micrographs are shown (Figure 2, panel E). Although immunofluorescence assays were performed with extracellular antibodies and without a permeabilization step, it could be possible that the fixation method employed here (10 min with 4% PFA at room temperature) affects the integrity of the plasma membrane. Therefore, the results of the immunofluorescence experiments should be viewed with caution. Together, these data indicate that Aβ25-35 exposure induces differential changes in mGluR proteins.
2.4. Modulation of PLC β1 Signalling Pathway after Aβ25-35 Exposure
As altered glutamatergic transmission has been suggested to have a pivotal role in many neurodegenerative diseases, we decided to further analyze the effect of Aβ25-35 exposure on the canonical transduction pathway mediated by Group I mGluR, the phospholipase C activation through Gq proteins.
We first tested whether gene expression of PLCβ1, the most highly expressed of PLCβ isoforms in the brain [], was modulated in the presence of Aβ25-35. We determined PLCβ1 gene expression by real-time RT-PCR with total RNA isolated from control and Aβ25-35 (25 µM, 24 or 48 h) treated cortical neurons. We detected a significant decrease in the expression levels of this gene after Aβ25-35 exposure (28% and 33% decrease at 24 and 48 h over control value, respectively, p < 0.05) (Figure 3, panel A).
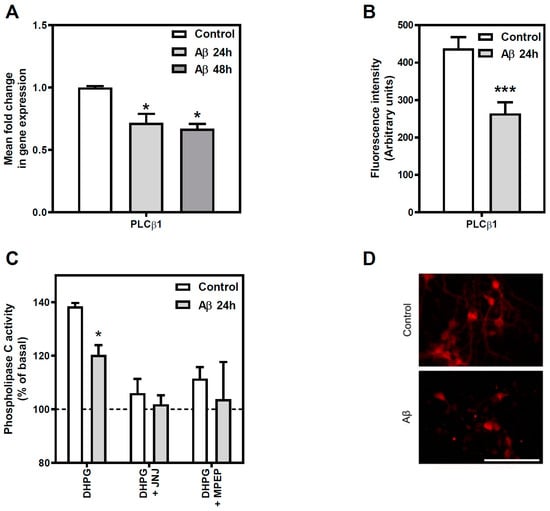
Figure 3.
Effect of Aβ25-35 exposure on Group I mGluR-mediated PLC pathway in cortical neurons. (A) Effect of Aβ25-35 treatment on PLCβ1 gene expression was measured using qPCR. Rat cortical neurons were exposed to 25 μM Aβ25-35 for 24 h and 48 h or maintained under control conditions prior to RNA isolation. Quantitative real time PCR assays were performed as described in Methods. Data are means ± SEM of, at least, three independent RNA isolations and their quantitative PCR replicates. * p < 0.05 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test. (B) Effect of Aβ25-35 treatment on PLCβ1 protein level was measured using immunohistochemistry. Data are means ± SEM of, at least, three independent experiments performed in duplicate using different preparations. *** p < 0.001 significantly different from untreated condition using two tailed Student´s t-test. (C) Group I mGluR-mediated PLC activity was measured under control and treated conditions. Stimulation of phosphoinositide breakdown was measured in intact neurons exposed to 25 μM Aβ25-35 for 24 h or maintained under control conditions. Group I mGluR-mediated PLC activity was stimulated using 30 μM DHPG for 10 min in control and Aβ-treated cortical neurons. The specificity of the assay was tested using the antagonists JNJ 16259685 and MPEP at concentrations of 0.5 µM and 1 µM, respectively. Results are expressed as a percentage of the basal (unstimulated) activity in the absence of DHPG (basal values: control 26.81 ± 7.27; Aβ25-35 treated 17.61 ± 4.85 pmol/mg·min). Data are means ± SEM of, at least, three independent experiments performed in duplicate using different cultures. All sets of data were significantly different (at least p < 0.05) from their corresponding basal value. * p < 0.05, significantly different from control value using two tailed Student´s t-test. (D) Representative images of immunochemistry experiments presented in panel B are shown for control and 25 μM Aβ25-35 exposed cells for 24 h. Bar represents 100 μm.
We then assessed whether PLCβ1 protein levels correlated with the observed decrease in its gene expression after Aβ25-35 exposure. For that purpose, we performed immunocytochemistry assays in cortical neurons fixed and permeabilized (Figure 3, panel B). The quantification of the images indicated that Aβ25-35 exposure reduced the total amount of PLCβ1 protein when compared to control cells (40% decrease over control value, p < 0.05). Representative micrographs of each condition quantified are shown in panel D of Figure 3.
To further analyze the Aβ25-35 effect on the main transduction pathway mediated by the group I mGluR, we performed enzymatic activity assays to determine the functionality of the whole system. Therefore, PLC activity was determined in basal and DHPG-stimulated conditions in cortical neurons exposed to Aβ25-35 (25 µM, 24 h) or maintained under control conditions (Figure 3, panel C). A significant decrease in PLC activity was observed in Aβ25-35 exposed cells when DHPG was used as an agonist (13% of decrease, p < 0.05), while no significant differences were observed when the activity was studied in basal conditions (basal activity: Control 26.8 ± 7.3 pmol/mg·min versus Aβ25-35 17.6 ± 4.8 pmol/mg·min). Therefore, Aβ25-35 exposure reduced the functionality of the Group I mGluR/PLC signalling pathway, but no modulation of PLC basal activity was found. Besides, 0.5 µM JNJ 16259685 (a highly potent selective mGluR1 antagonist) or 1 µM MPEP (a potent mGluR5 antagonist), added 10 min before the addition of DHPG, were used to distinguish the specific involvement of mGluR1 and mGluR5 on PLC stimulation. These antagonists blocked DHPG-elicited PLC stimulation in control cells and there were no differences between control and Aβ25-35 treated cells (Figure 3, panel C).
2.5. Effect of Aβ25-35 Exposure on Metabotropic Glutamate Receptors/Adenylyl Cyclase Pathway
To study whether proteins and gene expression variations detected in neurons were associated with alterations of their corresponding functionality, mGluR-mediated AC activity in control and Aβ25-35 (25 µM, 24 h) exposed neurons under basal and stimulated (5 µM forskolin, an activator of AC) conditions was determined. No differences were found in basal or forskolin-stimulated AC activity values between control and Aβ25-35-treated neurons (basal activity: control 2.91 ± 0.60 pmol/mg·min versus Aβ25-35 treated 2.33 ± 0.55 pmol/mg·min). Also, mGluR-mediated AC inhibition was performed using 100 µM APDC and 100 µM L-AP4, selective agonists for Group II and Group III mGluR, respectively, in the presence of 5 µM forskolin (Figure 4). While the ability to inhibit forskolin-mediated AC stimulation by Group II mGluR remained unaltered after Aβ25-35 exposure, Group III-mediated AC inhibition was incremented in cortical neurons exposed to Aβ25-35 (69% of increase over control value, p < 0.05).
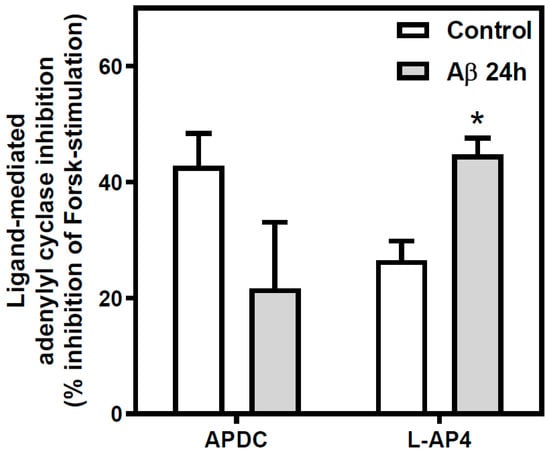
Figure 4.
Effect of Aβ25-35 treatment on Group II and III mGluR-mediated adenylyl cyclase pathway in cortical neurons. Inhibition of AC was measured in intact neurons exposed to 25 μM Aβ25-35 for 24 h or maintained under control conditions. Control and treated cells were incubated in the presence of forskolin (5 µM) and in the absence or in the presence of 100 μM (2R,4R)-APDC or 100 μM L-AP4, specific group II and group III mGluR agonists, respectively. Aβ25-35 exposure increased Group III-mediated inhibition of the forskolin-stimulated AC activity in cortical neurons. Data are means ± SEM of, at least, three independent experiments performed in duplicate using different preparations. All data were significantly different (at least p < 0.05) from their respective forskolin-stimulated value. Basal values: control 2.91 ± 0.60; Aβ25-35 treated 2.33 ± 0.55 pmol/mg·min. * p < 0.05 significantly different from untreated condition using two tailed Student´s t-test.
2.6. Effect of Aβ25-35 Exposure on Adenosine A1 and A2A Receptors
Adenosine A1R and A2AR levels were analyzed by radioligand binding assay using [3H]DPCPX or [3H]ZM241385, respectively, as radioligand in rat cortical neurons exposed to Aβ25-35 (25 µM, 24 or 48 h) or maintained under control conditions (Table 1). Total A1R density (Bmax) was markedly increased after Aβ25-35 exposure (265% over control value at 24 h, p < 0.01; 184% over control value at 48 h, p < 0.01), while A1R affinity (1/KD) was significantly reduced (between 4.7–6.5 less affinity than control value, p < 0.05). Total A2AR density (Bmax) was not altered after 24 h of Aβ25-35 exposure but it was significantly increased (80% over control value, p < 0.001) after 48 h of treatment. In contrast, no significant variation of A2AR affinity (1/KD) was observed. These data suggest that Aβ25-35 exposure induces an increase in total A1R density and a decrease in its affinity, while A2AR is also up-regulated, but later (48 h), without changes on receptor’s affinity.
2.7. Effect of Aβ25-35 Exposure on Adenosine Receptor Gene Expression
To determine whether variations observed in A1R and A2AR densities at the cell surface were due to modifications of gene expression, the expression of the three more common types of adenosine receptors, A1R, A2AR, and A2BR, were analyzed by real time PCR in neurons exposed to Aβ25-35 (25 µM, 24 or 48 h) or maintained under control conditions. In all tested conditions (Figure 5), A1R, A2AR, and A2BR gene expression were significantly increased (at least p < 0.05) by Aβ25-35 exposure. These results suggest that the increase in A1R and A2AR densities previously detected could be explained because of an increased gene expression.
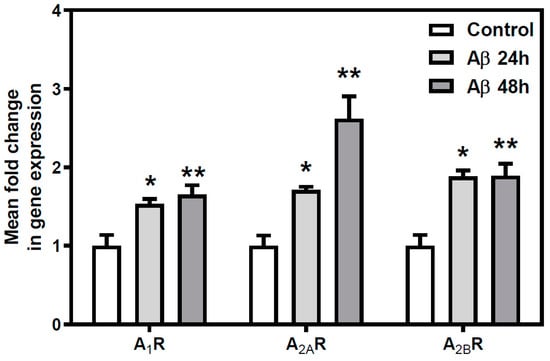
Figure 5.
Aβ25-35 effect on AR gene expression. Quantitative real-time PCR assays after Aβ25-35 exposure were performed to measure the expression of A1R, A2AR, and A2BR genes. Histograms represent mean ± SEM values obtained from at least three independent experiments performed in duplicate using different preparations. * p < 0.05 ** p < 0.01 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test.
2.8. Effect of Aβ25-35 Exposure on Adenosine Receptors-Mediated Adenylyl Cyclase Activity
Adenosine A1R- and A2AR-mediated AC activity was measured in cortical neurons exposed to Aβ25-35 (25 µM, 24 h) or maintained under control conditions. Aβ25-35 exposure did not alter basal or forskolin-stimulated cyclic AMP levels, as commented above. A1R-mediated inhibition of AC activity (56% increase, p < 0.01), measured as the ability of 1 µM CHA to inhibit forskolin-mediated AC stimulation, was significantly increased (Figure 6, panel A). Nonetheless, Aβ25-35 exposure did not alter A2AR-mediated stimulation of AC activity, using 1 µM CGS 21680 as a selective A2AR ligand (Figure 6, panel B).
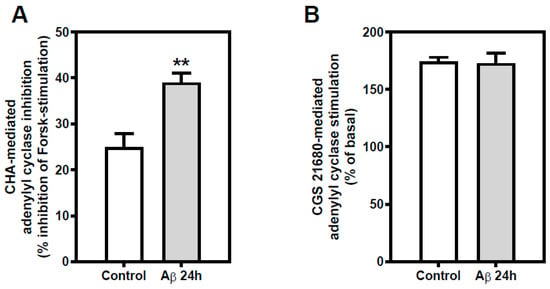
Figure 6.
Effect of Aβ25-35 exposure AR-mediated adenylyl cyclase pathway in cortical neurons. (A) Inhibitory effect of 1 µM CHA, selective A1R agonist, on forskolin-stimulated cyclic AMP accumulation in intact cortical neurons was measured. Aβ25-35 exposure in cortical neurons increased A1R-mediated AC inhibition. (B) Stimulatory effect of 1 µM CGS 21680, selective A2AR agonist, was measured in intact neurons exposed to 25 μM Aβ25-35 for 24 h or maintained under control conditions. Histograms represent mean ± SEM values obtained from at least three independent experiments performed in duplicate using different preparations. * p < 0.05 ** p < 0.01 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test.
2.9. Effect of Aβ25-35 Exposure on the Expression of Genes Coding for CREB and CREM
CREB and CREM transcriptional regulation factors gene expression was analyzed by real-time PCR in cortical neurons exposed to Aβ25-35 (25 µM, 24 or 48 h) or maintained under control conditions (Figure 7). Aβ25-35 exposure induced a decrease in the expression of genes encoding for CREB and CREM transcription factors in all tested conditions (at least p < 0.05). These results suggest that Aβ25-35 exposure could potentially affect molecular pathways regulated by CREB/CREM factors.
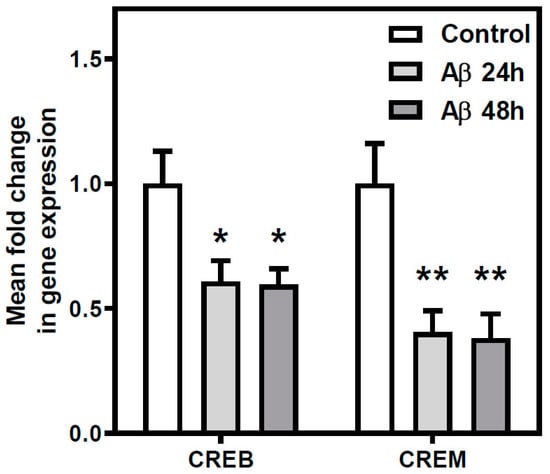
Figure 7.
Aβ25-35 effect on the expression of cyclic AMP-dependent transcription factors. The expression of the cAMP-dependent transcription factors CREB and CREM was measured using qPCR. Rat cortical neurons were exposed to 25 μM Aβ25-35 for 24 h, 48 h, or maintained under control conditions prior to RNA isolation. Aβ25-35 exposure reduced the expression of CREB and CREM. Histograms represent mean ± SEM values obtained from three independent experiments performed in duplicate using different preparations. * p < 0.05 and ** p < 0.01 significantly different from control value using one-way ANOVA and Bonferroni’s post hoc test.
2.10. Effect of Group I Metabotropic Glutamate and Adenosine A1 and A2A Receptors Ligands on Aβ25-35 Induced Toxicity
Finally, to determine the possible neuroprotective role of these receptors, we tested whether the activation or blockade of Group I mGluR, A1R, or A2AR exerted any influence on cortical neurons viability (Figure 8). For that purpose, we used DHPG as selective Group I agonist and AIDA as selective antagonist; CPA and DPCPX as selective A1R agonist and antagonist, respectively; and CGS21680 and ZM241385 as selective A2AR agonist and antagonist, respectively. These drugs were added separately 30 min before the addition of Aβ25-35 (25 µM, 24 h). It was observed that Group I mGluR blockade with AIDA partially prevented Aβ25-35-induced toxicity (12% of viability increase in Aβ25-35 treated neurons, p < 0.05), while Group I activation or A1R or A2AR activation or blockade had no significant effect on Aβ25-35 induced toxicity. These data suggest that the blockade of Group I mGluR has a neuroprotective effect on cortical neurons exposed to Aβ25-35.
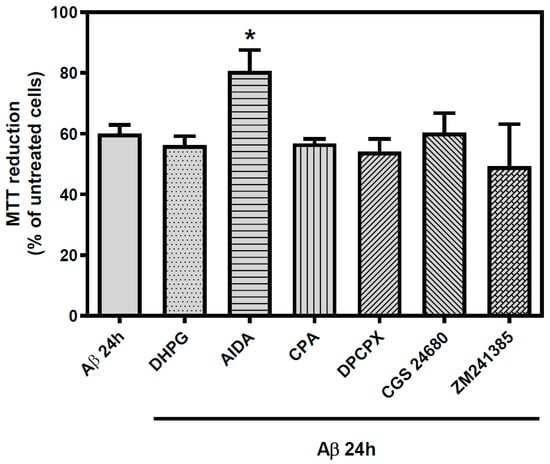
Figure 8.
Effect of Group I metabotropic glutamate receptors and adenosine receptors selective ligands on the toxicity exhibited by Aβ25-35 exposure. Rat cortical neurons were subjected to selective Group I mGluR and AR ligands prior to 25 μM Aβ25-35 for 24 h. DHPG was employed as selective Group I mGluR agonist and AIDA as selective antagonist. CPA was used as selective agonist of A1R, DPCPX as A1R antagonist, CGS 21680 as A2AR agonist, and ZM241385 as A2A antagonist. Each of the ligands were used at 100 µM and added 30 min before Aβ insult and maintained with amyloid peptide until the beginning of MTT quantification. Mean ± SEM values obtained are represented; each point was measured in triplicate employing, at least, three different cultures. * p < 0.05 significantly different from Aβ value using one-way ANOVA and Bonferroni’s post-hoc test.
3. Discussion
Understanding the biological mechanisms underlying early alterations in AD is a key point to gain insight into AD etiopathogenesis and to define the appropriate time windows for AD treatment. The alteration of Aβ homeostasis, due to an imbalance between Aβ production and clearance, may impact the fine-tuning of synaptic vesicle cycling, neurotransmitter release, and cell signalling, thus altering synaptic homeostasis [].
Our findings indicate that cortical neurons exposed to Aβ25-35, a peptide broadly used in in vitro and in vivo models of AD [,], undergo a significant change in the signalling pathways mediated by different mGluRs and ARs, and a reduction in the cell viability. In brief, (i) density of Group I mGluR was increased at the plasma membrane together with an increase in mGluR1 gene expression; however, the ability of these receptors to activate the PLC system resulted in being impaired. (ii) The ability of Group III mGluR to inhibit AC was increased. (iii) the density of A1R and A2AR was increased together with a higher gene expression; however, while the ability of A1R to inhibit AC was increased, the ability of A2AR to stimulate this system was unaffected by Aβ25-35 exposure. (iv) The gene expression of the transcription factors CREB and CREM was decreased. (v) Finally, the cell death evoked by Aβ25-35 exposure was partially prevented by pre-incubation with a Group I mGluR antagonist (Scheme 1).
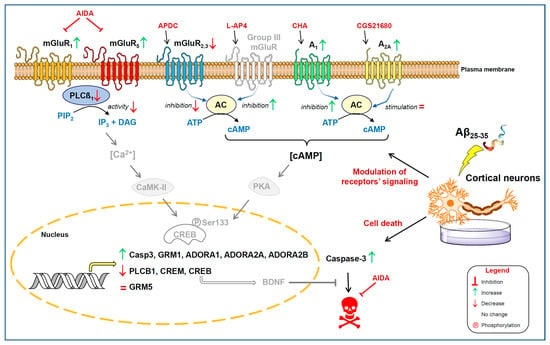
Scheme 1.
Early events after Aβ25-35 exposure. Density of metabotropic glutamate receptors (mGluR) and adenosine receptors (AR) are modulated at the plasma membrane (see Legend). Gene expression of indicated genes was also altered. Activation of phospholipase C (PLC) through mGluR was impaired. The ability of Group II (mGluR2,3) and Group III mGluR to inhibit adenylate cyclase (AC) activity was decreased and increased, respectively. The density of A1R and A2AR was increased together with a higher gene expression; however, while the ability of A1R to inhibit AC increased, the ability of A2AR to stimulate this system was unaffected by Aβ25-35 exposure. The gene expression of the transcription factors CREB and CREM was decreased. The cell death evoked by Aβ25-35 exposure was partially prevented by pre-incubation with a Group I mGluR antagonist (AIDA). Molecules or proteins not directly measured in the present study are shown in grey colour.
Although a neuroprotective role of Aβ [] that seems to be Aβ size-form specific [] was reported, it has been assumed that Aβ1-42 soluble peptide could induce synaptic deficits before plaque deposition []. Our research group has reported the modulation of adenosine receptors, which is proposed as a new therapeutic target to manage AD cognitive deficits [,,,] in a model of ageing and age-associated diseases [] at stages before the appearance of senile plaques []. Besides, mGluR are good candidates as molecular targets to reach neuroprotection in neurodegenerative diseases as they modulate (instead of mediate) excitatory synaptic transmission [,,,,].
The ability of physiologically-released Aβ, particularly in oligomeric forms, to control neuronal excitability by inducing long term depression (LTD) through NMDA receptors activation has been known for a long time []. Furthermore, Aβ could induce this phenomenon by its ability to block neuronal glutamate uptake, leading to increased glutamate levels at the synaptic cleft; excessive NMDA receptors activation and consequently glutamate receptors internalization; lower increases in [Ca2+]i; and synaptic depression (reviewed in []). This hypothesis correlates with previous publications of our group. We described that in the frontal cortex of AD brains there is a down-regulation of total mGluR and a desensitization of the calcium signalling pathway mediated by PLC []. Also, we demonstrated that the mGluR expressed in cortical neurons exposed in vitro to glutamate (1 µM L-Glu for 24 h or 100 µM L-Glu for 2 h) suffer a classical response to agonist and are down-regulated at the time that excitotoxic cell death is induced []. Nevertheless, these previous data contrast with the results reported in the present work, focused on the very early effects of Aβ presence. Although the functional significance of this phenomena is unknown at present, the increase in Group I mGluR reported here could likely be part of a compensatory mechanism that manages to recover part of the lost synaptic transmission among neurons elicited by the toxic effect of Aβ. Differences between laboratory models (e.g., in vitro models) and human brains have been reported before and have been assigned to the fact that while the former serves as an example of a pre-clinical stage of the disease, the latter represents an advanced stage of the disease [,].
There is strong evidence that Group I mGluR develops a prominent role in the Aβ–neuron interaction, which could explain the up-regulation reported here. In this sense, soluble oligomers of Aβ induced the abnormal accumulation of mGluR5 in the synaptic cleft, and in reactive astrocytes [], which produced synapse decline on hippocampal neurons []. Furthermore, although it was assumed that soluble Aβ oligomers disrupted synaptic plasticity by altering glutamate recycling at the synapse [], recently it was proposed that those events should be secondary and that mGluR5 have a much more central role in mediating Aβ disrupting effects than was previously believed []. Our results correlate with those studies suggesting the interaction between mGluR5 and Aβ as the starter of a positive feedback mechanism, which induces a further increase in Aβ levels and, consequently, further neurodegeneration, a mechanism which can be prevented by genetic deletion of GRM5, which also would reduce Aβ burdens and soluble oligomers [,]. Therefore, the blockade of those receptors could also prevent this positive feedback. Interestingly, in the same animal model (APPswe/PS1ΔE9 mice), an age-dependent increase of mGluR1 in the cortex was reported, which is also related to the levels of Aβ peptide []. As we previously reported that mGluR1 levels decrease in the human brain frontal cortex with the progression of AD pathology [], we suggest that mGluR1 levels could depend on the stage of disease and the cellular components (i.e., neurons, glia, etc.) mimicked by the experimental model.
In line with this, mGluR5 mRNA and protein level are upregulated in cultured astrocytes following 48 h of treatment with Aβ oligomers [,]. Moreover, it can be found a strong enrichment of mGluR5 on reactive astrocytes surrounding Aβ plaques and a rapid binding and clustering of Aβ oligomers over the astrocytic cell surface, which represents a diffusional trapping and clustering of mGluR5 within Aβ clusters, which in turn leads to an increased ATP release []. The mGluR5, with the participation of PrPC as co-receptor [,], has been included among the candidates for receptors of Aβ oligomers [].
Within the sequence of major pathogenic events leading to AD proposed by the amyloid cascade hypothesis, the very early increase in Aβ oligomers may directly injure the synapses and neurites of brain neurons, in addition to activating microglia and astrocytes []. Thus, a limitation of the present work could be the absence of other cell types also present in the whole brain compared to cortical neurons (e.g., absence of glial-neuronal interactions; however, it can be also helpful to avoid the presence of many other confusing factors in our experimental model that hinder our focus in the early events occurring in cortical neurons after Aβ peptide exposure. Therefore, these new data should be integrated with the effects described on other systems.
As the molecular mechanisms responsible for mGluR up-regulation are not understood, it is hard to explain the controversial effect of the specific antagonist (i.e., AIDA) on cell viability recovery. In fact, the precise role of Group I-specific drugs as neuroprotective agents or neurodegenerative facilitators has been discussed for a long time. Nowadays, the development of allosteric modulators seems to be a promising way to handle neurodegenerative disorders [], although it is assumed that Group I mGluR antagonists are expected to behave as neuroprotective agents []. Different roles have been proposed for mGluR and their specific ligands depending on the system studied. Thus, mGluR1 could function as a dependence receptor, and while its overexpression is neurotoxic in rat cerebellar neurons, mouse cortical neurons or rat cortical astrocytes, the decrease of its expression is neuroprotective []. This neuroprotection by reduced receptor expression correlates with experiments presented here where the blockade of Group I receptors is partially neuroprotective. MGluR1 blockade might be a potential enhancer of GABA release [,], which could be responsible for the neuroprotective role reached by selective mGluR1 antagonists under various toxic conditions like ischemia [,] or in clinical environments for the treatment of anxiety, depression [] or schizophrenia []. Moreover, selective blockade of mGluR5 is neuroprotective in vitro against Aβ toxicity [] or in vivo against MPTP effects [,], with promising clinical perspectives also in other CNS-related diseases (reviewed in []).
The role of Aβ peptide in calcium signalling is also controversial. Differences between the experimental models that used non-oligomerizable forms of Aβ and the ones that employ Aβ burdens have been reported. In the first case, soluble Aβ peptide impairs PKC signalling [] and decreases the NMDA receptor-mediated calcium signalling []. In the second case, some neurons in the vicinity of Aβ plaques experimented with an increase in intracellular calcium [,], and those neurons were directly related to the learning impairment observed in animal models. We have not measured calcium levels in the present study. However, we detected a decreased mGluR/PLC signalling after Aβ25-35 exposure. Furthermore, we previously reported this abnormal mGluR/PLC signalling in Diffuse Lewy body disease, a neurodegenerative disease with some AD-related hallmarks []. Those apparent controversial results concerning calcium levels must be further studied taking into account the dual role of glutamate signalling in physiology and pathology [] and the possible role that modulation of Gq/11 proteins under different conditions may have in Group I mGluR-mediated calcium signalling via PLC [].
In the present work, the A1R density at the plasma membrane was modulated by Aβ25-35 before (24 h) than A2AR (48 h) and the grade of change was higher for A1R. This suggests a prominent role for A1R in the regulation of A2AR. Similarly, A1R activation was the starter of a coordinated program of re-adaptation of both A1R- and A2AR-mediated pathways found during hypoxia in rat C6 glioma cells, which were resistant to cell death elicited by hypoxic insult. Interestingly, CREB and CREM transcription factors were also decreased []. A1R was found to be significantly increased in human neuroblastoma SH-SY5Y cells treated with Aβ25-35 and in the brain tissue of 5xFAD mice when Aβ25-35 was directly injected into the lateral ventricles []. Furthermore, these results are consistent with the significant increase of both A1R and A2AR previously detected in the frontal cortex of AD patients from stages I to VI of Braak [], although the different expression and activity of adenosine receptors seem to be brain region-specific (for a review see [,]).
The efficacy of caffeine (AR antagonist) against AD and AD-related cognitive impairment was reviewed, focusing on the proposed protective mechanisms of action []. There is evidence that caffeine and A2AR antagonists afford protection against Aβ-induced amnesia in vivo [] and prevent the neuronal cell death caused by exposure of rat cultured cerebellar granule neurons to 25 µM Aβ25-35 for 48 h []. However, a neuroprotective role for A1R antagonism cannot be ruled out. In line with this, the blockade of both A1R and A2AR was responsible for beneficial effects of caffeine in human neuroblastoma SH-SY5Y cells exposed to Aβ25-35 alone [] or combined with AlCl3 [], probably because A1R blockade might potentiate A2AR-mediated protection by promoting the recovery of Ca2+ homeostasis []. Neuroprotection against some neurodegenerative disorders can be induced via promoting CREB/BDNF signalling pathway []. To further complicate this scenario, it was suggested that Aβ monomers (not oligomers), by activating the IGF-IR-stimulated PI3-K/AKT pathway, induce the activation of CREB in neurons and sustain BDNF transcription and release []. The upregulation of A1R reported here could be responsible for the lower levels of CREB expression and the downregulation of the anti-apoptotic CREB-driven gene expression. However, as DPCPX (i.e., A1R antagonism) was ineffective against cortical neuron cell death, a possible role for A1R and its enhanced inhibition of cAMP generation could be counteracting the increase of A2AR and its role in stimulating cAMP levels. Preclinical studies, clinical trials, and reviews suggest that increasing cAMP with phosphodiesterase inhibitors is disease-modifying in AD [].
The present study provides strong evidence that one of the early events that takes place when cortical neurons are exposed to Aβ25-35, is the up-regulation of Group I mGluR and the desensitization of their main transduction system; the activation of PLC. Interestingly, the pre-treatment with an antagonist of these receptors is enough to reduce cellular damage. Besides, adenosine receptors (mainly A1R) are also early increased by Aβ25-35 exposure and their signalling pathway modulated in accordance, leading to enhanced inhibition of the formation of cAMP and, perhaps, a lower expression of transcriptional factors like CREB and CREM.
The strategies against AD based on lowering Aβ levels or reducing Aβ production in human trials are not as effective as first expected [,], so there is a chance to improve those strategies by focusing on the early events occurring in AD brains rather than the neurodegeneration caused by the amyloid plaques [,], and targeting adenosine receptors and/or Group I mGluR. Even the latter has the potential to mediate, at least in part, the influence of Aβ peptide on neurons. Of interest, apart from the interplay between Aβ and AR [,,] or mGluR [,,], the widely reported cross-talk between adenosine and metabotropic glutamate receptors could be helpful in the therapeutic intervention based on these receptors []. Moreover, different to other GPCRs that may have a neuroprotective role, Group I mGluR does not desensitize and down-regulate quickly, as does A1R [], so there is a window of time in which these receptors could be considered potential targets for AD. Furthermore, the clear up-regulation of Group I mGluR on Aβ-treated neurons shows it is a promising diagnostic tool to detect the beginning of AD (promising imaging studies have been carried out in rodents []), and we would not have to wait a long time (usually 10–20 years) to observe the classical neurodegenerative phenotypes in AD patients, where neurodegeneration is too high to improve any kind of neuroprotective therapy []. The role of mGluRs in synaptic plasticity and their modulation as a possible strategy for AD intervention deserves further exploration, as recently reviewed []. Overall, this study increases our knowledge of the very early events that take place in neurons after soluble Aβ exposure and could provide new ways of targeting AD. Future research should study the molecular mechanisms underlying the processes described here that will help us to better understand the nature of these early interactions of Aβ with cortical neurons.
4. Materials and Methods
4.1. Materials
L-[3,4-3H]-glutamic acid (L-[3H]Glu, 52 Ci/mmol) and Inositol-1,4,5-trisphosphate (Inositol-1-[3H](N) ([3H]IP3, 24.1 Ci/mmol) were purchased from Perkin Elmer (Boston, MA, USA). Cyclopentyl-1,3-dypropylxanthine,8-[dipropy-2,3-3H(N)] ([3H]DPCPX, 120 Ci/mmol) was purchased from Amersham (Madrid, Spain). L-glutamic acid (L-Glu), (S)-3,5-dihydroxyphenylglycine (DHPG), (RS)-1-Aminoindan-1,5-dicarboxylic acid (AIDA), (3,4-Dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclohexyl)-methanone (JNJ 16259685), 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP), (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate (APDC), L-(+)-2-Amino-4-phosphonobutyric acid (L-AP4), ([2-3H](4-(2-[7-amino-2-(2-fury1) [1,2,4] triazolo [2,3-a] [1,3,5] triazin-5-ylamino]ethyl)phenol) ([3H]ZM241385, 27.4 Ci/mmol), 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), 2-[p-(2-carboxyethyl) phenylamino]-50-N-ethylcarboxyamide adenosine (CGS21680) and 4-(2-[7-Amino-2-(2-furyl) [1,2,4] triazolo [2,3-a] [1,3,5] triazin-5-ylamino] ethyl)phenol (ZM241385) were from Tocris (Bristol, UK). (RS)-α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), 2-Carboxy-3-carboxymethyl-4-isopropenylpyrrolidine (kainic acid), (R)-2-(Methylamino)succinic acid (NMDA), threo-2-Amino-3-hydroxysuccinic acid (β-OH-Asp), N6-cyclopentyladenosine (CPA), N6-cyclohexyladenosine (CHA), calf intestine adenosine deaminase (ADA; EC 3.5.4.4), forskolin, cytosine β-D-arabinofuranoside (AraC), Aβ25-35, Aβ1-42, and 3-[4,5-dimetylthiazol-2-yl]-2,5-dipheniltetrazolium bromide (MTT) were obtained from Sigma (Madrid, Spain). Liquid scintillation solutions were purchased from Perkin Elmer (Boston, MA, EEUU). Liquid scintillation solutions were purchased from PerkinElmer (Boston, MA, USA). Chemicals, culture media, and culture plates used to obtain and maintain cellular cultures were acquired from Gibco BRL (Barcelona, Spain) unless otherwise stated. All other products were of analytical grade. All the drugs employed in this study were prepared, stored at −80 °C, and thawed only once. In all cases, appropriate controls were carried out to avoid the solvent effect.
4.2. Primary Culture of Cortical Neurons
Primary cortical neuronal cultures were prepared using Wistar rat foetuses on embryonic day 18 using a protocol described previously [] with minor modifications []. Briefly, foetuses were isolated and placed in PBS supplemented with 6 mM glucose and 1% BSA. Cortical hemispheres were dissected under sterile conditions and incubated with papain (30 U/mL; Sigma, Madrid, Spain) for 5 min at 37 °C. Tissue was mechanically dissociated with a glass Pasteur pipette, then DNase was added, and dissociated cells were filtered through a 70 µm cell strainer (BD Falcon, Madrid, Spain). Cells were collected by centrifugation (300× g for 6 min) and resuspended in MEM (Minimum Essential Medium) supplemented with 2.2 g/L NaHCO3, 10 mL/L Glutamax I, 2.6 g/L HEPES, 10 mL/L Antibiotic-Antimycotic mix, and 10% decomplemented horse serum at a density of 4·105 cells/mL. Neurons were plated at a density of 2.6·105 cells/well on poly-D-lysine-coated 24-well plates (binding, PCR and enzymatic assays), on 12-mm-diameter poly-D-lysine-coated coverslips (immunochemistry), or at a density of 8·104 cells/well on poly-D-lysine-coated 96-weel plates (viability assays). The following day medium was replaced with Neurobasal medium supplemented with B27. At 2 days in vitro (DIV), 5 µM AraC was added to inhibit glial growth, which was less than 5%. Cultures were kept at 37 °C in a 5% CO2 atmosphere. During the following weeks, half of the medium was changed once a week. All experiments were performed on neurons at 14–18 DIV. All experiments followed the European Community Council Directives (86/609/EEC) about animal experimentation, those of the Experimentation Animal Committee of Castilla-La Mancha University, and all efforts were performed to minimize the number of animals and their suffering.
4.3. Drug Treatments and Evaluation of Cell Death
Aβ25-35 and Aβ1-42 were water-soluble peptides, however, while Aβ25-35, the functional domain of Aβ required for both neurotrophic and neurotoxic effects, was directly added to neuronal cultures after its solubilisation, Aβ1-42 was solved in water and maintained for 24 h at 37 °C before its addition to promote its aggregation. To test the effect of mGluR ligands on cell death induced by Aβ in neuronal cultures, these drugs were added 30 min before the addition of Aβ25-35 and maintained during Aβ exposure. Unless otherwise indicated, all chemicals were dissolved in water.
Cell viability was determined on 96-well plates using an in vitro toxicology assay kit based on a mitochondrial-dependent reaction that transforms 3-[4,5-dimetylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) into formazan crystals. Briefly, at the end of treatments, cells were incubated in culture medium with MTT solution (5 mg/mL) at 37 °C for 3 h. After incubation, MTT solubilization solution (10% Triton X-100 plus 0.1 M HCl in isopropanol anhydrous) was added into the wells to dissolve formazan crystals and the absorbance of each well was measured at 570 nm and 690 nm, according to the manufacturer’s instructions.
4.4. Caspase 3 Activity Assay
A commercial kit (Molecular Probes, Barcelona, Spain) was used to determine Caspase 3 activity following the manufacturer’s instructions. Briefly, cortical neurons growth in 24-well plates were exposed to Aβ25-35 or maintained under control conditions. Cells were lysed and lysates centrifuged to clear cell debris. Then, 50 µL of test buffer containing Z-DEVD-Rhodamine 110, DTT, EDTA, PIPES, and CHAPS was added to the supernatants obtained in the proportions indicated by the manufacturer. After 30 min of incubation at room temperature, the absorbance of each sample was measured on a plate reader (excitation/emission 496/520 nm).
4.5. Quantification of Metabotropic Glutamate and Adenosine Receptors in Intact Cells by Radioligand Binding Assay
Metabotropic glutamate receptors were determined using the radioligand L-[3H]glutamate, as described previously []. Radioligand binding assays using intact cells were performed in 24-well plates. Briefly, cells were washed with serum-free DMEM buffered with 20 mM HEPES pH 7.4 and incubated for 60 min at 37 °C in a final volume of 250 µL, in the presence of 100 μM AMPA, 100 μM NMDA, and 100 μM kainate, to block glutamate binding to these receptor types; 10 μM of the glutamate uptake inhibitor D,L-threo-β-hydroxyaspartic acid; and increasing concentrations of L-[3H] glutamate (200 nM to 1.2 µM). Non-labeled L-Glutamate in a concentration of 105 times higher than the radioligand was used to determine non-specific binding. After incubation, cells were washed with 500 µL of ice-cold buffer and disrupted with 0.2% SDS. Well contents were then transferred to vials and a scintillation liquid mixture was added to measure radioactivity. At least two wells from each plate were reserved for protein concentration measurement.
Concerning adenosine receptor assessment by radioligand binding, [3H]DPCPX and [3H]ZM241385 were employed as selective A1R and A2AR radioligands, as described previously [], to determine A1R and A2AR density, respectively. Briefly, primary cortical neurons growth in 24-well plates were washed with serum-free DMEM and pre-incubated with 2 U/mL ADA at 37 °C for 30 min to remove endogenous adenosine. After incubation, the radioligands [3H]DPCPX (1–20 nM) or [3H] ZM241385 (1–20 nM) were added in the absence or the presence of CPA in a concentration 104 times higher than the radioligand, to obtain non-specific binding to A1R, or 5 mM theophylline, to obtain non-specific binding to A2AR. A specific adenosine uptake inhibitor (1 µM dipyridamole) was added to the reaction mixture to block adenosine receptor ligands’ binding to adenosine transporters. After incubation at 25 °C for 2 h in a final volume of 250 µL, cells were washed with 500 µL of ice-cold buffer and disrupted with 0.2% SDS. Well contents were then transferred to vials and a scintillation liquid mixture was added to measure radioactivity. Two wells from each plate were reserved for protein concentration measurement.
4.6. Extracellular Targeting of mGluR1, mGluR5 and mGluR2,3 by Immunochemistry
The quantitative analysis of the levels of Group I mGluR and mGluR2,3 at the plasma membrane was performed with extracellular targeted polyclonal antibodies (mGluR1, mGluR5 and mGluR2,3, Alomone Labs, Jerusalem, Israel), as previously described [,,] with minor modifications. Briefly, after 2 weeks in culture, the culture medium was removed and cells washed with Locke Buffer (LB, pH 7.4). Cells were then fixed with 4% paraformaldehyde for 10 min at room temperature and washed with three 10-min intervals with LB.
Nonspecific staining was suppressed by blocking with 3% normal goat serum in LB for 60 min. Thereafter, the cells were incubated overnight at 4 °C in the same blocking buffer containing rabbit anti-mGluR1 (1:200) or anti-mGluR5 (1:200). Thereafter, cells were washed with LB, mounted following standard procedures using ProLong Gold as an antifade reagent, and stored in cold and dark conditions.
4.7. PLCβ1 Immunocytochemistry
For PLCβ1 immunostaining, the protocol described above was followed with the introduction of modified steps. Before the nonspecific staining blockade with 3% normal goat serum, cells were pre-treated with Triton 0.25% in LB for 10 min to induce membrane permeabilization. Cells were incubated overnight in blocking buffer with mouse anti-PLCβ1 monoclonal antibody (1:500) from Millipore (Bedford, MA, USA). The following day, after three LB washes (10 min each), cortical neurons were exposed to Cy3-conjugated goat anti-mouse IgG (1:600). In all immunochemical procedures, internal controls were carried out where primary antibodies were not added to the blocking buffer.
4.8. Microscopy Imaging
To evaluate the degree of fluorescence intensity in cortical neurons, fluorescence was measured to estimate the protein expression in the different experimental conditions analyzed. Images (448.6 µm × 335.08 µm) obtained with a digital camera (Leica DFC350FX R2), attached to a Leica DMI6000B (Leica Microsystems, Wetzlar, Germany) fluorescent microscope, were used for quantification (20x HCX PL FLUOTAR 0.4 dry objective). For whole-cell immunostaining, six ROIs were randomly distributed in five different images of each condition (N = 30 per experimental condition; 1324.16 µm2 each ROI). Fluorescence intensity was estimated by averaging mean grey values. These ROIs have enough size to include one to four cells. Data were normalized to the total number of cells in the analyzed fields. The mean fluorescence background, calculated in the unstained regions between neurons, was subtracted from the fluorescence intensity of each data point. For cell body and dendrite localized immunostaining, 10 ROIs were analysed in the same five images of each condition used for whole-cell immunostaining analysis. ImageJ 1.53 e (NIH, Bethesda, Maryland, USA) was used to measure cell fluorescence after selection of cell and dendrite portions using selection tools (freeform and rectangle, respectively). In “set measurements” display, the area-integrated intensity and mean grey value were used to calculate Correct Total Cell Fluorescence (CTCF) as CTCF = Integrated Density— (area of selected cell × Mean fluorescence of background readings). This method is based on QBI Advanced Microscopy Facility of The University of Queensland. All the samples were processed together. Acquisition parameters were adjusted to prevent fluorescence from saturating the image in any condition, and these parameters were maintained with the subsequent images.
4.9. Determination of Phospholipase C Activity
This method was previously reported [] and is divided into two well-defined steps:
IP3 accumulation: culture medium was discharged, and cells were washed twice with DMEM containing 20 mM HEPES, pH 7.4. After 20 min at 37 °C, different ligands were added to cells for 10 min at 37 °C to analyse their effect on PLC activity in a final reaction volume of 300 µL. The enzymatic reaction was stopped and cells lysed by adding 50 µL 2.8 M perchloric acid and placing plates on ice for 30 min. The medium was neutralized with 70 µL 1 M Tris-HCl, 2 M KOH, and 60 mM EGTA, transferred to Eppendorf tubes and centrifuged at 12,000× g for 10 min. The supernatant was then collected to determine the IP3 level.
IP3 level determination: IP3 levels in cells were determined as previously reported by Palmer [] and modified by Gerwins []. Incubation of [3H]IP3 (0.2 pmol; 3000–5000 cpm) in assay buffer (100 mM Tris-HCl, 4 mM EDTA, 4 mM EGTA, 4 mg/mL BSA, pH 9.0), binding protein (500–600 µg), and 25–50 µL of supernatant obtained from IP3 accumulation assay was carried out. Samples were incubated at 4 °C for 60 min and centrifuged at 12,000× g for 10 min. Pellets were suspended in 100 µL 0.2% SDS and transferred to scintillation vials to measure radioactivity. A standard curve was made with known [3H]IP3 concentrations (0.25–10 µM). Nonspecific binding was determined in the presence of 10 µM unlabeled IP3. Binding protein was obtained from bovine suprarenal capsules according to Palmer’s protocol [].
4.10. Determination of Adenylyl Cyclase Activity
AC activity was determined as previously reported by Murphy [] with minor modifications []. Briefly, control or treated cells were pre-incubated in serum-free DMEM buffered with 20 mM HEPES, pH 7.4, supplemented with 100 µM Ro 20-1724 and 2 U/mL ADA, for 10 min at 37 °C. AC activity was stimulated using specific ligands for 15 min at 37 °C in a final volume of 250 µL. The reaction was stopped by adding 500 µL 0.1 M HCl in absolute ethanol. Once centrifuged at 12,000× g for 10 min, supernatants were evaporated in speed-vac and resuspended in 150 µL assay buffer (50 mM Tris-HCl, 4 mM EDTA). Fifty microliters of the sample were used to determine cAMP accumulation using protein kinase A as a cAMP binding protein and [3H]cAMP as radioligand. A cAMP standard curve (0–16 pmol) was prepared in the same buffer. Incubation was stopped by rapid filtration through Whatman GF/B filters.
4.11. Preparation of Total RNA and cDNA
Total RNA was extracted using an ABI 6100 Nucleic Acid PrepStation according to the manufacturer’s protocol (Applied Biosystems Inc., Foster City, CA, USA). Total RNA from cells was isolated and stored at −80 °C. The quality of isolated RNA was estimated by the ratio A260/A280 and was in the range of 1.9–2.1. RNA integrity was evaluated by agarose gel electrophoresis according to MIQE guidelines [] using the “bleach gel” method []. RNA concentrations were determined from the A260. One microgram of total RNA was reverse transcribed using Applied Biosystems High-Capacity cDNA Archive Kit.
4.12. Quantitative Real-Time RT-PCR Analysis
To assess relative gene expression in neurons, quantitative real-time RT-PCR analysis was performed with an Applied Biosystems Prism 7500 Fast Sequence Detection System using TaqMan® universal PCR master mix according to the manufacturer’s specifications. The TaqMan probes and primers for GRM1 (assay ID: Rn00566625_m1), GRM5 (assay ID: Rn00566628_m1), ADORA1 (assay ID:Rn00567668_m1), ADORA2A (assay ID: Rn00583935_m1), ADORA2B (assay ID:Rn00567697_m1), PI-PLCβ1 (assay ID: Rn01514511_m1), CREB1 (assay ID: Rn00578826_m1), CREM (assay ID: Rn00565271_m1), Caspase3 (assay ID: Rn00563902_m1), and ACTβ (Rn00667869_m1) were validated assay-on-demand gene expression products. ACTβ gene was used as an endogenous control. A non-fluorescent quencher and the minor groove binder were linked at the 3′ end of the probe as quenchers. The thermal cycler conditions were as follows: hold for 20″ at 95 °C, followed by two-step PCR for 40 cycles of 95 °C for 3″ followed by 60 °C for 30″. Levels of RNA expression were determined using the 7500 Fast System SDS software version 1.3.1 (Applied Biosystems) according to the 2−ΔΔCt method. Briefly, expression results of a gene were normalized to internal control β-actin and relatively to a calibrator, consisting of the mean expression level of the control gene as follows: 2−ΔΔCt = 2-((Ct target gene − Ct β-actin)sample − (Ct target gene − Ct β-actin)calibrator). The results from three independent repeat assays, performed in different plates each using different cDNA’s from the cultures analyzed, were averaged to produce a single mean quantity value for each mRNA.
4.13. Protein Determination
Protein concentration was measured by the method of Lowry, using bovine serum albumin as standard.
4.14. Statistics and Data Analysis
Statistical analysis was performed using non paired two-tailed Student’s t-test or one-way ANOVA, followed by Bonferroni’s multiple comparisons test, as appropriate. Differences between mean values were considered statistically significant at p < 0.05. Nonlinear regression curve fit (one site binding hyperbola) was performed for binding data using GraphPad Prism 8 program for Windows (GraphPad Software, San Diego, CA, USA).
5. Conclusions
We show strong evidence that one of the early events that takes place after Aβ25-35 exposure is the up-regulation of adenosine A1R, A2AR, and group I mGluR, and the different impacts on their corresponding signaling pathways. These results emphasize the importance of deciphering the early events and the possible involvement of metabotropic glutamate and adenosine receptors in AD physiopathology.
Author Contributions
Conceptualization: M.M. and J.L.A.; Formal analysis: C.A.C. and J.L.A.; Funding acquisition: M.M.; Investigation: C.A.C., I.B.-Y., D.A.L.-N.; Writing—original draft: C.A.C. and M.M.; Writing—review & editing: M.M. and J.L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministerio de Ciencia e Innovación (grant PID2019-109206GB-I00), by UCLM (grant 2020-GRIN-29108 cofinanced with the European Union FEDER), and by Junta de Comunidades de Castilla-La Mancha (JCCM) (grant SBPLY/19/180501/000251, cofinanced with the European Union FEDER).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Selkoe, D.J. Clearing the brain’s amyloid cobwebs. Neuron 2001, 32, 177–180. [Google Scholar] [CrossRef]
- Huber, G.; Martin, J.R.; Loffler, J.; Moreau, J.L. Involvement of amyloid precursor protein in memory formation in the rat: An indirect antibody approach. Brain Res. 1993, 603, 348–352. [Google Scholar] [CrossRef]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid beta-protein: Synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012, 2, a006338. [Google Scholar] [CrossRef]
- Plant, L.D.; Boyle, J.P.; Smith, I.F.; Peers, C.; Pearson, H.A. The production of amyloid beta peptide is a critical requirement for the viability of central neurons. J. Neurosci. 2003, 23, 5531–5535. [Google Scholar] [CrossRef]
- Giuffrida, M.L.; Caraci, F.; Pignataro, B.; Cataldo, S.; De Bona, P.; Bruno, V.; Molinaro, G.; Pappalardo, G.; Messina, A.; Palmigiano, A.; et al. Beta-amyloid monomers are neuroprotective. J. Neurosci. 2009, 29, 10582–10587. [Google Scholar] [CrossRef]
- Savory, J.; Ghribi, O.; Herman, M.M. Is amyloid beta-peptide neurotoxic or neuroprotective and what is its role in the binding of metal ions? Neurobiol. Aging 2002, 23, 1089–1092. [Google Scholar] [CrossRef]
- Copani, A. The underexplored question of beta-amyloid monomers. Eur. J. Pharmacol. 2017, 817, 71–75. [Google Scholar] [CrossRef]
- Gouras, G.K.; Tampellini, D.; Takahashi, R.H.; Capetillo-Zarate, E. Intraneuronal beta-amyloid accumulation and synapse pathology in Alzheimer’s disease. Acta Neuropathol. 2010, 119, 523–541. [Google Scholar] [CrossRef]
- Pike, C.J.; Burdick, D.; Walencewicz, A.J.; Glabe, C.G.; Cotman, C.W. Neurodegeneration induced by beta-amyloid peptides in vitro: The role of peptide assembly state. J. Neurosci. 1993, 13, 1676–1687. [Google Scholar] [CrossRef]
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotrophic and neurotoxic effects of amyloid beta protein: Reversal by tachykinin neuropeptides. Science 1990, 250, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, Y.G.; Marlatt, M.W.; Smith, M.A.; Kosenko, E.A. Subcellular and metabolic examination of amyloid-beta peptides in Alzheimer disease pathogenesis: Evidence for Abeta(25-35). Exp. Neurol. 2010, 221, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Bhute, S.; Sarmah, D.; Datta, A.; Rane, P.; Shard, A.; Goswami, A.; Borah, A.; Kalia, K.; Dave, K.R.; Bhattacharya, P. Molecular Pathogenesis and Interventional Strategies for Alzheimer’s Disease: Promises and Pitfalls. ACS Pharmacol. Transl. Sci. 2020, 3, 472–488. [Google Scholar] [CrossRef]
- Ayton, S.; Bush, A.I. Beta-amyloid: The known unknowns. Ageing Res. Rev. 2021, 65, 101212. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.; Olmo, R.P.; Ferguson, S.S. Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Battaglia, G.; Sortino, M.A.; Spampinato, S.; Molinaro, G.; Copani, A.; Nicoletti, F.; Bruno, V. Metabotropic glutamate receptors in neurodegeneration/neuroprotection: Still a hot topic? Neurochem. Int. 2012, 61, 559–565. [Google Scholar] [CrossRef]
- Jakaria, M.; Park, S.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef]
- Renner, M.; Lacor, P.N.; Velasco, P.T.; Xu, J.; Contractor, A.; Klein, W.L.; Triller, A. Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron 2010, 66, 739–754. [Google Scholar] [CrossRef]
- Canas, P.M.; Simoes, A.P.; Rodrigues, R.J.; Cunha, R.A. Predominant loss of glutamatergic terminal markers in a beta-amyloid peptide model of Alzheimer’s disease. Neuropharmacology 2014, 76 Pt A, 51–56. [Google Scholar] [CrossRef]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; AP, I.J.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 53, 527–552. [Google Scholar] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Perez, S.; Barrachina, M.; Ferrer, I.; Martin, M. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol. 2008, 18, 211–219. [Google Scholar] [CrossRef]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Leon-Navarro, D.A.; Albasanz, J.L.; Martin, M. Functional Cross-Talk between Adenosine and Metabotropic Glutamate Receptors. Curr. Neuropharmacol. 2019, 17, 422–437. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Dalfo, E.; Ferrer, I.; Martin, M. Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol. Dis. 2005, 20, 685–693. [Google Scholar] [CrossRef]
- Castillo, C.A.; Albasanz, J.L.; León, D.; Jordán, J.; Pallás, M.; Camins, A.; Martín, M. Age-related expression of adenosine receptors in brain from the senescence-accelerated mouse. Exp. Gerontol. 2009, 44, 453–461. [Google Scholar] [CrossRef]
- Sanchez-Melgar, A.; Albasanz, J.L.; Pallas, M.; Martin, M. Resveratrol Differently Modulates Group I Metabotropic Glutamate Receptors Depending on Age in SAMP8 Mice. ACS Chem. Neurosci. 2020, 11, 1770–1780. [Google Scholar] [CrossRef]
- Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef]
- Fagiani, F.; Lanni, C.; Racchi, M.; Govoni, S. (Dys)regulation of Synaptic Activity and Neurotransmitter Release by beta-Amyloid: A Look Beyond Alzheimer’s Disease Pathogenesis. Front. Mol. Neurosci. 2021, 14, 635880. [Google Scholar] [CrossRef]
- Iloun, P.; Hooshmandi, E.; Gheibi, S.; Kashfi, K.; Ghasemi, R.; Ahmadiani, A. Roles and Interaction of the MAPK Signaling Cascade in Abeta25-35-Induced Neurotoxicity Using an Isolated Primary Hippocampal Cell Culture System. Cell Mol. Neurobiol. 2020. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin Protects Against Abeta25-35-Induced Hippocampal HT22 Cells Apoptosis Through Mediating ROS/p38/Caspase 3-Dependent Pathway. Front. Pharmacol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Mucke, L.; Masliah, E.; Yu, G.Q.; Mallory, M.; Rockenstein, E.M.; Tatsuno, G.; Hu, K.; Kholodenko, D.; Johnson-Wood, K.; McConlogue, L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: Synaptotoxicity without plaque formation. J. Neurosci. 2000, 20, 4050–4058. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Cieslak, M.; Wojtczak, A. Role of purinergic receptors in the Alzheimer’s disease. Purinergic Signal. 2018, 14, 331–344. [Google Scholar] [CrossRef]
- Burnstock, G.; Krugel, U.; Abbracchio, M.P.; Illes, P. Purinergic signalling: From normal behaviour to pathological brain function. Prog. Neurobiol. 2011, 95, 229–274. [Google Scholar] [CrossRef]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine receptors and neurological disease: Neuroprotection and neurodegeneration. Handb. Exp. Pharmacol. 2009, 535–587. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Castillo, C.A.; Barrachina, M.; Ferrer, I.; Martín, M. Modulation of Signal Transduction Pathways in Senescence-Accelerated Mice P8 Strain: A Useful Tool for Alzheimer’s Disease Research. In The Clinical Spectrum of Alzheimer’s Disease -The Charge toward Comprehensive Diagnostic and Therapeutic Strategies; InTechOpen: London, UK, 2011. [Google Scholar]
- Srivastava, A.; Das, B.; Yao, A.Y.; Yan, R. Metabotropic Glutamate Receptors in Alzheimer’s Disease Synaptic Dysfunction: Therapeutic Opportunities and Hope for the Future. J. Alzheimers Dis. 2020, 78, 1345–1361. [Google Scholar] [CrossRef]
- Nicoletti, F.; Orlando, R.; Di Menna, L.; Cannella, M.; Notartomaso, S.; Mascio, G.; Iacovelli, L.; Matrisciano, F.; Fazio, F.; Caraci, F.; et al. Targeting mGlu Receptors for Optimization of Antipsychotic Activity and Disease-Modifying Effect in Schizophrenia. Front. Psychiatry 2019, 10, 49. [Google Scholar] [CrossRef]
- Hovelso, N.; Sotty, F.; Montezinho, L.P.; Pinheiro, P.S.; Herrik, K.F.; Mork, A. Therapeutic potential of metabotropic glutamate receptor modulators. Curr. Neuropharmacol. 2012, 10, 12–48. [Google Scholar] [CrossRef]
- Kamenetz, F.; Tomita, T.; Hsieh, H.; Seabrook, G.; Borchelt, D.; Iwatsubo, T.; Sisodia, S.; Malinow, R. APP processing and synaptic function. Neuron 2003, 37, 925–937. [Google Scholar] [CrossRef]
- Castillo, C.A.; Leon, D.A.; Ballesteros-Yanez, I.; Iglesias, I.; Martin, M.; Albasanz, J.L. Glutamate differently modulates metabotropic glutamate receptors in neuronal and glial cells. Neurochem. Res. 2010, 35, 1050–1063. [Google Scholar] [CrossRef]
- Ashe, K.H.; Zahs, K.R. Probing the biology of Alzheimer’s disease in mice. Neuron 2010, 66, 631–645. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Partridge, V.; Leon, W.C.; Canneva, F.; Allard, S.; Arvanitis, D.N.; Vercauteren, F.; Houle, D.; Ducatenzeiler, A.; Klein, W.L.; et al. Transgenic mice as a model of pre-clinical Alzheimer’s disease. Curr. Alzheimer. Res. 2011, 8, 4–23. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Kowalewski, J.M.; Renner, M.; Bousset, L.; Koulakoff, A.; Melki, R.; Giaume, C.; Triller, A. Beta-amyloid and ATP-induced diffusional trapping of astrocyte and neuronal metabotropic glutamate type-5 receptors. Glia 2013, 61, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble amyloid-beta oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef]
- Kumar, A.; Dhull, D.K.; Mishra, P.S. Therapeutic potential of mGluR5 targeting in Alzheimer’s disease. Front. Neurosci. 2015, 9, 215. [Google Scholar] [CrossRef]
- Hu, N.W.; Nicoll, A.J.; Zhang, D.; Mably, A.J.; O’Malley, T.; Purro, S.A.; Terry, C.; Collinge, J.; Walsh, D.M.; Rowan, M.J. mGlu5 receptors and cellular prion protein mediate amyloid-beta-facilitated synaptic long-term depression in vivo. Nat. Commun. 2014, 5, 3374. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Esseltine, J.L.; DeVries, R.A.; Cregan, S.P.; Ferguson, S.S. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 2014, 7, 40. [Google Scholar] [CrossRef]
- Ostapchenko, V.G.; Beraldo, F.H.; Guimaraes, A.L.; Mishra, S.; Guzman, M.; Fan, J.; Martins, V.R.; Prado, V.F.; Prado, M.A. Increased prion protein processing and expression of metabotropic glutamate receptor 1 in a mouse model of Alzheimer’s disease. J. Neurochem. 2013, 127, 415–425. [Google Scholar] [CrossRef]
- Casley, C.S.; Lakics, V.; Lee, H.G.; Broad, L.M.; Day, T.A.; Cluett, T.; Smith, M.A.; O’Neill, M.J.; Kingston, A.E. Up-regulation of astrocyte metabotropic glutamate receptor 5 by amyloid-beta peptide. Brain Res. 2009, 1260, 65–75. [Google Scholar] [CrossRef]
- Lim, D.; Iyer, A.; Ronco, V.; Grolla, A.A.; Canonico, P.L.; Aronica, E.; Genazzani, A.A. Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 2013, 61, 1134–1145. [Google Scholar] [CrossRef]
- Haas, L.T.; Salazar, S.V.; Kostylev, M.A.; Um, J.W.; Kaufman, A.C.; Strittmatter, S.M. Metabotropic glutamate receptor 5 couples cellular prion protein to intracellular signalling in Alzheimer’s disease. Brain 2016, 139, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Mroczko, B.; Groblewska, M.; Litman-Zawadzka, A.; Kornhuber, J.; Lewczuk, P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J. Neural. Transm. (Vienna) 2018, 125, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Foster, D.J.; Conn, P.J. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef]
- Pshenichkin, S.; Dolinska, M.; Klauzinska, M.; Luchenko, V.; Grajkowska, E.; Wroblewski, J.T. Dual neurotoxic and neuroprotective role of metabotropic glutamate receptor 1 in conditions of trophic deprivation—Possible role as a dependence receptor. Neuropharmacology 2008, 55, 500–508. [Google Scholar] [CrossRef]
- Battaglia, G.; Bruno, V.; Pisani, A.; Centonze, D.; Catania, M.V.; Calabresi, P.; Nicoletti, F. Selective blockade of type-1 metabotropic glutamate receptors induces neuroprotection by enhancing gabaergic transmission. Mol. Cell Neurosci. 2001, 17, 1071–1083. [Google Scholar] [CrossRef]
- Landucci, E.; Boscia, F.; Gerace, E.; Scartabelli, T.; Cozzi, A.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Involvement of endocannabinoid signaling in the neuroprotective effects of subtype 1 metabotropic glutamate receptor antagonists in models of cerebral ischemia. Int. Rev. Neurobiol. 2009, 85, 337–350. [Google Scholar] [CrossRef]
- Pellegrini-Giampietro, D.E. The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol. Sci. 2003, 24, 461–470. [Google Scholar] [CrossRef]
- Lesage, A.; Steckler, T. Metabotropic glutamate mGlu1 receptor stimulation and blockade: Therapeutic opportunities in psychiatric illness. Eur. J. Pharmacol. 2010, 639, 2–16. [Google Scholar] [CrossRef]
- Bruno, V.; Ksiazek, I.; Battaglia, G.; Lukic, S.; Leonhardt, T.; Sauer, D.; Gasparini, F.; Kuhn, R.; Nicoletti, F.; Flor, P.J. Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 2000, 39, 2223–2230. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Bogenpohl, J.W.; Alagille, D.; Delevich, K.; Tamagnan, G.; Votaw, J.R.; Wichmann, T.; Smith, Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain 2011, 134, 2057–2073. [Google Scholar] [CrossRef]
- Morin, N.; Morissette, M.; Gregoire, L.; Gomez-Mancilla, B.; Gasparini, F.; Di Paolo, T. Chronic treatment with MPEP, an mGlu5 receptor antagonist, normalizes basal ganglia glutamate neurotransmission in L-DOPA-treated parkinsonian monkeys. Neuropharmacology 2013, 73, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Fazio, F.; Ulivieri, M.; Volpi, C.; Gargaro, M.; Fallarino, F. Targeting metabotropic glutamate receptors for the treatment of neuroinflammation. Curr. Opin. Pharmacol. 2018, 38, 16–23. [Google Scholar] [CrossRef]
- Tyszkiewicz, J.P.; Yan, Z. Beta-Amyloid peptides impair PKC-dependent functions of metabotropic glutamate receptors in prefrontal cortical neurons. J. Neurophysiol. 2005, 93, 3102–3111. [Google Scholar] [CrossRef] [PubMed]
- Chalimoniuk, M.; Strosznajder, J.B. Aging modulates nitric oxide synthesis and cGMP levels in hippocampus and cerebellum. Effects of amyloid beta peptide. Mol. Chem. Neuropathol. 1998, 35, 77–95. [Google Scholar] [CrossRef]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 2008, 321, 1686–1689. [Google Scholar] [CrossRef]
- Kuchibhotla, K.V.; Goldman, S.T.; Lattarulo, C.R.; Wu, H.Y.; Hyman, B.T.; Bacskai, B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 2008, 59, 214–225. [Google Scholar] [CrossRef]
- Dalfo, E.; Albasanz, J.L.; Martin, M.; Ferrer, I. Abnormal metabotropic glutamate receptor expression and signaling in the cerebral cortex in diffuse Lewy body disease is associated with irregular alpha-synuclein/phospholipase C (PLCbeta1) interactions. Brain Pathol. 2004, 14, 388–398. [Google Scholar] [CrossRef]
- Ikonomidou, C.; Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002, 1, 383–386. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Young, K.W.; Ennion, S.J.; Kew, J.N.; Nahorski, S.R.; Challiss, R.A. Altered expression of G(q/11alpha) protein shapes mGlu1 and mGlu5 receptor-mediated single cell inositol 1,4,5-trisphosphate and Ca(2+) signaling. Mol. Pharmacol. 2006, 69, 174–184. [Google Scholar] [CrossRef]
- Castillo, C.A.; Leon, D.; Ruiz, M.A.; Albasanz, J.L.; Martin, M. Modulation of adenosine A1 and A2A receptors in C6 glioma cells during hypoxia: Involvement of endogenous adenosine. J. Neurochem. 2008, 105, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Tang, M.; Zhao, Y.; Wang, X. Amyloid beta and tau are involved in sleep disorder in Alzheimer’s disease by orexin A and adenosine A(1) receptor. Int. J. Mol. Med. 2019, 43, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Londzin, P.; Zamora, M.; Kakol, B.; Taborek, A.; Folwarczna, J. Potential of Caffeine in Alzheimer’s Disease-A Review of Experimental Studies. Nutrients 2021, 13, 537. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and adenosine A(2a) receptor antagonists prevent beta-amyloid (25-35)-induced cognitive deficits in mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Porciuncula, L.O.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br. J. Pharmacol. 2003, 138, 1207–1209. [Google Scholar] [CrossRef]
- Keshavarz, M.; Farrokhi, M.R.; Amiri, A. Caffeine Neuroprotective Mechanism Against beta-Amyloid Neurotoxicity in SHSY5Y Cell Line: Involvement of Adenosine, Ryanodine, and N-Methyl-D-Aspartate Receptors. Adv. Pharm. Bull. 2017, 7, 579–584. [Google Scholar] [CrossRef]
- Giunta, S.; Andriolo, V.; Castorina, A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int. J. Biochem. Cell Biol. 2014, 54, 122–136. [Google Scholar] [CrossRef]
- Goel, R.; Bhat, S.A.; Hanif, K.; Nath, C.; Shukla, R. Angiotensin II Receptor Blockers Attenuate Lipopolysaccharide-Induced Memory Impairment by Modulation of NF-kappaB-Mediated BDNF/CREB Expression and Apoptosis in Spontaneously Hypertensive Rats. Mol. Neurobiol. 2018, 55, 1725–1739. [Google Scholar] [CrossRef]
- Zimbone, S.; Monaco, I.; Giani, F.; Pandini, G.; Copani, A.G.; Giuffrida, M.L.; Rizzarelli, E. Amyloid Beta monomers regulate cyclic adenosine monophosphate response element binding protein functions by activating type-1 insulin-like growth factor receptors in neuronal cells. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Sanders, O.; Rajagopal, L. Phosphodiesterase Inhibitors for Alzheimer’s Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale. J. Alzheimers Dis. Rep. 2020, 4, 185–215. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Mandelkow, E.; Selkoe, D.J. Alzheimer disease in 2020. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Dar, K.B.; Bhat, A.H.; Amin, S.; Reshi, B.A.; Zargar, M.A.; Masood, A.; Ganie, S.A. Elucidating Critical Proteinopathic Mechanisms and Potential Drug Targets in Neurodegeneration. Cell Mol. Neurobiol. 2020, 40, 313–345. [Google Scholar] [CrossRef]
- Rahman, A. The role of adenosine in Alzheimer’s disease. Curr. Neuropharmacol. 2009, 7, 207–216. [Google Scholar] [CrossRef]
- Ul Islam, B.; Khan, M.S.; Jabir, N.R.; Kamal, M.A.; Tabrez, S. Elucidating Treatment of Alzheimer’s Disease via Different Receptors. Curr. Top Med. Chem. 2017, 17, 1400–1407. [Google Scholar] [CrossRef]
- Dal Pra, I.; Armato, U.; Chiarini, A. Family C G-Protein-Coupled Receptors in Alzheimer’s Disease and Therapeutic Implications. Front. Pharmacol. 2019, 10, 1282. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Barth, V.N.; Liow, J.S.; Zoghbi, S.S.; Clark, D.T.; Rhoads, E.; Siuda, E.; Heinz, B.A.; Nisenbaum, E.; Dressman, B.; et al. Evaluation in vitro and in animals of a new 11C-labeled PET radioligand for metabotropic glutamate receptors 1 in brain. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 245–253. [Google Scholar] [CrossRef][Green Version]
- Janssens, N.; Lesage, A.S. Glutamate receptor subunit expression in primary neuronal and secondary glial cultures. J. Neurochem. 2001, 77, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.A.; Leon, D.A.; Ballesteros-Yanez, I.; Albasanz, J.L.; Martin, M. Glutamate differently modulates excitatory and inhibitory adenosine receptors in neuronal and glial cells. Neurochem. Int. 2010, 57, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Spelta, V.; Sim, J.; North, R.A.; Surprenant, A. Differential assembly of rat purinergic P2 × 7 receptor in immune cells of the brain and periphery. J. Biol. Chem. 2001, 276, 23262–23267. [Google Scholar] [CrossRef]
- Shruti, S.; Urban-Ciecko, J.; Fitzpatrick, J.A.; Brenner, R.; Bruchez, M.P.; Barth, A.L. The brain-specific Beta4 subunit downregulates BK channel cell surface expression. PLoS ONE 2012, 7, e33429. [Google Scholar] [CrossRef]
- Tsou, K.; Brown, S.; Sanudo-Pena, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef]
- Palmer, S.; Hughes, K.T.; Lee, D.Y.; Wakelam, M.J. Development of a novel, Ins(1,4,5)P3-specific binding assay. Its use to determine the intracellular concentration of Ins(1,4,5)P3 in unstimulated and vasopressin-stimulated rat hepatocytes. Cell Signal. 1989, 1, 147–156. [Google Scholar] [CrossRef]
- Gerwins, P. Modification of a competitive protein binding assay for determination of inositol 1,4,5-trisphosphate. Anal. Biochem. 1993, 210, 45–49. [Google Scholar] [CrossRef]
- Murphy, M.G.; Moak, C.M.; Byczko, Z.; MacDonald, W.F. Adenosine-dependent regulation of cyclic AMP accumulation in primary cultures of rat astrocytes and neurons. J. Neurosci. Res. 1991, 30, 631–640. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Aranda, P.S.; LaJoie, D.M.; Jorcyk, C.L. Bleach gel: A simple agarose gel for analyzing RNA quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).