Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma
Abstract
:1. Introduction
2. Results and Discussion
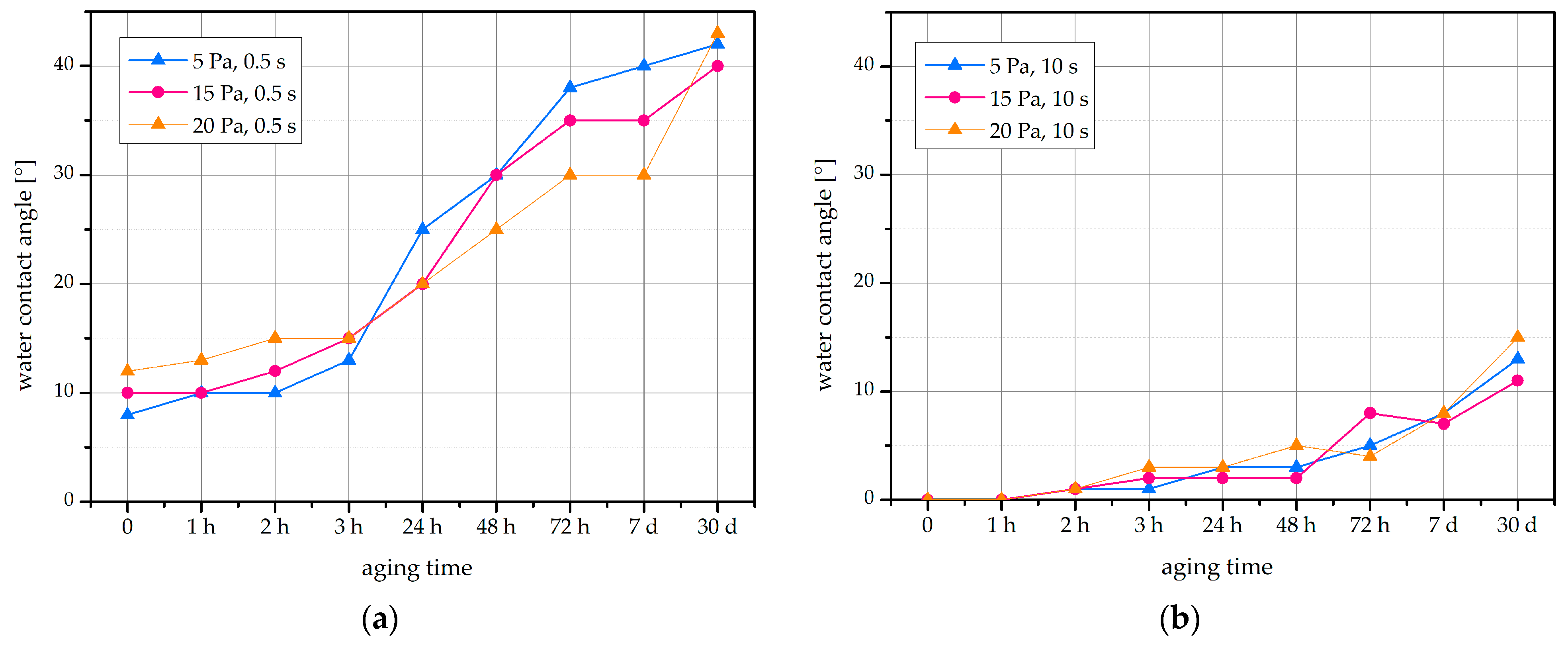
2.1. Water Contact Angle
2.2. Hydrophobic Recovery
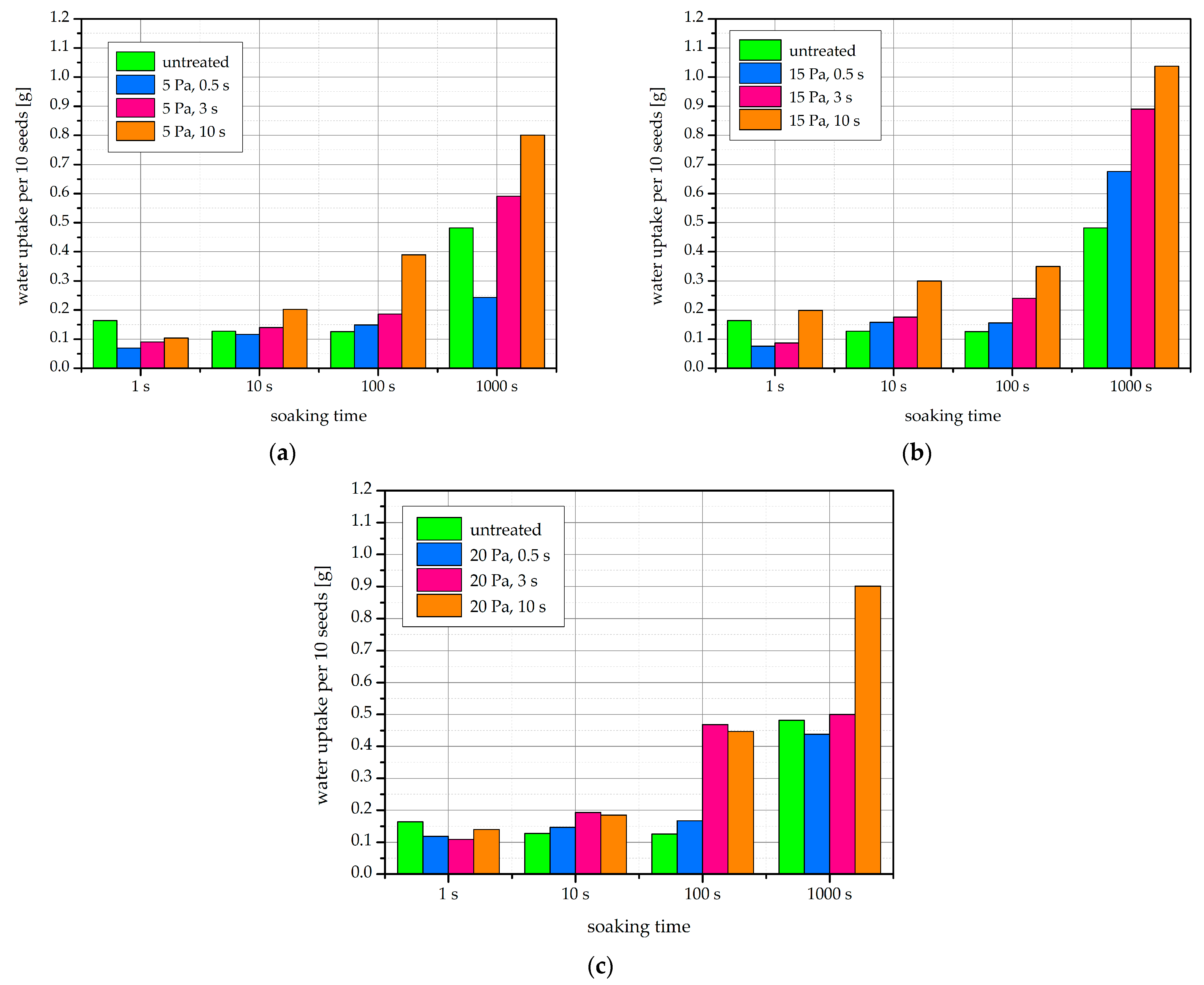
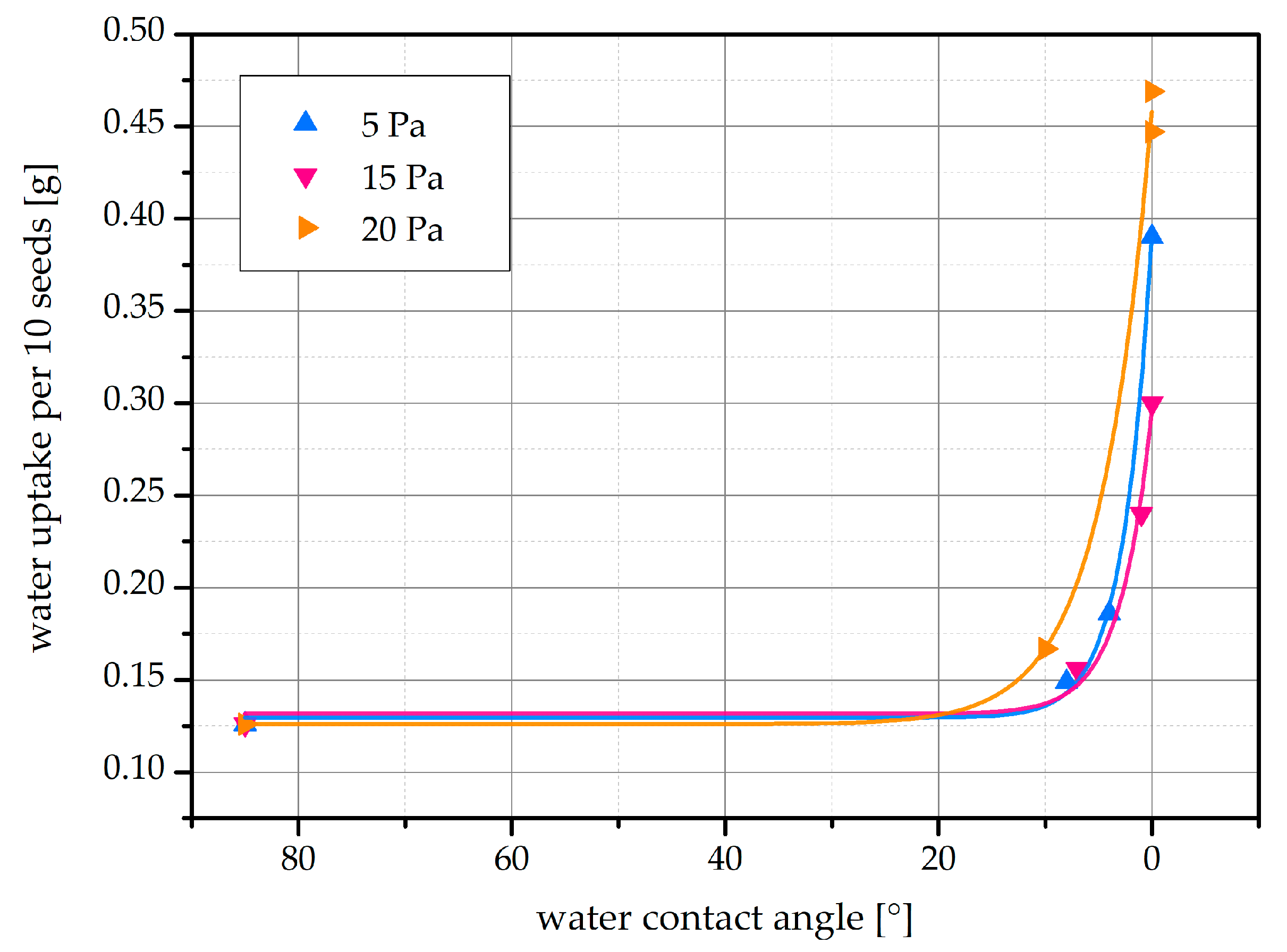
2.3. Water Uptake
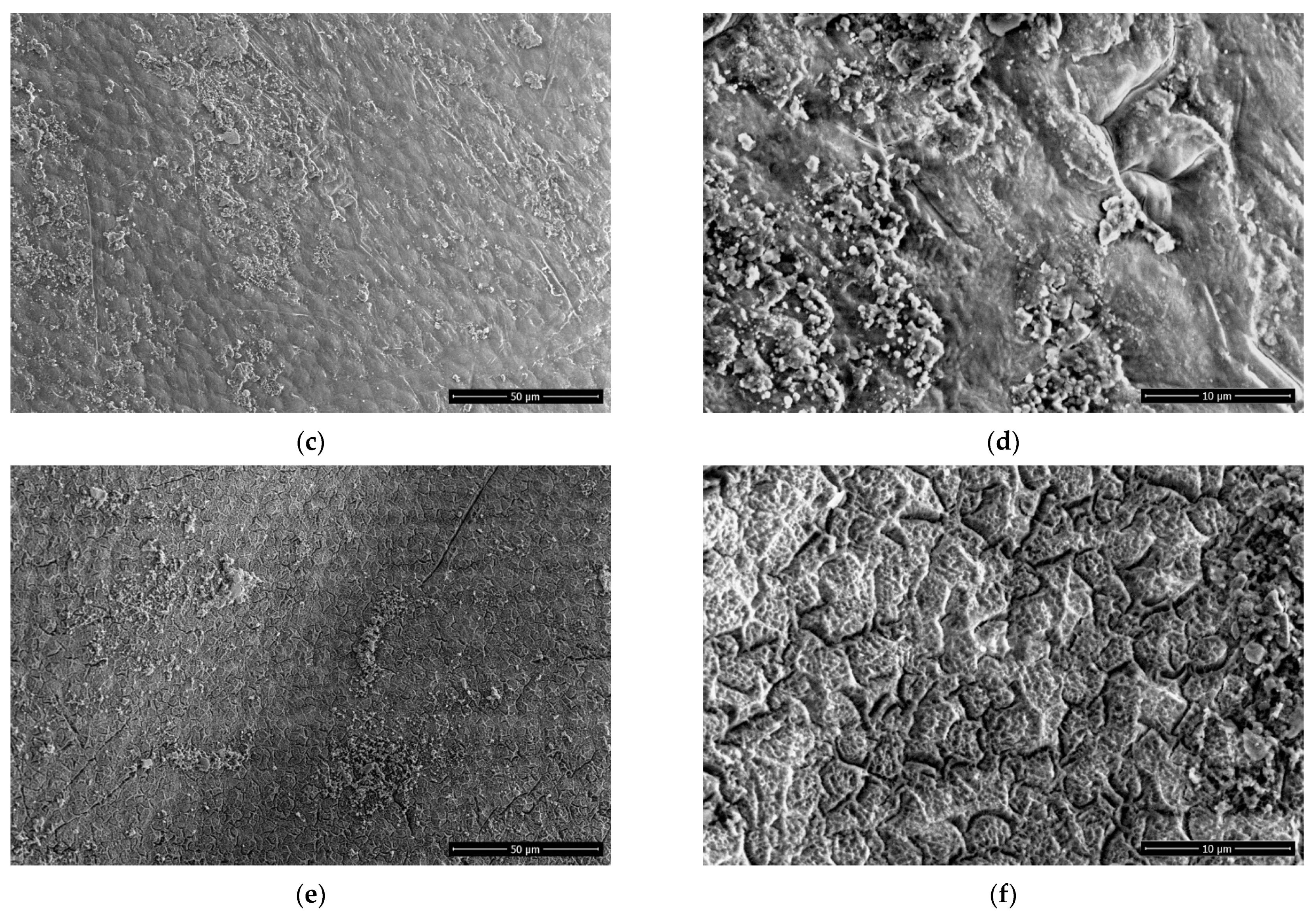
2.4. Scanning Electron Microscopy
2.5. X-ray Photoelectron Spectroscopy
2.6. Germination Percentage
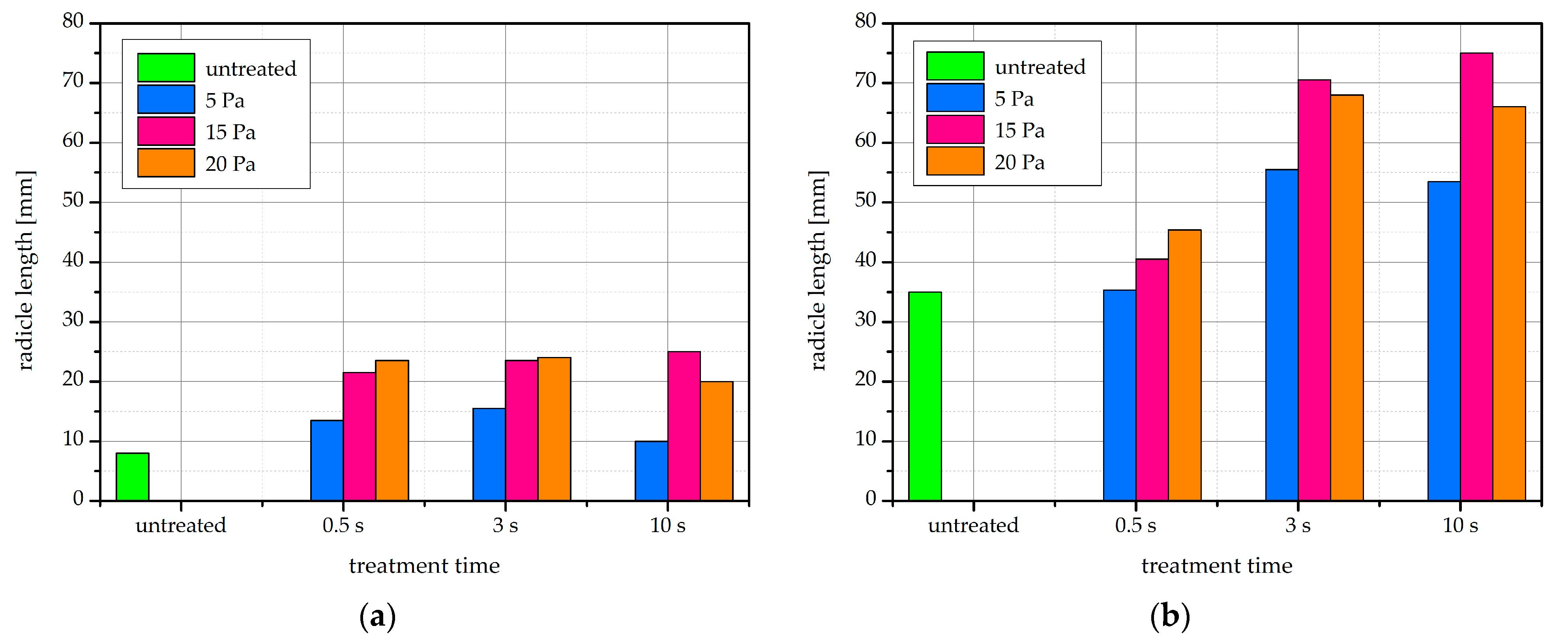
2.7. Radicle Length
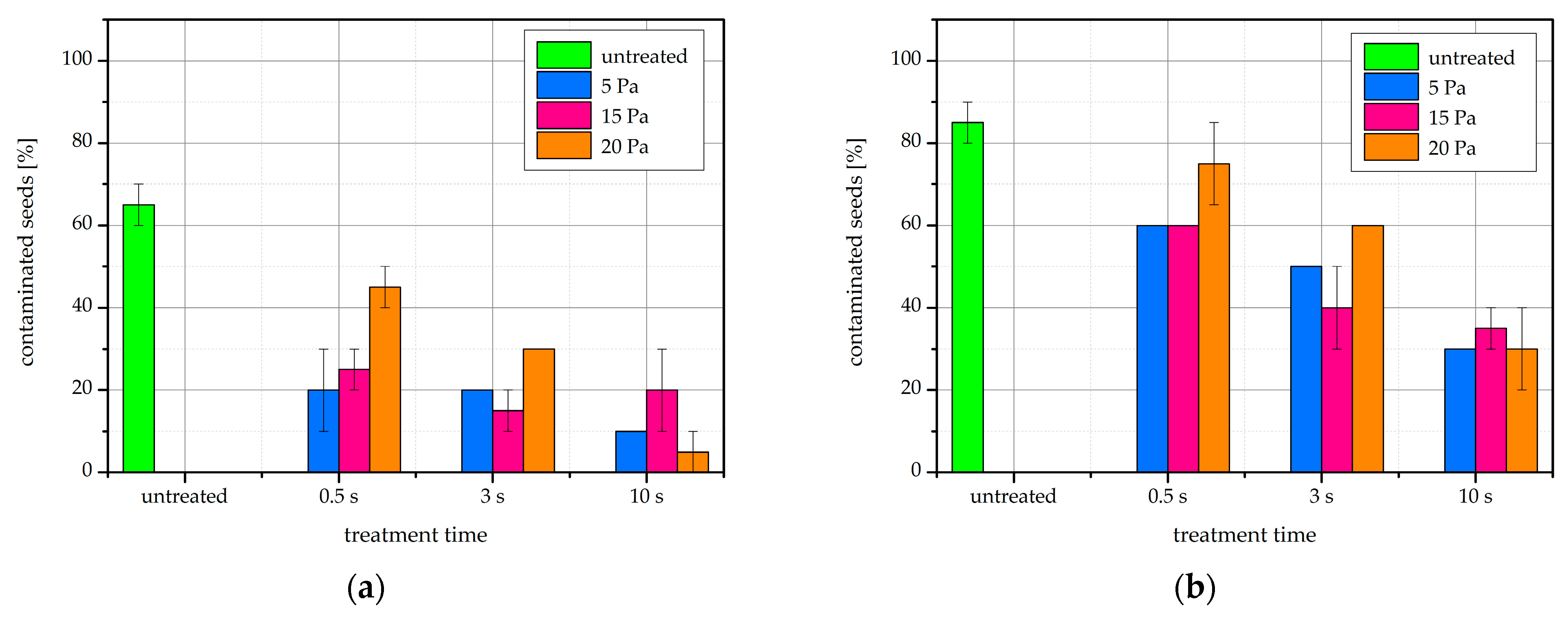
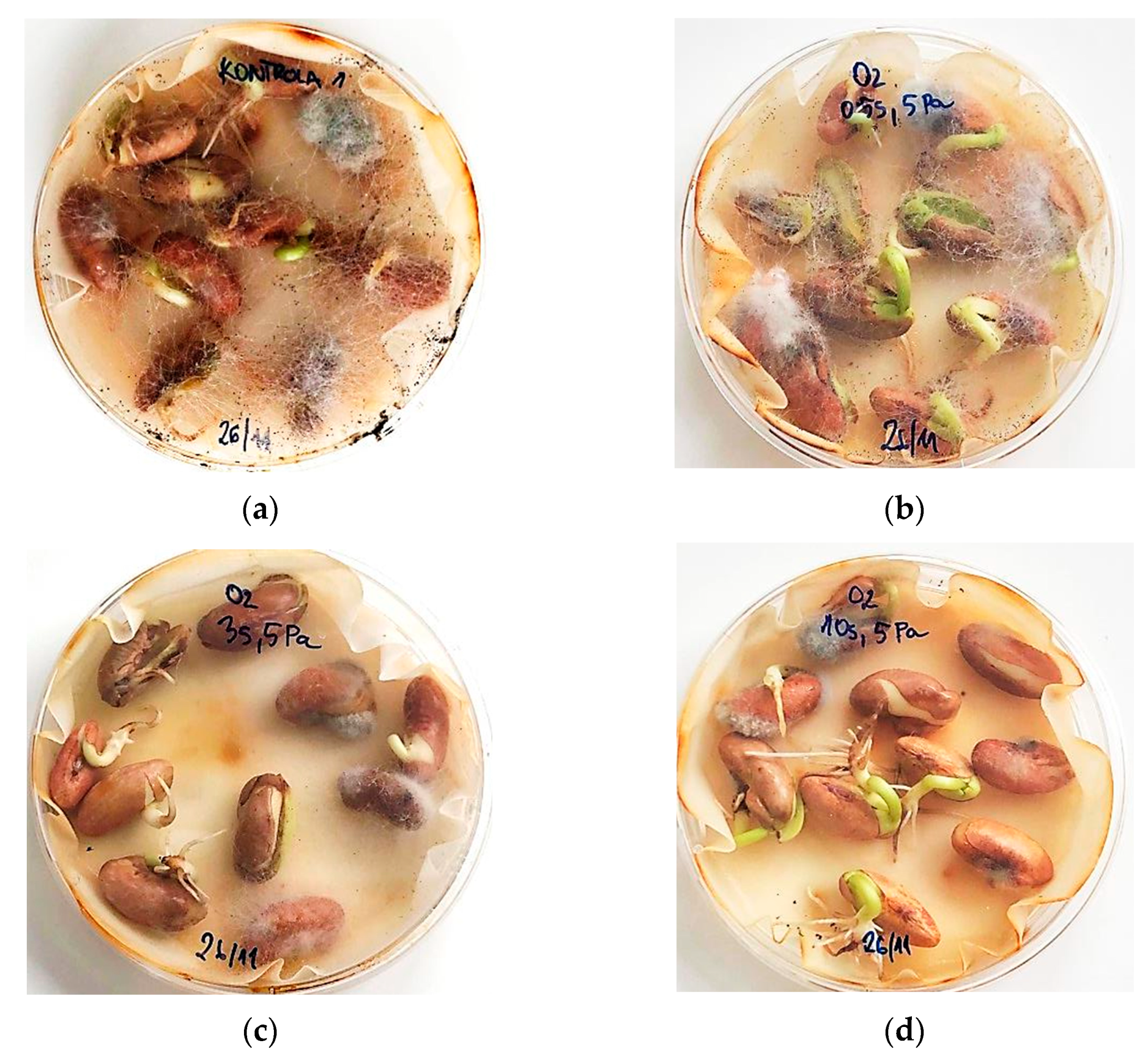
2.8. Fungal Contamination
3. Materials and Methods
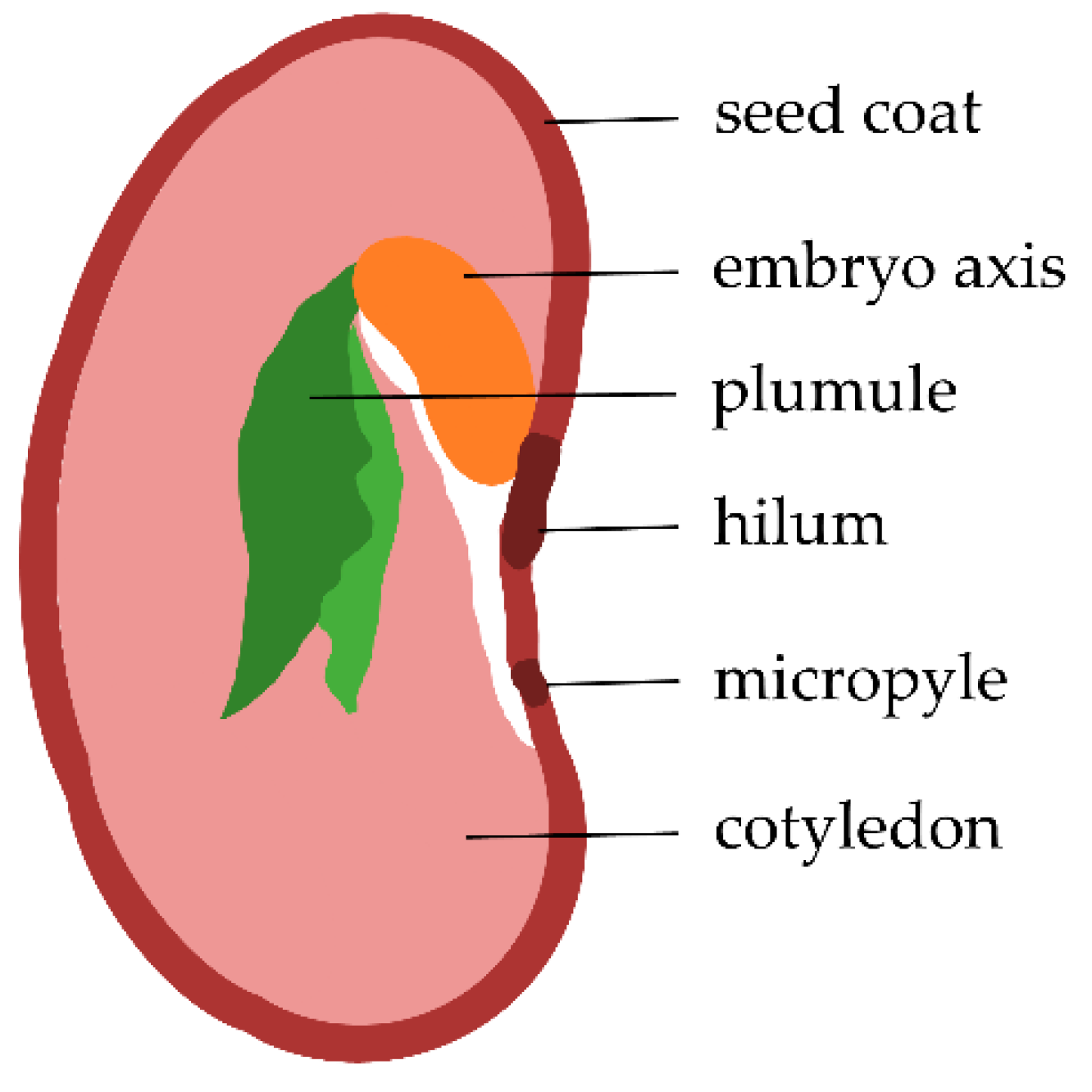
3.1. Seed Material
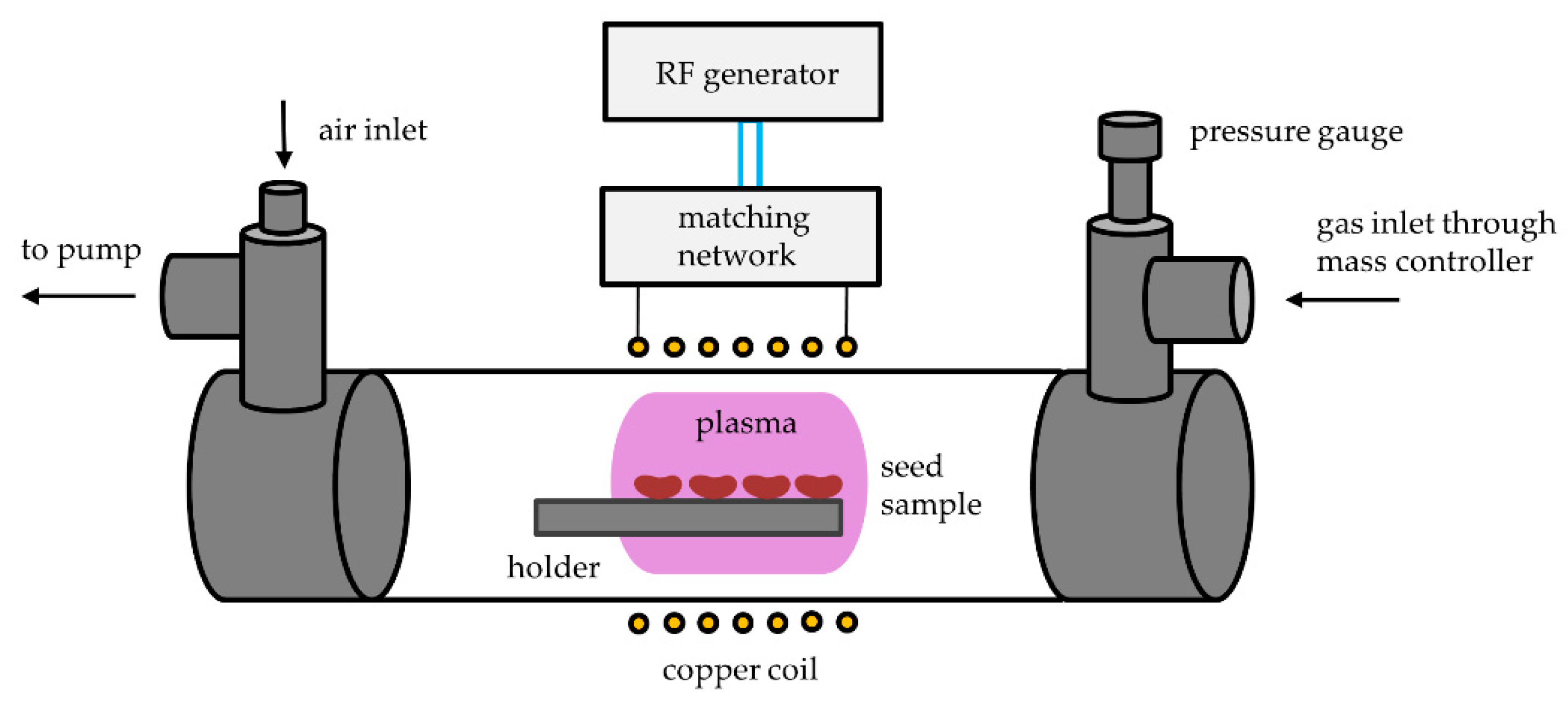
3.2. Plasma Reactor
3.3. Plasma Treatment
3.4. Water Contact Angle
3.5. Water Uptake
3.6. Scanning Electron Microscopy
3.7. X-ray Photoelectron Spectroscopy
3.8. Germination and Radicle Length
3.9. Fungal Contamination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sathe, S.K.; Deshpande, S.S. Beans. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Trugo, L., Finglas, P.M., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 403–412. [Google Scholar]
- Faostat Crops Data: Beans, Dry. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 27 April 2021).
- Hema, M.; Sreenivasulu, P.; Patil, B.L.; Kumar, P.L.; Reddy, D.V. Tropical food legumes: Virus diseases of economic importance and their control. Adv. Virus Res. 2014, 90, 431–505. [Google Scholar] [CrossRef]
- Marcenaro, D.; Valkonen, J.P. Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. Inta rojo) in nicaragua. PLoS ONE 2016, 11, e0168662. [Google Scholar] [CrossRef] [Green Version]
- Diseases of Bean (Phaseolus Vulgaris L.). Available online: https://www.apsnet.org/edcenter/resources/commonnames/Pages/Bean.aspx (accessed on 14 June 2021).
- Gupta, S.K.; Singh, G. Major Fungal French Bean Diseases: Epidemiology and Management. In Emerging Trends in Plant Pathology; Singh, K.P., Jahagirdar, S., Sarma, B.K., Eds.; Springer: Singapore, 2021; pp. 141–174. [Google Scholar]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Hoppanová, L.; Medvecká, V.; Dyblíková, J.; Hudecova, D.; Kaliňáková, B.; Kryštofová, S.; Zahoranová, A. Low-temperature plasma applications in chemical fungicide treatment reduction. Acta Chim. Slovaca 2020, 13, 26–33. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňaková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2016, 36, 397–414. [Google Scholar] [CrossRef]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of cold atmospheric pressure plasma on maize seeds: Enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza Sativa L.) seed sterilization and germination enhancement via atmospheric hybrid nonthermal discharge plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef]
- Pérez Pizá, M.C.; Prevosto, L.; Zilli, C.; Cejas, E.; Kelly, H.; Balestrasse, K. Effects of non-thermal plasmas on seed-borne diaporthe/phomopsis complex and germination parameters of soybean seeds. Innov. Food Sci. Emerg. Technol. 2018, 49, 82–91. [Google Scholar] [CrossRef]
- Rüntzel, C.L.; da Silva, J.R.; da Silva, B.A.; Moecke, E.S.; Scussel, V.M. Effect of cold plasma on black beans (Phaseolus Vulgaris L.), fungi inactivation and micro-structures stability. Emir. J. Food Agric. 2019, 31, 864–873. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus Vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Rojas, J.; Barillas, L.; Ching-Baltodano, R.; Poveda-Orozco, F.; Vargas, V.I. Low-temperature plasma irradiation to improve germination and vigor in seeds of coriandrum sativum, lycopersicon lycopersicum, phaseolus vulgaris and raphanus sativus. In Proceedings of the 16th Latin American Workshop on Plasma Physics, Mexico City, Mexico, 4–8 September 2017; pp. 67–72. [Google Scholar]
- Judee, F.; Dufour, T. Seed-packed dielectric barrier device for plasma agriculture: Understanding its electrical properties through an equivalent electrical model. J. Appl. Phys. 2020, 128, 044901. [Google Scholar] [CrossRef]
- Souza, F.H.D.D.E.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in fabaceae. Rev. Bras. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef] [Green Version]
- The Seed of a Typical Dicotyledonous Plant: The Bean. Available online: http://www.bio.miami.edu/dana/docs/seeds.html (accessed on 25 May 2021).
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum Sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some indian kidney bean (Phaseolus Vulgaris L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Zaplotnik, R.; Vesel, A.; Mozetič, M. Transition from e to h mode in inductively coupled oxygen plasma: Hysteresis and the behaviour of oxygen atom density. Europhys. Lett. 2011, 95, 55001. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, Y.; Makabe, T. Review and current status: E ⇌ h mode transition in low-temperature icp and related electron dynamics. Plasma Sour. Sci. Technol. 2021, 30, 023001. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Kim, K.-H.; Moon, J.-H.; Chung, C.-W. Electrical and plasma characterization of a hybrid plasma source combined with inductively coupled and capacitively coupled plasmas for o atom generation. Phys. Plasmas 2020, 27, 093504. [Google Scholar] [CrossRef]
- Meichsner, J.; Wegner, T. Evaluation of oxygen species during e–h transition in inductively coupled rf plasmas: Combination of experimental results with global model. Eur. Phys. J. D 2018, 72, 85. [Google Scholar] [CrossRef] [Green Version]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the vuv radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sour. Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Shapira, Y.; Multanen, V.; Whyman, G.; Bormashenko, Y.; Chaniel, G.; Barkay, Z.; Bormashenko, E. Plasma treatment switches the regime of wetting and floating of pepper seeds. Colloids Surf. B Biointerfaces 2017, 157, 417–423. [Google Scholar] [CrossRef]
- Sadhu, S.; Thirumdas, R.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on the enzymatic activity in germinating mung beans (Vigna Radiate). LWT 2017, 78, 97–104. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [Green Version]
- Fridman, A. Plasma Chemistry; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Mozetič, M. Plasma-stimulated super-hydrophilic surface finish of polymers. Polymers 2020, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Zaplotnik, R.; Mozetič, M.; Primc, G. Surface modification of ps polymer by oxygen-atom treatment from remote plasma: Initial kinetics of functional groups formation. Appl. Surf. Sci. 2021, 561, 150058. [Google Scholar] [CrossRef]
- Srisonphan, S. Tuning surface wettability through hot carrier initiated impact ionization in cold plasma. ACS Appl. Mater. Interfaces 2018, 10, 11297–11304. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (cucumis sativus l.) and pepper (capsicum annuum l.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Volkov, A.G.; Hairston, J.S.; Marshall, J.; Bookal, A.; Dholichand, A.; Patel, D. Plasma seeds: Cold plasma accelerates Phaseolus vulgaris seed imbibition, germination, and speed of seedling growth. Plasma Med. 2020, 10, 139–158. [Google Scholar] [CrossRef]
- Volkov, A.G.; Bookal, A.; Hairston, J.S.; Patel, D. Radio frequency plasma capacitor can increase rates of seeds imbibition, germination, and radicle growth. Funct. Plant. Biol. 2021, 48, 312–320. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Khatami, S.; Ahmadinia, A. Increased germination and growth rates of pea and zucchini seed by fsg plasma. J. Theor. Appl. Phys. 2018, 12, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Phan, L.; Yoon, S.; Moon, M.-W. Plasma-based nanostructuring of polymers: A review. Polymers 2017, 9, 417. [Google Scholar] [CrossRef]
- Dhayal, M.; Lee, S.-Y.; Park, S.-U. Using low-pressure plasma for carthamus tinctorium l. Seed surface modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Rohn, S.; Kroh, L.W.; Geyer, M.; Schlüter, O. Surface morphology and chemical composition of lamb’s lettuce (Valerianella Locusta) after exposure to a low-pressure oxygen plasma. Food Chem. 2010, 122, 1145–1152. [Google Scholar] [CrossRef]
- Baldanov, B.B.; Ranzhurov, T.V.; Sordonova, M.N.; Budazhapov, L.V. Changes in the properties and surface structure of grain seeds under the influence of a glow discharge at atmospheric pressure. Plasma Phys. Rep. 2020, 46, 110–114. [Google Scholar] [CrossRef]
- Gao, X.T.; Zhang, A.; Heroux, P.; Sand, W.; Sun, Z.Y.; Zhan, J.X.; Wang, C.H.; Hao, S.Y.; Li, Z.Y.; Li, Z.Y.; et al. Effect of dielectric barrier discharge cold plasma on pea seed growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar] [CrossRef]
- Molina, R.; Lalueza, A.; López-Santos, C.; Ghobeira, R.; Cools, P.; Morent, R.; de Geyter, N.; González-Elipe, A.R. Physicochemical surface analysis and germination at different irrigation conditions of dbd plasma-treated wheat seeds. Plasma Process. Polym. 2020, 18, e2000086. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sengupta, A.; Shaw, S.; Sarkar, S.; Pal, D.; Das, U.K. Interrelation between surface wax alkanes from red kidney bean (Phaseolus Vulgaris L.) seeds and adzuki bean weevil [callosobruchus chinensis (f.)] (coleoptera: Bruchidae). Legum. Res. 2020. [Google Scholar] [CrossRef]
- Zeisler-Diehl, V.V.; Barthlott, W.; Schreiber, L. Plant Cuticular Waxes: Composition, Function, and Interactions with Microorganisms. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Wilkes, H., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–16. [Google Scholar]
- Vesel, A.; Semenič, T. Etching rates of different polymers in oxygen plasma. Mater. Tehnol. 2012, 46, 227–231. [Google Scholar]
- Molina, R.; López-Santos, C.; Gómez-Ramírez, A.; Vilchez, A.; Espinós, J.P.; González-Elipe, A.R. Influence of irrigation conditions in the germination of plasma treated nasturtium seeds. Sci. Rep. 2018, 8, 16442. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [Green Version]
- Varnagiris, S.; Vilimaite, S.; Mikelionyte, I.; Urbonavicius, M.; Tuckute, S.; Milcius, D. The combination of simultaneous plasma treatment with mg nanoparticles deposition technique for better mung bean seeds germination. Processes 2020, 8. [Google Scholar] [CrossRef]
- Morar, R.; Munteanu, R.; Simion, E.; Munteanu, I.; Dascalescu, L. Electrostatic treatment of bean seeds. IEEE Trans. Ind. Appl. 1999, 35, 208–212. [Google Scholar] [CrossRef]
- Lonlua, R.; Sarapirom, S. The effect of low-pressure plasma treatment on sunflower seed germination and sprouts growth rate. J. Phys. Conf. Ser. 2019, 1380, 012157. [Google Scholar] [CrossRef]
- Seed and Seedling Biology. Available online: https://extension.psu.edu/seed-and-seedling-biology (accessed on 3 May 2021).
- Pérez-Pizá, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, e01495. [Google Scholar] [CrossRef] [Green Version]
- Araújo, S.d.S.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant. Sci. 2016, 7, 646. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Tóth, B.; Téren, J. Mycotoxin producing fungi and mycotoxins in foods in hungary in the period 1994–2002. Acta Aliment. 2005, 34, 267–275. [Google Scholar] [CrossRef]
- Hojnik, N.; Cvelbar, U.; Tavcar-Kalcher, G.; Walsh, J.L.; Krizaj, I. Mycotoxin decontamination of food: Cold atmospheric pressure plasma versus classic decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef]
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Kloti, D.; Buttner-Mainik, A.; Adamcik, J.; von Rohr, P.R.; Schuppler, M. Characterization of efficiency and mechanisms of cold atmospheric pressure plasma decontamination of seeds for sprout production. Front. Microbiol. 2018, 9, 3164. [Google Scholar] [CrossRef] [Green Version]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Hashizume, H.; Ohta, T.; Takeda, K.; Ishikawa, K.; Hori, M.; Ito, M. Oxidation mechanism of penicillium digitatum spores through neutral oxygen radicals. Jpn. J. Appl. Phys. 2014, 53, 010209. [Google Scholar] [CrossRef]
- Ono, R.; Uchida, S.; Hayashi, N.; Kosaka, R.; Soeda, Y. Inactivation of bacteria on plant seed surface by low-pressure rf plasma using a vibrating stirring device. Vacuum 2017, 136, 214–220. [Google Scholar] [CrossRef]
- Bol’shakov, A.A.; Cruden, B.A.; Mogul, R.; Rao, M.V.V.S.; Sharma, S.P.; Khare, B.N.; Meyyappan, M. Radio-frequency oxygen plasma as a sterilization source. AIAA J. 2004, 42, 823–832. [Google Scholar] [CrossRef]
- Mravlje, J.; Regvar, M.; Staric, P.; Mozetic, M.; Vogel-Mikus, K. Cold plasma affects germination and fungal community structure of buckwheat seeds. Plants 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]





| t | p | C | N | O | Si | Mg | Ca |
|---|---|---|---|---|---|---|---|
| Untreated | 75.3 | 4.4 | 17.7 | 2.8 | / | / | |
| 0.5 s | 5 Pa | 50.2 | 3.4 | 36.6 | 2.0 | 2.5 | 2.4 |
| 15 Pa | 66.4 | 4.6 | 24.9 | 1.6 | 0.8 | 0.8 | |
| 20 Pa | 57.6 | 4.9 | 32.0 | 1.7 | 1.3 | 1.4 | |
| 3 s | 5 Pa | 49.1 | 4.6 | 35.9 | 1.7 | 2.1 | 2.0 |
| 15 Pa | 53.1 | 2.1 | 37.4 | 1.6 | 3.0 | 2.2 | |
| 20 Pa | 61.4 | 4.6 | 29.2 | 2.5 | 0.8 | 1.1 | |
| 10 s | 5 Pa | 49.3 | 3.1 | 38.7 | 2.7 | 2.4 | 2.7 |
| 15 Pa | 45.1 | 2.6 | 40.9 | 1.8 | 2.2 | 2.8 | |
| 20 Pa | 49.4 | 4.5 | 39.0 | 2.9 | 1.6 | 2.1 | |
| p (Pa) | PO (W) | PREF (W) | PEFF (W) |
|---|---|---|---|
| 5 | 400 | 80 | 320 |
| 15 | 400 | 70 | 330 |
| 20 | 400 | 60 | 340 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recek, N.; Holc, M.; Vesel, A.; Zaplotnik, R.; Gselman, P.; Mozetič, M.; Primc, G. Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma. Int. J. Mol. Sci. 2021, 22, 6672. https://doi.org/10.3390/ijms22136672
Recek N, Holc M, Vesel A, Zaplotnik R, Gselman P, Mozetič M, Primc G. Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma. International Journal of Molecular Sciences. 2021; 22(13):6672. https://doi.org/10.3390/ijms22136672
Chicago/Turabian StyleRecek, Nina, Matej Holc, Alenka Vesel, Rok Zaplotnik, Peter Gselman, Miran Mozetič, and Gregor Primc. 2021. "Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma" International Journal of Molecular Sciences 22, no. 13: 6672. https://doi.org/10.3390/ijms22136672
APA StyleRecek, N., Holc, M., Vesel, A., Zaplotnik, R., Gselman, P., Mozetič, M., & Primc, G. (2021). Germination of Phaseolus vulgaris L. Seeds after a Short Treatment with a Powerful RF Plasma. International Journal of Molecular Sciences, 22(13), 6672. https://doi.org/10.3390/ijms22136672









