Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation
Abstract
1. Introduction
2. Results
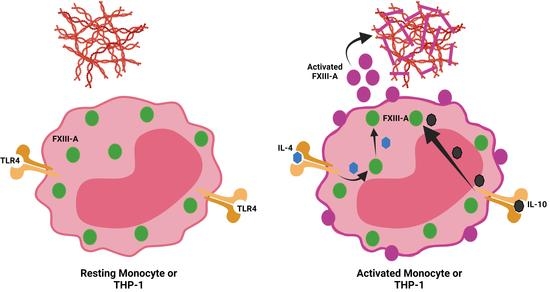
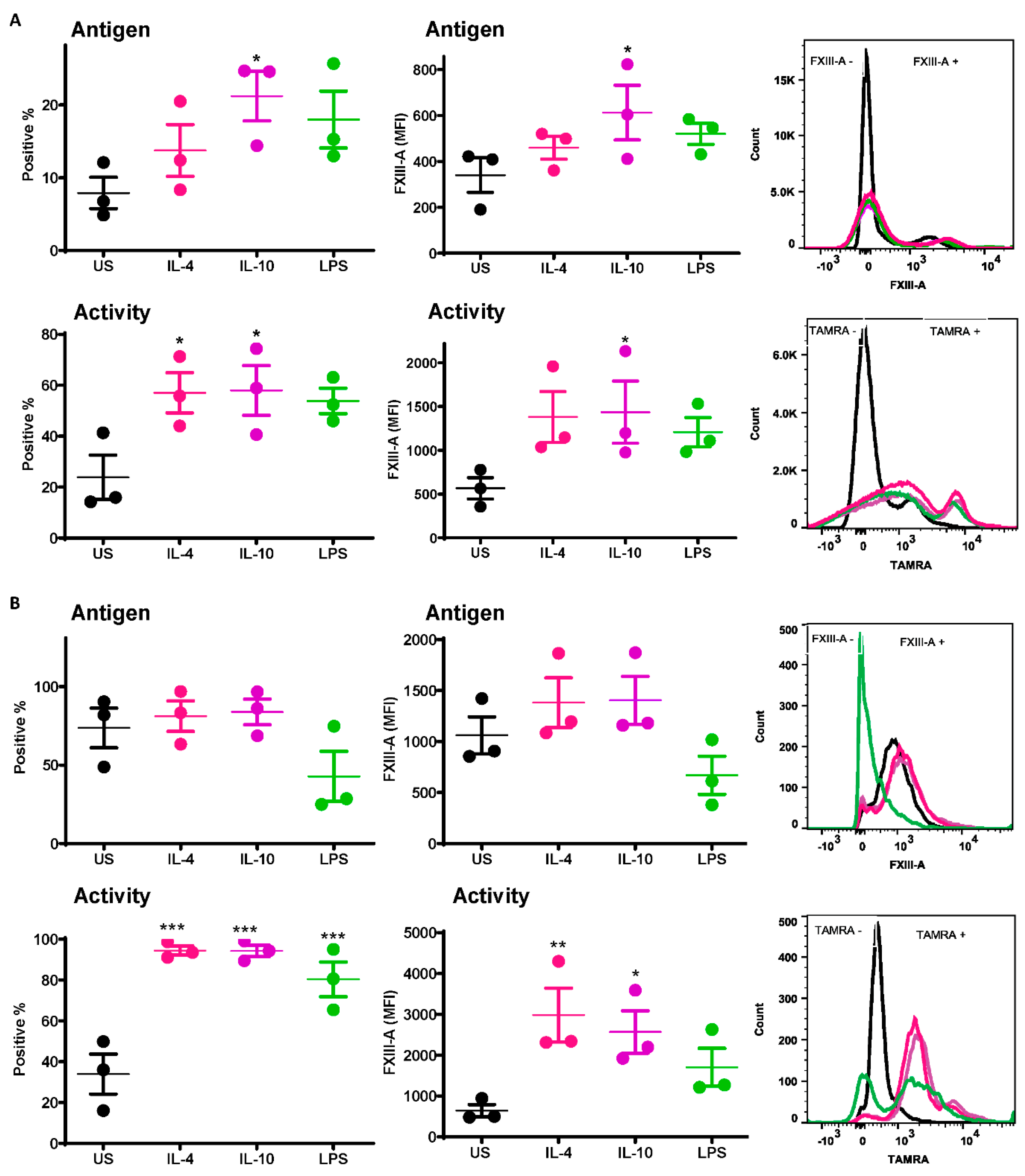
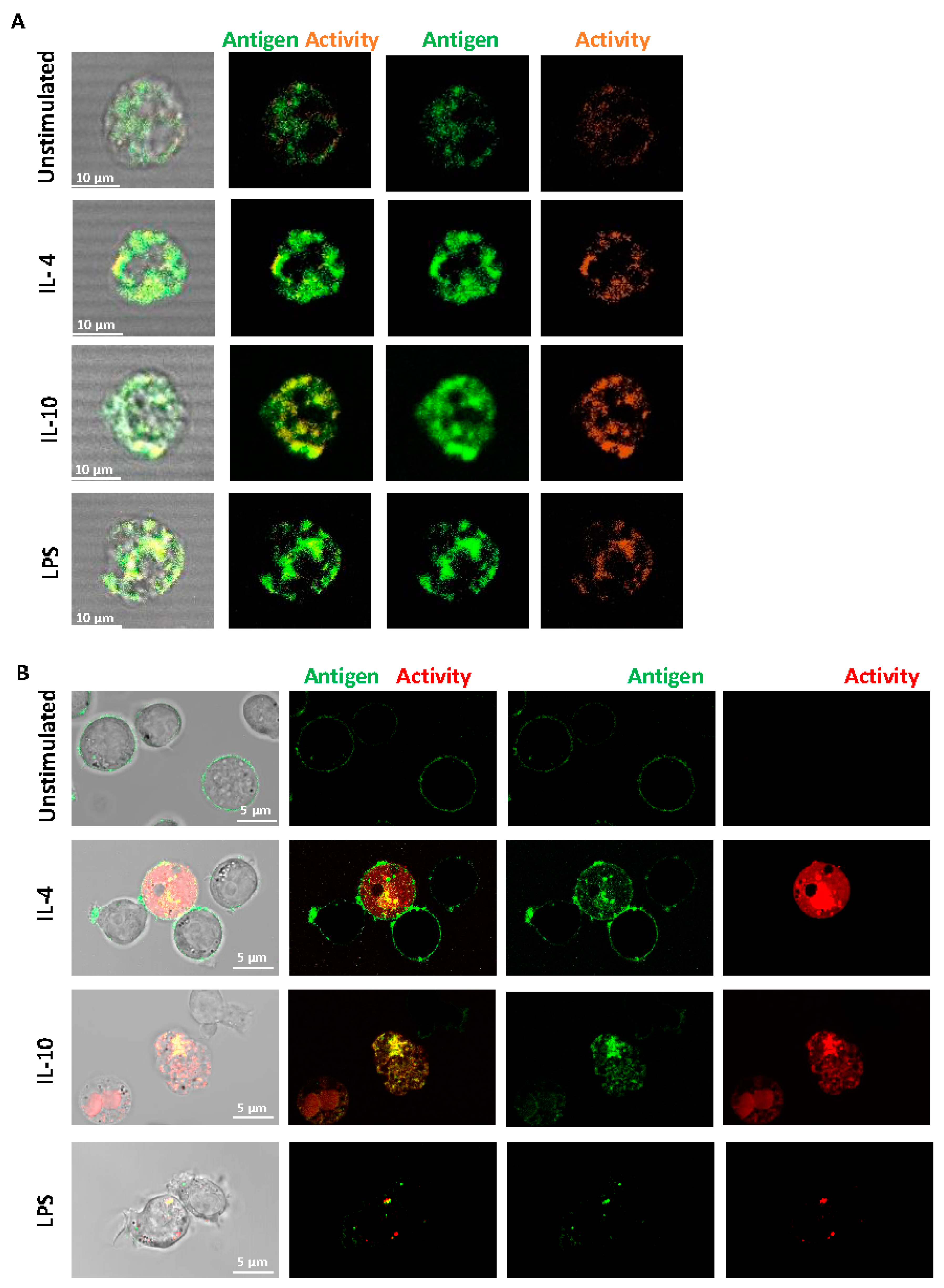
2.1. FXIII-A Antigen and Activity Are Exposed on the Surface of Human Monocytes and THP-1 Cells
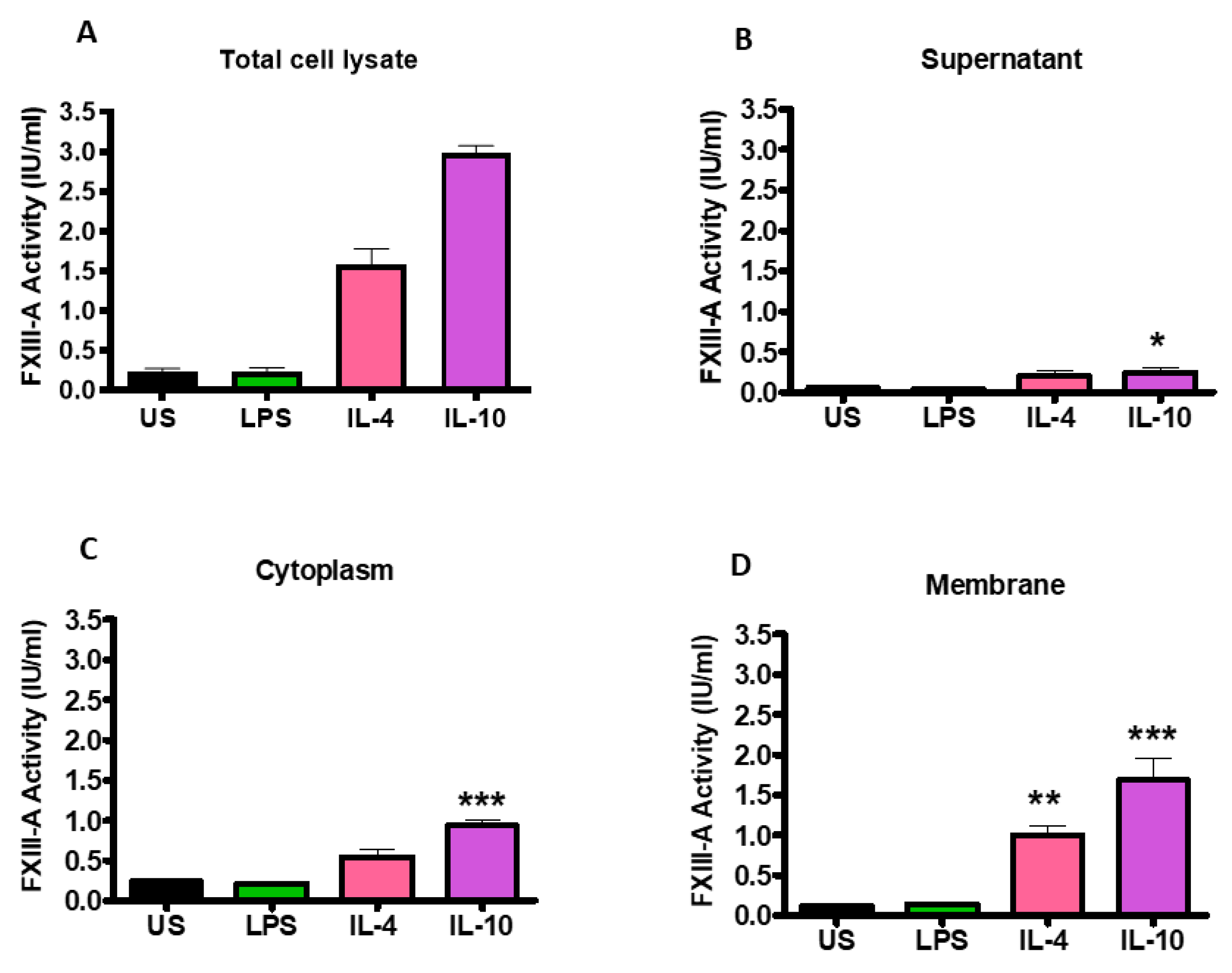
2.2. FXIII-A Activity in IL-4- or IL-10-Activated THP-1 Cells Primarily Localized to the Cell Membrane
2.3. Monocytes Are Incorporated in Thrombi
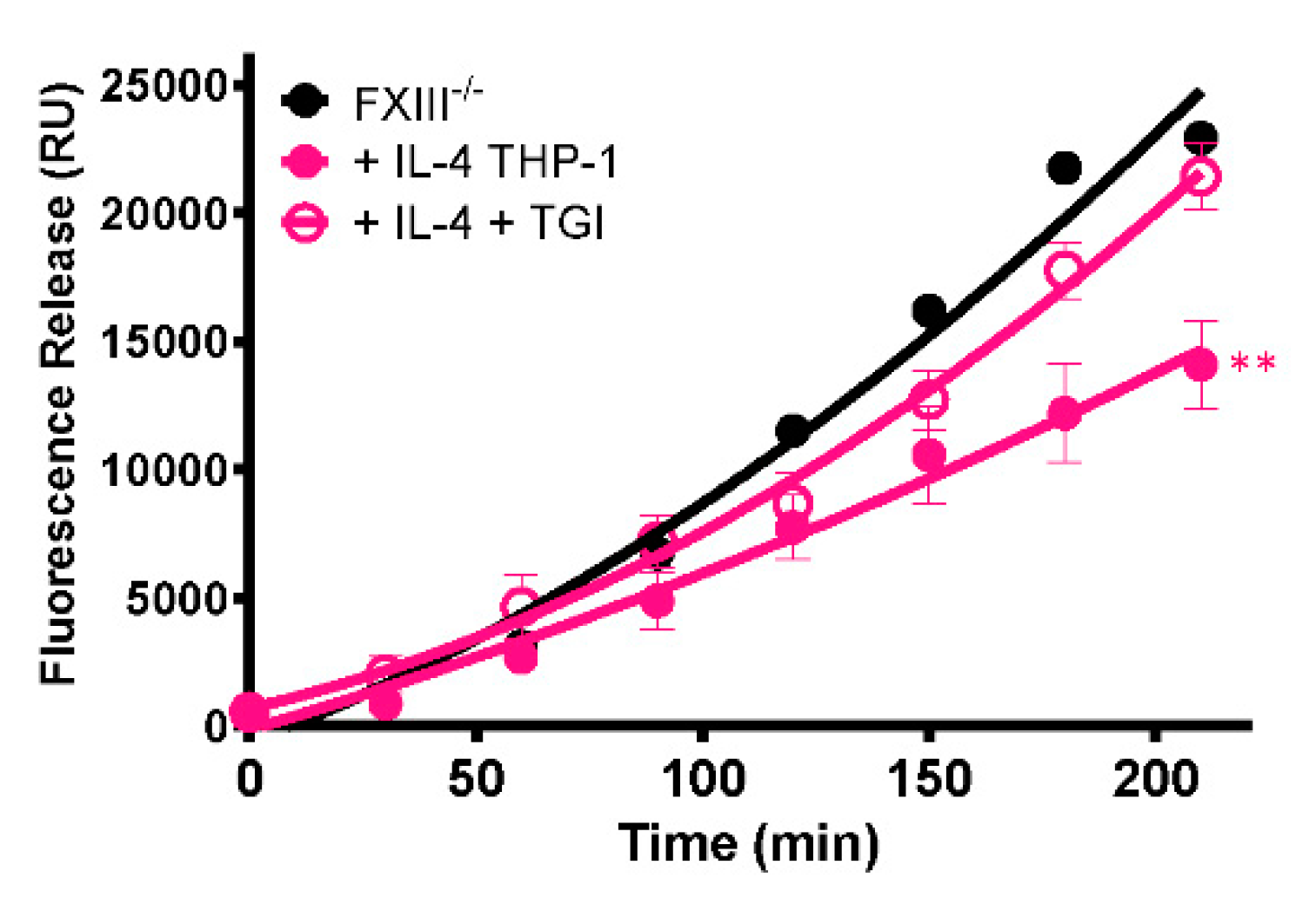
2.4. Monocytes Enhance Thrombus Stability in a Transglutaminase-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Blood Collection and Preparation of Serum
4.2. Isolation of Peripheral Blood Mononuclear Cells
4.3. Culture and Stimulation of Isolated Monocytes and THP-1
4.4. Preparation of THP-1 Cell Fractions
4.5. Flow Cytometry
4.6. Fluorescent Confocal Microscopy
4.7. FXIII Activity Assay
4.8. Thrombus Formation, Lysis and Fixation
4.9. Immunohistochemistry
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muszbek, L.; Yee, V.C.; Hevessy, Z. Blood coagulation factor XIII: Structure and function. Thromb. Res. 1999, 94, 271–305. [Google Scholar] [CrossRef]
- Muszbek, L.; Bereczky, Z.; Bagoly, Z.; Komaromi, I.; Katona, E. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol. Rev. 2011, 91, 931–972. [Google Scholar] [CrossRef]
- Dardik, R.; Solomon, A.; Loscalzo, J.; Eskaraev, R.; Bialik, A.; Goldberg, I.; Schiby, G.; Inbal, A. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arter. Thromb. Vasc. Biol. 2003, 23, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.S.M.; Whyte, C.S.; Mutch, N.J. Factor XIII-A: An Indispensable “Factor” in Haemostasis and Wound Healing. Int. J. Mol. Sci. 2021, 22, 3055. [Google Scholar] [CrossRef] [PubMed]
- Polgar, J.; Hidasi, V.; Muszbek, L. Non-proteolytic activation of cellular protransglutaminase (placenta macrophage factor XIII). Biochem. J. 1990, 267, 557–560. [Google Scholar] [CrossRef]
- Muszbek, L.; Polgar, J.; Boda, Z. Platelet factor XIII becomes active without the release of activation peptide during platelet activation. Thromb. Haemost. 1993, 69, 282–285. [Google Scholar] [CrossRef]
- Kaetsu, H.; Hashiguchi, T.; Foster, D.; Ichinose, A. Expression and release of the a and b subunits for human coagulation factor XIII in baby hamster kidney (BHK) cells. J. Biochem. 1996, 119, 961–969. [Google Scholar] [CrossRef]
- Cordell, P.A.; Kile, B.T.; Standeven, K.F.; Josefsson, E.C.; Pease, R.J.; Grant, P.J. Association of coagulation factor XIII-A with Golgi proteins within monocyte-macrophages: Implications for subcellular trafficking and secretion. Blood 2010, 115, 2674–2681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poon, M.C.; Russell, J.A.; Low, S.; Sinclair, G.D.; Jones, A.R.; Blahey, W.; Ruether, B.A.; Hoar, D.I. Hemopoietic origin of factor XIII A subunits in platelets, monocytes, and plasma. Evidence from bone marrow transplantation studies. J. Clin. Investig. 1989, 84, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Inbal, A.; Muszbek, L.; Lubetsky, A.; Katona, E.; Levi, I.; Karpati, L.; Nagler, A. Platelets but not monocytes contribute to the plasma levels of factor XIII subunit A in patients undergoing autologous peripheral blood stem cell transplantation. Blood Coagul. Fibrinolysis 2004, 15, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Beckers, C.M.L.; Simpson, K.R.; Griffin, K.J.; Brown, J.M.; Cheah, L.T.; Smith, K.A.; Vacher, J.; Cordell, P.A.; Kearney, M.T.; Grant, P.J.; et al. Cre/lox Studies Identify Resident Macrophages as the Major Source of Circulating Coagulation Factor XIII-A. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1494–1502. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Lionikiene, A.S.; Fraser, S.R.; Whyte, C.S.; Booth, N.A.; Mutch, N.J. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood 2014, 124, 3982–3990. [Google Scholar] [CrossRef]
- Stalker, T.J.; Welsh, J.D.; Tomaiuolo, M.; Wu, J.; Colace, T.V.; Diamond, S.L.; Brass, L.F. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood 2014, 124, 1824–1831. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Cook, C.; Thomas, S.; Hall, A.J.; Hubbard, R.; Vallance, P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006, 367, 1075–1079. [Google Scholar] [CrossRef]
- von Bruhl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Kollnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Laurance, S.; Bertin, F.R.; Ebrahimian, T.; Kassim, Y.; Rys, R.N.; Lehoux, S.; Lemarie, C.A.; Blostein, M.D. Gas6 Promotes Inflammatory (CCR2(hi)CX3CR1(lo)) Monocyte Recruitment in Venous Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Muszbek, L.; Adany, R.; Szegedi, G.; Polgar, J.; Kavai, M. Factor XIII of blood coagulation in human monocytes. Thromb. Res. 1985, 37, 401–410. [Google Scholar] [CrossRef]
- Kradin, R.L.; Lynch, G.W.; Kurnick, J.T.; Erikson, M.; Colvin, R.B.; Mcdonagh, J. Factor-Xiii-a Is Synthesized and Expressed on the Surface of U937-Cells and Alveolar Macrophages. Blood 1987, 69, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.A.; Orekhov, A.N. Monocyte Activation in Immunopathology: Cellular Test for Development of Diagnostics and Therapy. J. Immunol. Res. 2016. [Google Scholar] [CrossRef]
- Fraser, S.R.; Booth, N.A.; Mutch, N.J. The antifibrinolytic function of factor XIII is exclusively expressed through alpha-antiplasmin cross-linking. Blood 2011, 117, 6371–6374. [Google Scholar] [CrossRef]
- Mutch, N.J.; Koikkalainen, J.S.; Fraser, S.R.; Duthie, K.M.; Griffin, M.; Mitchell, J.; Watson, H.G.; Booth, N.A. Model thrombi formed under flow reveal the role of factor XIII-mediated cross-linking in resistance to fibrinolysis. J. Thromb. Haemost. JTH 2010, 8, 2017–2024. [Google Scholar] [CrossRef]
- Bosshart, H.; Heinzelmann, M. THP-1 cells as a model for human monocytes. Ann. Transl. Med. 2016, 4, 438. [Google Scholar] [CrossRef]
- Tugal, D.; Liao, X.; Jain, M.K. Transcriptional control of macrophage polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Torocsik, D.; Bardos, H.; Nagy, L.; Adany, R. Identification of factor XIII-A as a marker of alternative macrophage activation. Cell Mol. Life Sci. 2005, 62, 2132–2139. [Google Scholar] [CrossRef]
- Grassi, F.; Dezutter-Dambuyant, C.; McIlroy, D.; Jacquet, C.; Yoneda, K.; Imamura, S.; Boumsell, L.; Schmitt, D.; Autran, B.; Debre, P.; et al. Monocyte-derived dendritic cells have a phenotype comparable to that of dermal dendritic cells and display ultrastructural granules distinct from Birbeck granules. J. Leukoc. Biol. 1998, 64, 484–493. [Google Scholar] [CrossRef]
- Sabat, R.; Grutz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Adany, R.; Antal, M. Three different cell types can synthesize factor XIII subunit A in the human liver. Thromb. Haemost. 1996, 76, 74–79. [Google Scholar]
- Yoshida, K.; Ono, M.; Sawada, H. Lipopolysaccharide-induced vacuoles in macrophages: Their origin is plasma membrane-derived organelles and endoplasmic reticulum, but not lysosomes. J. Endotoxin. Res. 1999, 5, 127–137. [Google Scholar] [CrossRef]
- Wong, A.O.; Marthi, M.; Mendel, Z.I.; Gregorka, B.; Swanson, M.S.; Swanson, J.A. Renitence vacuoles facilitate protection against phagolysosomal damage in activated macrophages. Mol. Biol. Cell 2018, 29, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Sarvary, A.; Szucs, S.; Balogh, I.; Becsky, A.; Bardos, H.; Kavai, M.; Seligsohn, U.; Egbring, R.; Lopaciuk, S.; Muszbek, L.; et al. Possible role of factor XIII subunit A in Fcgamma and complement receptor-mediated phagocytosis. Cell Immunol. 2004, 228, 81–90. [Google Scholar] [CrossRef]
- Cohen, I.; Young-Bandala, L.; Blankenberg, T.A.; Siefring, G.E., Jr.; Bruner-Lorand, J. Fibrinoligase-catalyzed cross-linking of myosin from platelet and skeletal muscle. Arch Biochem. Biophys. 1979, 192, 100–111. [Google Scholar] [CrossRef]
- Cohen, I.; Blankenberg, T.A.; Borden, D.; Kahn, D.R.; Veis, A. Factor XIIIa-catalyzed cross-linking of platelet and muscle actin. Regulation by nucleotides. Biochim. Biophys. Acta 1980, 628, 365–375. [Google Scholar] [CrossRef]
- Asijee, G.M.; Muszbek, L.; Kappelmayer, J.; Polgar, J.; Horvath, A.; Sturk, A. Platelet vinculin: A substrate of activated factor XIII. Biochim. Biophys. Acta 1988, 954, 303–308. [Google Scholar] [CrossRef]
- Freund, K.F.; Doshi, K.P.; Gaul, S.L.; Claremon, D.A.; Remy, D.C.; Baldwin, J.J.; Pitzenberger, S.M.; Stern, A.M. Transglutaminase inhibition by 2-[(2-oxopropyl)thio]imidazolium derivatives: Mechanism of factor XIIIa inactivation. Biochemistry 1994, 33, 10109–10119. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Kaartinen, M.T. Transglutaminase activity regulates differentiation, migration and fusion of osteoclasts via affecting actin dynamics. J. Cell Physiol. 2018, 233, 7497–7513. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Downing, L.J.; Strieter, R.M.; Kadell, A.M.; Wilke, C.A.; Austin, J.C.; Hare, B.D.; Burdick, M.D.; Greenfield, L.J.; Wakefield, T.W. IL-10 regulates thrombus-induced vein wall inflammation and thrombosis. J. Immunol. 1998, 161, 1471–1476. [Google Scholar]
- Caligiuri, G.; Rudling, M.; Ollivier, V.; Jacob, M.P.; Michel, J.B.; Hansson, G.K.; Nicoletti, A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 2003, 9, 10–17. [Google Scholar] [CrossRef]
- Poredos, P.; Jezovnik, M.K. In patients with idiopathic venous thrombosis, interleukin-10 is decreased and related to endothelial dysfunction. Heart Vessel. 2011, 26, 596–602. [Google Scholar] [CrossRef]
- Pajkrt, D.; van der Poll, T.; Levi, M.; Cutler, D.L.; Affrime, M.B.; van den Ende, A.; ten Cate, J.W.; van Deventer, S.J. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood 1997, 89, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Obi, A.T.; Elfline, M.A.; Hogikyan, E.; Luke, C.E.; Henke, S.; Coleman, D.; Henke, P.K. Alterations in macrophage phenotypes in experimental venous thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 2016, 4, 463–471. [Google Scholar] [CrossRef]
- Soendergaard, C.; Kvist, P.H.; Seidelin, J.B.; Nielsen, O.H. Tissue-regenerating functions of coagulation factor XIII. J. Thromb. Haemost. JTH 2013, 11, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Vanscheidt, W.; Hasler, K.; Wokalek, H.; Niedner, R.; Schopf, E. Factor XIII-deficiency in the blood of venous leg ulcer patients. Acta Derm Venereol. 1991, 71, 55–57. [Google Scholar]
- Inbal, A.; Lubetsky, A.; Krapp, T.; Castel, D.; Shaish, A.; Dickneitte, G.; Modis, L.; Muszbek, L.; Inbal, A. Impaired wound healing in factor XIII deficient mice. Thromb. Haemost. 2005, 94, 432–437. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Hu, K.; Frantz, S.; Jaffer, F.A.; Tung, C.H.; Hiller, K.H.; Voll, S.; Nordbeck, P.; Sosnovik, D.; Gattenlohner, S.; et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 2006, 113, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Gierhake, F.W.; Papastavrou, N.; Zimmermann, K.; Bohn, H.; Schwick, H.G. Prophylaxis of post-operative disturbances of wound healing with factor XIII substitution (author’s transl). Dtsch Med Wochenschr. 1974, 99, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.; Gossage, J.A.; Modarai, B.; Burnand, K.G.; Sisson, T.H.; Murdoch, C.; Smith, A. Monocyte urokinase-type plasminogen activator up-regulation reduces thrombus size in a model of venous thrombosis. J. Vasc. Surg. 2009, 50, 1127–1134. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, F.S.M.; Whyte, C.S.; Tuncay, A.; Williams, M.-L.; Wilson, H.M.; Mutch, N.J. Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. Int. J. Mol. Sci. 2021, 22, 6591. https://doi.org/10.3390/ijms22126591
Alshehri FSM, Whyte CS, Tuncay A, Williams M-L, Wilson HM, Mutch NJ. Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. International Journal of Molecular Sciences. 2021; 22(12):6591. https://doi.org/10.3390/ijms22126591
Chicago/Turabian StyleAlshehri, Fahad S. M., Claire S. Whyte, Ahmet Tuncay, Maria-Louise Williams, Heather M. Wilson, and Nicola J. Mutch. 2021. "Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation" International Journal of Molecular Sciences 22, no. 12: 6591. https://doi.org/10.3390/ijms22126591
APA StyleAlshehri, F. S. M., Whyte, C. S., Tuncay, A., Williams, M.-L., Wilson, H. M., & Mutch, N. J. (2021). Monocytes Expose Factor XIII-A and Stabilize Thrombi against Fibrinolytic Degradation. International Journal of Molecular Sciences, 22(12), 6591. https://doi.org/10.3390/ijms22126591