Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity
Abstract
1. Introduction
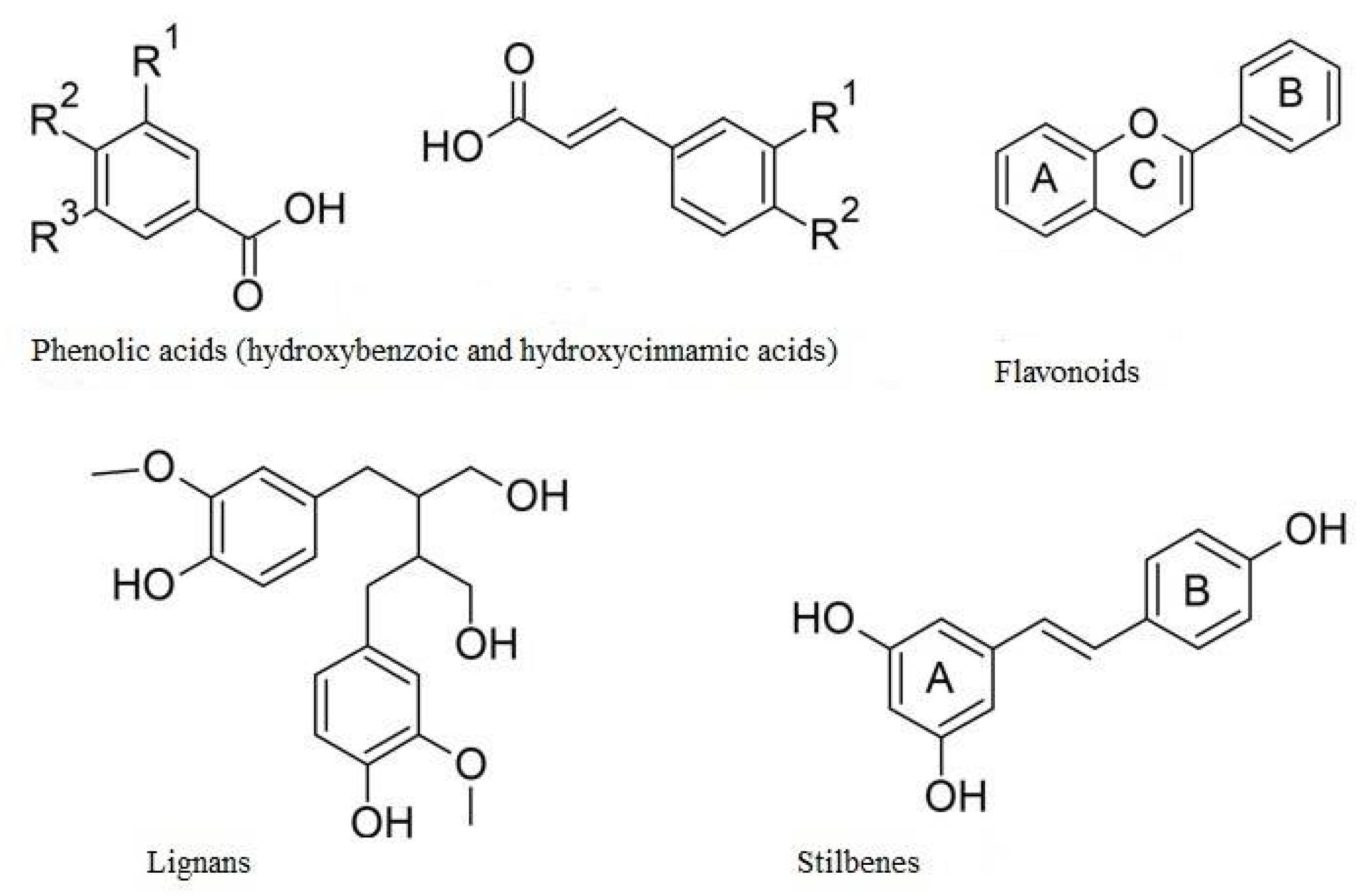
2. Polyphenols
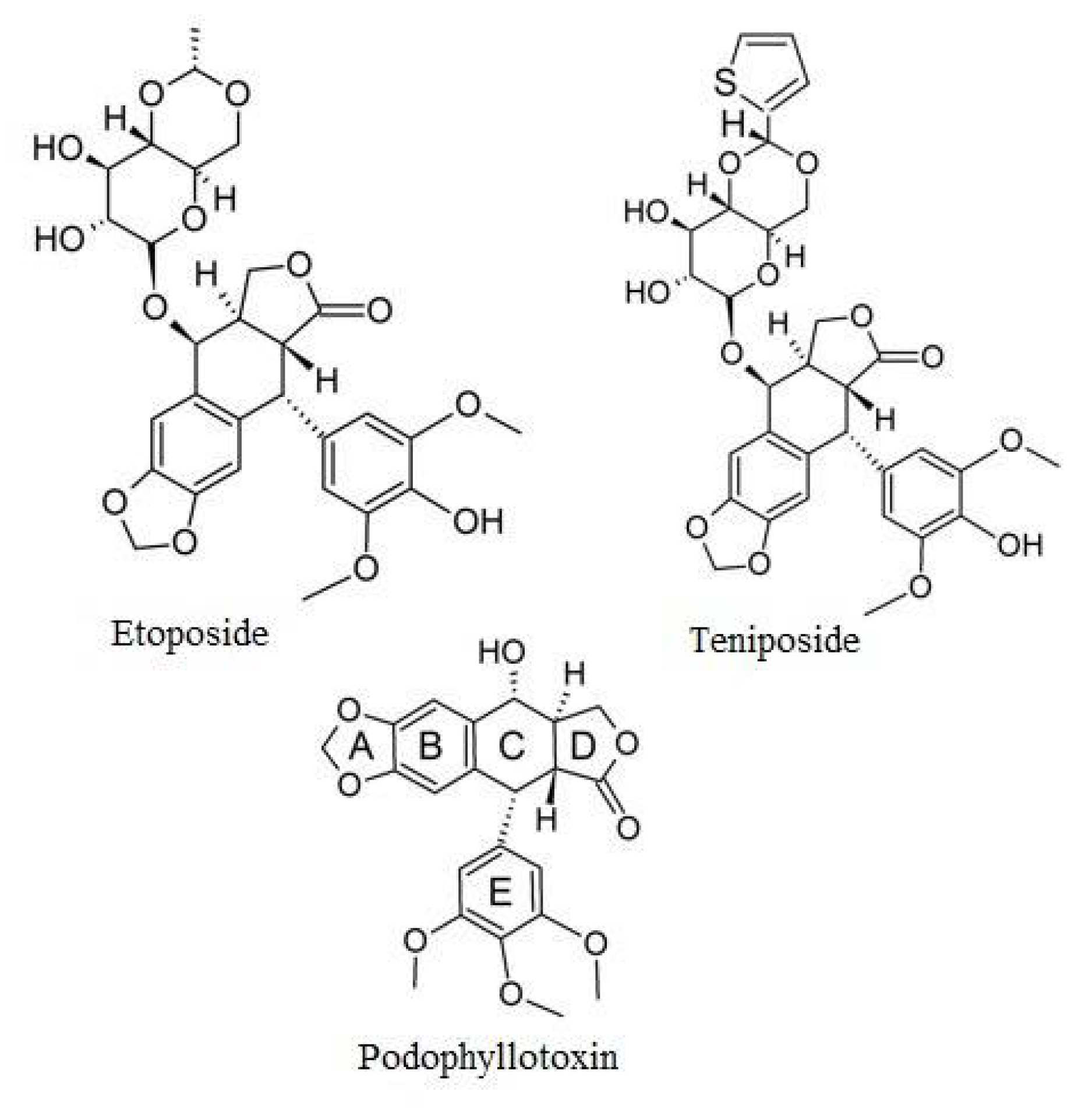
3. Etoposide
4. Polyphenols as Poisons of Topoisomerase II
5. Polyphenols as Modulators of Etoposide Activity
5.1. In Vitro Models
5.2. In Vivo Models
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMPK | (AMP)-activated protein kinase |
| BNML | Brown Norway Acute Myeloid Leukaemia |
| CSC | cancer stem cells |
| DISC | death-inducing signaling complex |
| EC | (−)-epicatechin |
| ECG | (−)-epicatechin gallate |
| EGC | (−)-epigallocatechin |
| EGCG | (−)-epigallocatechin gallate |
| FasL | Fas ligand |
| FasR | Fas receptor |
| GSH | glutathione |
| HSCs | haemopoietic stem cell lines |
| LSD | lowest significant dose |
| MLL | Mixed Lineage Leukemia gene |
| NSCLC | non-small cell lung cancer cells |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| STACs | SIRT1-activating compounds |
| t-AML | acute myelocytic leukemia |
| t-MDS | treatment-related myelodysplastic syndromes |
| TopoI | topoisomerase I |
| TopoII | topoisomerase II |
References
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Giuliano, C.; Cerri, S.; Blandini, F. Potential therapeutic effects of polyphenols in Parkinson’s disease: In vivo and in vitro pre-clinical studies. Neural Regen. Res. 2021, 16, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wei, J.; Zhao, C.; Li, G. Natural Polyphenols Targeting Senescence: A Novel Prevention and Therapy Strategy for Cancer. Int. J. Mol. Sci. 2020, 21, 684. [Google Scholar] [CrossRef]
- Alaswad, H.A.; Mahbub, A.A.; Le Maitre, C.L.; Jordan-Mahy, N. Molecular Action of Polyphenols in Leukaemia and Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 3085. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef] [PubMed]
- Bandele, O.J.; Clawson, S.J.; Osheroff, N. Dietary polyphenols as topoisomerase II poisons: B ring and C ring substituents determine the mechanism of enzyme-mediated DNA cleavage enhancement. Chem. Res. Toxicol. 2008, 21, 1253–1260. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic Interactions Between Anticancer Chemotherapeutics and Phenolic Compounds and Anticancer Synergy Between Polyphenols. Postepy Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Najar, I.A.; Johri, R.K. Pharmaceutical and pharmacological approaches for bioavailability enhancement of etoposide. J. Biosci. 2014, 39, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.; Sekhar, A.; Patil, V.M.; Menon, N.; Joshi, A.; Kapoor, A.; Prabhash, K. Systemic therapy for limited stage small cell lung carcinoma. J. Thorac. Dis. 2020, 12, 6275–6290. [Google Scholar] [CrossRef] [PubMed]
- Alsdorf, W.; Seidel, C.; Bokemeyer, C.; Oing, C. Current pharmacotherapy for testicular germ cell cancer. Expert Opin. Pharmacother. 2019, 20, 837–850. [Google Scholar] [CrossRef]
- Economides, M.P.; McCue, D.; Borthakur, G.; Pemmaraju, N. Topoisomerase II inhibitors in AML: Past, present, and future. Expert Opin. Pharmacother. 2019, 20, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Corsello, A.; Locantore, P.; Papi, G.; Pontecorvi, A.; Corsello, S.M. Medical Approaches in Adrenocortical Carcinoma. Biomedicines 2020, 8, E551. [Google Scholar] [CrossRef]
- Atienza, D.M.; Vogel, C.L.; Trock, B.; Swain, S.M. Phase II study of oral etoposide for patients with advanced breast cancer. Cancer 1995, 76, 2485–2490. [Google Scholar] [CrossRef]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Romano, A.; Maurizi, P.; Mastrangelo, S.; Attinà, G. Temozolomide and oral etoposide in children with recurrent malignant brain tumors. Drugs Context. 2020, 9. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, N.L.; Hsieh, T.S. New mechanistic and functional insights into DNA topoisomerases. Annu Rev. Biochem. 2013, 82, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614, Erratum in: Front. Pharmacol. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Chamani, E.; Rabbani-Chadegani, A.; Zahraei, Z. Spectroscopic detection of etoposide binding to chromatin components: The role of histone proteins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cong, Y.; Li, H.M.; Li, S.; Shen, Y.; Qi, Q.; Zhang, Y.; Li, Y.Z.; Tang, Y.J. Challenges and potential for improving the drug ability of podophyllotoxin-derived drugs in cancer chemotherapy. Nat. Prod. Rep. 2020, 8. [Google Scholar] [CrossRef]
- Wang, P.; Song, J.H.; Song, D.K.; Zhang, J.; Hao, C. Role of death receptor and mitochondrial pathways in conventional chemotherapy drug induction of apoptosis. Cell Signal. 2006, 18, 1528–1535. [Google Scholar] [CrossRef]
- Sinha, B.K. Role of Oxygen and Nitrogen Radicals in the Mechanism of Anticancer Drug Cytotoxicity. J. Cancer Sci. Ther. 2020, 12, 10–18. [Google Scholar]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Polyphenols act synergistically with doxorubicin and etoposide in leukaemia cell lines. Cell Death Discov. 2015, 1, 15043. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W.; Szade, K.; Bukowska-Straková, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Devel. Ther. 2016, 10, 557–570. [Google Scholar] [CrossRef][Green Version]
- Ermakova, S.P.; Kang, B.S.; Choi, B.Y.; Choi, H.S.; Schuster, T.F.; Ma, W.Y.; Bode, A.M.; Dong, Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006, 66, 9260–9269. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yang, Q.; Wang, T.; Cao, Y.; Jiang, Q.Y.; Ma, H.D.; Sun, H.W.; Hou, M.X.; Yang, Y.P.; Feng, F. Rhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agents. Biochim. Biophys. Acta 2016, 1860, 1417–1430. [Google Scholar] [CrossRef]
- Ruíz, G.; Valencia-González, H.A.; León-Galicia, I.; García-Villa, E.; García-Carrancá, A.; Gariglio, P. Inhibition of RAD51 by siRNA and Resveratrol Sensitizes Cancer Stem Cells Derived from HeLa Cell Cultures to Apoptosis. Stem Cells Int. 2018, 2018, 2493869. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kwak, D.W.; Lin, S.K.; Kim, H.M.; Kim, Y.M.; Park, O.J. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann. N. Y. Acad. Sci. 2007, 1095, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Zarnani, A.H.; Zand, H.; Koohdani, F.; Jeddi-Tehrani, M.; Vafa, M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur. J. Pharmacol. 2013, 718, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Heiduschka, G.; Bigenzahn, J.; Brunner, M.; Thurnher, D. Resveratrol synergistically enhances the effect of etoposide in HNSCC cell lines. Acta Otolaryngol. 2014, 134, 1071–1078. [Google Scholar] [CrossRef]
- Li, Z.M.; Jiang, W.Q.; Zhu, Z.Y.; Zhu, X.F.; Zhou, J.M.; Liu, Z.C.; Yang, D.J.; Guang, Z.Z. Synergistic cytotoxicity of Bcl-xL inhibitor, gossypol and chemotherapeutic agents in non-Hodgkin’s lymphoma cells. Cancer Biol. Ther. 2008, 7, 51–60. [Google Scholar] [CrossRef]
- Noda, C.; He, J.; Takano, T.; Tanaka, C.; Kondo, T.; Tohyama, K.; Yamamura, H.; Tohyama, Y. Induction of apoptosis by epigallocatechin-3-gallate in human lymphoblastoid B cells. Biochem. Biophys. Res. Commun. 2007, 362, 951–957. [Google Scholar] [CrossRef]
- Ferreira de Oliveira, J.M.P.; Pacheco, A.R.; Coutinho, L.; Oliveira, H.; Pinho, S.; Almeida, L.; Fernandes, E.; Santos, C. Combination of etoposide and fisetin results in anti-cancer efficiency against osteosarcoma cell models. Arch. Toxicol. 2018, 92, 1205–1214. [Google Scholar] [CrossRef]
- Yu, L.L.; Wu, J.G.; Dai, N.; Yu, H.G.; Si, J.M. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol. Rep. 2011, 26, 1197–1203. [Google Scholar] [CrossRef]
- Jiang, H.; Geng, D.; Liu, H.; Li, Z.; Cao, J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug Deliv. 2016, 23, 3665–3673. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, C.; Nair, S.M.; Escalon, E.; Melnick, S.J. Potentiation of etoposide and temozolomide cytotoxicity by curcumin and turmeric force™ in brain tumor cell lines. J. Complement. Integr. Med. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Krishnakumar, S. Synergistic Effect of Curcumin in Combination with Anti-cancer Agents in Human Retinoblastoma Cancer Cell Lines. Curr. Eye Res. 2015, 40, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Zupkó, I.; Chang, F.R.; Hunyadi, A.; Wu, C.C.; Weng, T.S.; Wang, H.C. Dietary flavonoid derivatives enhance chemotherapeutic effect by inhibiting the DNA damage response pathway. Toxicol. Appl. Pharmacol. 2016, 311, 99–105. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol derivatives isolated from Lens culinaris Medik. reduce DNA damage induced by etoposide in peripheral blood mononuclear cells. Toxicol. Res. (Camb). 2019, 8, 896–907. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol and Its Glycoside Derivatives as Modulators of Etoposide Activity in HL-60 Cells. Int. J. Mol. Sci. 2021, 22, 3520. [Google Scholar] [CrossRef]
- Papiez, M.A. The influence of curcumin on the action of etoposide in a rat acute myeloid leukemia cell line. Folia Med. Cracov. 2013, 53, 61–72. [Google Scholar]
- Zhang, M.; Liu, H.; Guo, R.; Ling, Y.; Wu, X.; Li, B.; Roller, P.P.; Wang, S.; Yang, D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem. Pharmacol. 2003, 66, 93–103. [Google Scholar] [CrossRef]
- Samuel, T.; Fadlalla, K.; Mosley, L.; Katkoori, V.; Turner, T.; Manne, U. Dual-mode interaction between quercetin and DNA-damaging drugs in cancer cells. Anti-Cancer Res. 2012, 32, 61–71. [Google Scholar]
- Felix, C.A.; Kolaris, C.P.; Osheroff, N. Topoisomerase II and the etiology of chromosomal translocations. DNA Repair (Amst). 2006, 5, 1093–1108. [Google Scholar] [CrossRef]
- Barjesteh van Waalwijk van Doorn-Khosrovani, S.; Janssen, J.; Maas, L.M.; Godschalk, R.W.; Nijhuis, J.G.; van Schooten, F.J. Dietary flavonoids induce MLL translocations in primary human CD34+ cells. Carcinogenesis 2007, 28, 1703–1709. [Google Scholar] [CrossRef]
- Biechonski, S.; Gourevich, D.; Rall, M.; Aqaqe, N.; Yassin, M.; Zipin-Roitman, A.; Trakhtenbrot, L.; Olender, L.; Raz, Y.; Jaffa, A.J.; et al. Quercetin alters the DNA damage response in human hematopoietic stem and progenitor cells via TopoII- and PI3K-dependent mechanisms synergizing in leukemogenic rearrangements. Int. J. Cancer 2017, 140, 864–876. [Google Scholar] [CrossRef]
- Ortiz-Sánchez, E.; Santiago-López, L.; Cruz-Domínguez, V.B.; Toledo-Guzmán, M.E.; Hernández-Cueto, D.; Muñiz-Hernández, S.; Garrido, E.; Cantú De León, D.; García-Carrancá, A. Characterization of cervical cancer stem cell-like cells: Phenotyping, stemness, and human papilloma virus co-receptor expression. Oncotarget 2016, 7, 31943–31954. [Google Scholar] [CrossRef]
- Yadav, A.K.; Desai, N.S. Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects. Stem Cell Rev. Rep. 2019, 15, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Syu, J.J.; Chen, J.C.; Wang, T.J.; Chang, P.Y.; Chen, C.Y.; Jian, Y.T.; Jian, Y.J.; Lin, Y.W. Resveratrol Enhances Etoposide-Induced Cytotoxicity through Down-Regulating ERK1/2 and AKT-Mediated X-ray Repair Cross-Complement Group 1 (XRCC1) Protein Expression in Human Non-Small-Cell Lung Cancer Cells. Basic Clin. Pharmacol. Toxicol. 2015, 117, 383–391. [Google Scholar] [CrossRef]
- Saleh, E.M.; El-awady, R.A.; Eissa, N.A.; Abdel-Rahman, W.M. Antagonism between curcumin and the topoisomerase II inhibitor etoposide: A study of DNA damage, cell cycle regulation and death pathways. Cancer Biol. Ther. 2012, 13, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Bandele, O.J.; Osheroff, N. The efficacy of topoisomerase II-targeted anti-cancer agents reflects the persistence of drug-induced cleavage complexes in cells. Biochemistry 2008, 47, 11900–11908. [Google Scholar] [CrossRef]
- Papież, M.A.; Krzyściak, W. The antioxidant quercetin protects HL-60 cells with high myeloperoxidase activity against pro-oxidative and apoptotic effects of etoposide. Acta Biochim. Pol. 2014, 61, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Papież, M.A. The effect of quercetin on oxidative DNA damage and myelosuppression induced by etoposide in bone marrow cells of rats. Acta Biochim. Pol. 2014, 61, 7–11. [Google Scholar] [CrossRef]
- Sonnemann, J.; Kahl, M.; Siranjeevi, P.M.; Blumrich, A.; Blümel, L.; Becker, S.; Wittig, S.; Winkler, R.; Krämer, O.H.; Beck, J.F. Reverse chemomodulatory effects of the SIRT1 activators resveratrol and SRT1720 in Ewing’s sarcoma cells: Resveratrol suppresses and SRT1720 enhances etoposide- and vincristine-induced anti-cancer activity. J. Cancer Res. Clin. Oncol. 2016, 142, 17–26. [Google Scholar] [CrossRef]
- Mahbub, A.A.; Le Maitre, C.L.; Haywood-Small, S.L.; Cross, N.A.; Jordan-Mahy, N. Glutathione is key to the synergistic enhancement of doxorubicin and etoposide by polyphenols in leukaemia cell lines. Cell Death Dis. 2015, 6, e2028. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef] [PubMed]
- Papiez, M.A.; Krzyściak, W. The dual effect of curcumin on etoposide action in leukemic and healthy bone marrow cells of rats with acute myeloid leukemia. Folia Med. Cracov. 2014, 54, 71–79. [Google Scholar] [PubMed]
- Papiez, M.A.; Bukowska-Straková, K.; Krzysciak, W.; Baran, J. (-)-Epicatechin enhances etoposide-induced antileukaemic effect in rats with acute myeloid leukaemia. Anti-Cancer Res. 2012, 32, 2905–2913. [Google Scholar]
- Amin, A.; Gali-Muhtasib, H.; Ocker, M.; Schneider-Stock, R. Overview of major classes of plant-derived anti-cancer drugs. Int. J Biomed. Sci. 2009, 5, 1–11. [Google Scholar] [PubMed]
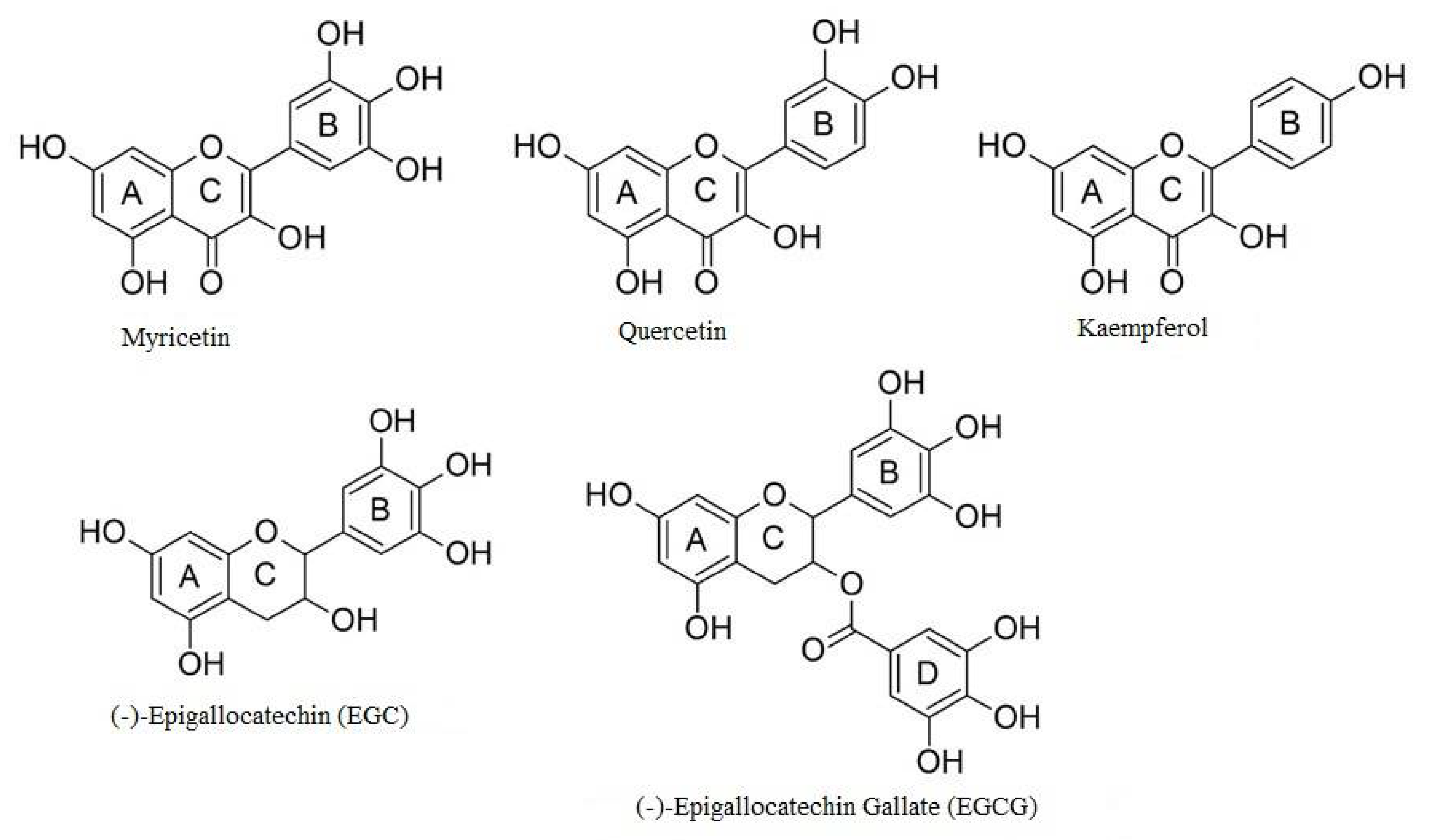
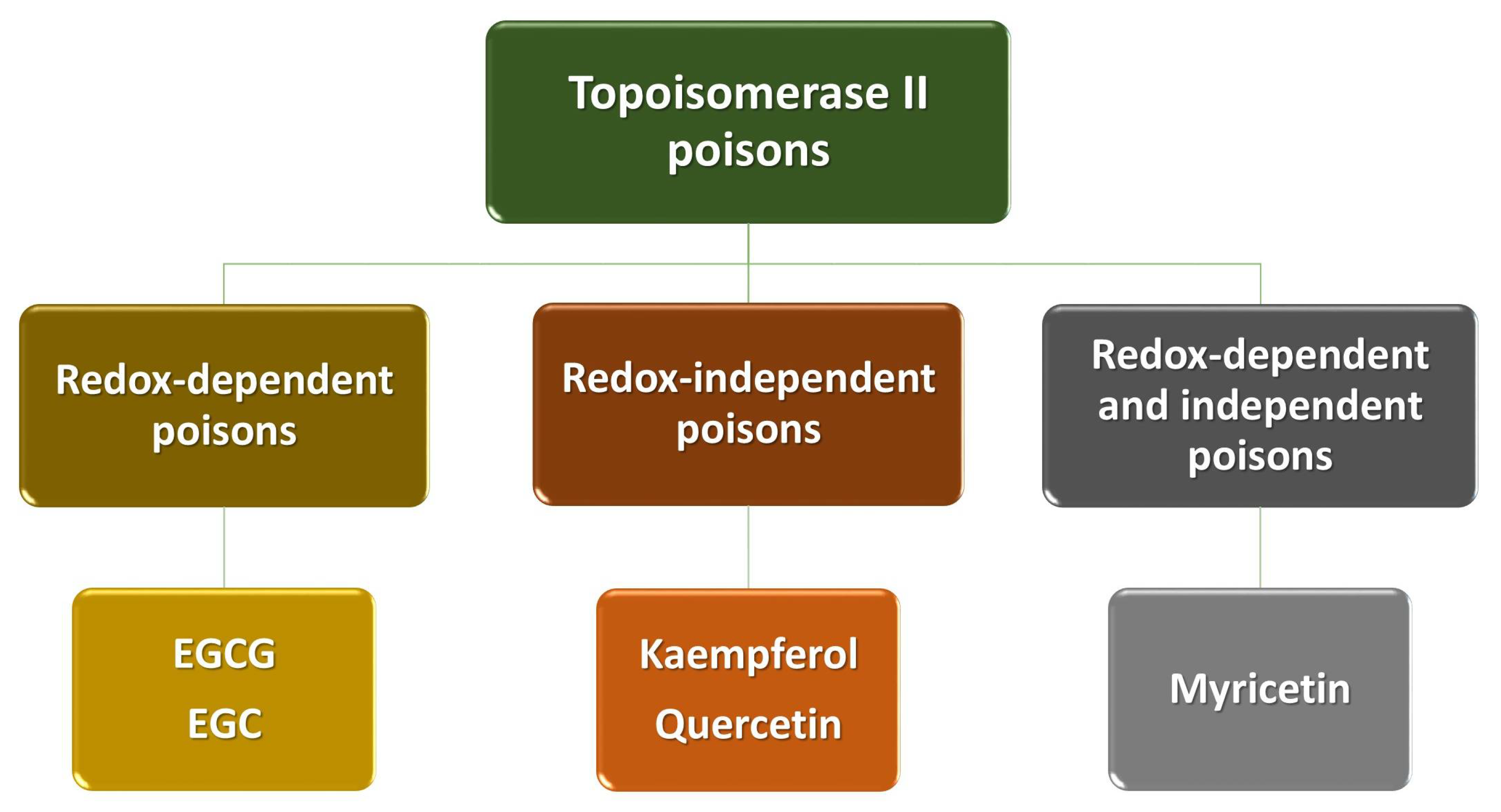
| Polyphenol | Proposed Mechanisms of Action |
|---|---|
| EGCG EGC |
|
| Kaempferol Quercetin |
|
| Myricetin |
|
| Polyphenol | In Vitro Model | Dose of Polyphenol | Dose of Etoposide | Interaction with Etoposide | Ref. |
|---|---|---|---|---|---|
| Apigenin | CCRF-CEM | LSD | LSD | ATP level ↑; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] |
| Jurkat | LSD | LSD | ATP level ↑; caspase-3 and 9 activity ↑; level of cells in S phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| KG-1a | LSD | LSD | ATP level ↑; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑; | [29] | |
| THP-1 | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| Catechin | MDA-MB-231 | 10–40 µM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| Curcumin | HL-60 | 20 µM | 3–10 µM | apoptosis ↑; phosphorylation of the histone H2AX induced by etoposide ↑; ROS generation ↑ | [30] |
| SGC7901 | 1 mg | 5 mg | cytotoxicity induced by etoposide ↑ | [41] | |
| Weri-Rb1 and Y79 | 5–10 µM | 0.1–20 µg/mL | etoposide-induced cytotoxicity ↑; level of apoptotic cells ↑; caspase 3 activity ↑; level of the cells in the G0/G1 phase of the cell cycle ↓ | [43] | |
| LT12 | 1–20 µM | 1–40 µM | level of cells arrested in the G2/M phase ↑; DNA damage ↑; number of apoptotic cells ↑ | [47] | |
| MCF-7, HepG2, HCT116, HeLa | 10 µg/mL | 1 µg/mL | cytotoxicity of etoposide ↓; level of MCF-7 cells in S phase of cell cycle ↑; level of HCT116 and HeLa cells in the G2/M phase ↑; | [56] | |
| U-87MG | 37.33 µg/mL (IC50) | 6.5 µg/mL | cytotoxicity induced by etoposide ↑; BAX/Bcl-2 ratio ↑; expression of p10 and p53 ↓ | [42] | |
| SGC7901 | 10–160 µM | 2–200 µM | etoposide-induced cytotoxicity ↑; phosphorylation of IκBα ↓; level of apoptotic cells ↑; Bcl-2 and Bcl-xL expression ↓; attenuated the activation of NF-κB | [40] | |
| Cyanidin | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| EGCG | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| Ramos | 7.5 µM | 0.02 µg/mL | apoptosis induced by etoposide ↑ | [38] | |
| MDA-MB-231 and T-47D | 10 µM | 0.1 µM | interferes with the formation of the anti-apoptotic GRP78-caspase-7 complex, which leads to an increase etoposide-induced apoptosis; suppresses the transformed phenotype of breast cancer cells treated with etoposide | [31] | |
| Emodin | CCRF-CEM | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] |
| Jurkat | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| KG-1a | LSD | LSD | caspase-9 activity ↑ | [29] | |
| THP-1 | LSD | LSD | caspase-9 activity ↑ | [29] | |
| Fisetin | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| MG-63 and Saos-2 | 5–150 µM | 0.5–10 µM | shows negative-to-positive interactions on the inhibition of cell proliferation depending on the relative concentrations; level of cells in G2-phase of the cell cycle ↑; cells in G1-phase ↓; levels of cyclins B1 and E1 ↓ | [39] | |
| Gossypol | Ramos | 12 µM | 20 µM | apoptosis in a time-dependent manner via activation of caspase-3 signaling ↑; enhances cytosolic cytochrome c release ↑ | [37] |
| Genistein | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| CEM | 50 µM | 0–200 µM | no impact on the cytotoxicity and genotoxicity induced by etoposide | [57] | |
| Kaempferol | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| HL-60 | 10–50 µg/mL | 1 µM | DNA damage induced by etoposide ↑ | [45] | |
| HL-60 | 10–50 µg/mL | 1–10 µM | sensitivity of cells to etoposide ↑; ROS generation ↓ | [46] | |
| Naringenin | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| Quercetin | MDA-MB-231 | 10–40 μM | 1 µM | inhibition of etoposide-induced Chk1 Ser345 phosphorylation | [44] |
| HL-60 | 0.5–100 µM | 1–10 µM | ROS generation ↓; apoptosis ↓ | [58] | |
| LT12 | 1–20 µM | 5 µM | oxidative DNA damage ↓ | [59] | |
| HCT116 | 50 µM | 50 µM | cyclin B1 level ↓; abrogates the increase in levels of p53 or its targets BAX and p21 induced by etoposide | [49] | |
| HSPCs | 50 µM | 10 µM | frequencies of MLL rearrangements in human HSPCs ↑ | [52] | |
| CCRF-CEM | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| Jurkat | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| KG-1a | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| THP-1 | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S and G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| Resveratrol | WE-68, SK-ES-1 and SK-N-MC | 5–10 µM | 0.1–1 µM | etoposide-induced p21 expression in WE-68 cells ↓; etoposide-induced cell death ↓ | [60] |
| SCC25, CAL27 and FaDu | 40 µM | 10 µM | etoposide-induced apoptosis ↑ | [36] | |
| HepG2, HCT-116 | 12.5–100 µM | 1–10 µM | etoposide-induced p53 expression ↑; anti-proliferative effects of etoposide ↑ | [35] | |
| HT-29 | 50–400 µM | 100–500 µM | cell death induced by etoposide ↑; ROS generation ↑; chemosensitivity of cells ↑; AMPK ↑ | [34] | |
| Cancer stem cells (CSC) from HeLa | 137 µM | 5.8 µg/mL | sensitizes cervical CSC cells to etoposide treatment by RAD51 inhibition | [33] | |
| Rhamnetin | HepG2 | 3 µM | 120 nM | level of cells in S phase of cell cycle ↑; IC50 value of etoposide ↓ | [32] |
| Rhein | CCRF-CEM | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] |
| Jurkat | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| KG-1a | LSD | LSD | caspase-9 activity ↑; glutathione level ↑ | [29] | |
| THP-1 | LSD | LSD | caspase-9 activity ↑; glutathione level ↑ | [29] | |
| cis-Stilbene | CCRF-CEM | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in S phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] |
| Jurkat | LSD | LSD | ATP level ↓; caspase-3 and 9 activity ↑; level of cells in G2/M phase of cell cycle ↑; glutathione level ↓; γH2AX foci ↑ | [29] | |
| KG-1a | LSD | LSD | caspase-9 activity ↑; glutathione level ↑ | [29] | |
| THP-1 | LSD | LSD | caspase-9 activity ↑; glutathione level ↑ | [29] | |
| Taurin | MCF-7, HepG2, U251, HeLaand HCT116 | 10–50 µg/mL | 1 µg/mL | no effect on etoposide cytotoxicity | [56] |
| Polyphenol | In Vivo Model | Dose of Polyphenol | Dose of Etoposide | Interaction with Etoposide | Ref. |
|---|---|---|---|---|---|
| Curcumin | Brown Norway rats with acute myeloid leukemia (BNML) | 100 and 200 mg/kg | 50 mg/kg |
| [30] |
| BALB/c mice bearing SGC7901 cells xenografts | 1 mg | 5 mg |
| [41] | |
| BNML rats | 200 mg/kg | 50 mg/kg |
| [63] | |
| (−)-Epicatechin | Brown Norway rats with acute myeloid leukemia (BNML) | 40 mg/kg | 50 mg/kg |
| [64] |
| Quercetin | Bone marrow cells from BN/CrlCmd rats | 100 mg/kg | 50 mg/kg |
| [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluska, M.; Woźniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. https://doi.org/10.3390/ijms22126602
Kluska M, Woźniak K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. International Journal of Molecular Sciences. 2021; 22(12):6602. https://doi.org/10.3390/ijms22126602
Chicago/Turabian StyleKluska, Magdalena, and Katarzyna Woźniak. 2021. "Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity" International Journal of Molecular Sciences 22, no. 12: 6602. https://doi.org/10.3390/ijms22126602
APA StyleKluska, M., & Woźniak, K. (2021). Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. International Journal of Molecular Sciences, 22(12), 6602. https://doi.org/10.3390/ijms22126602







