Different Expressions of HIF-1α and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice
Abstract
:1. Introduction
2. Results
2.1. Physiological and Behavioral Alterations
2.2. HIF-1α Expressions in the Liver, Kidney and Brain After Acute Hypoxic Exposure
2.3. Locations of HIF-1α Protein in the Liver, Kidney and Brain of Hypoxic Mice
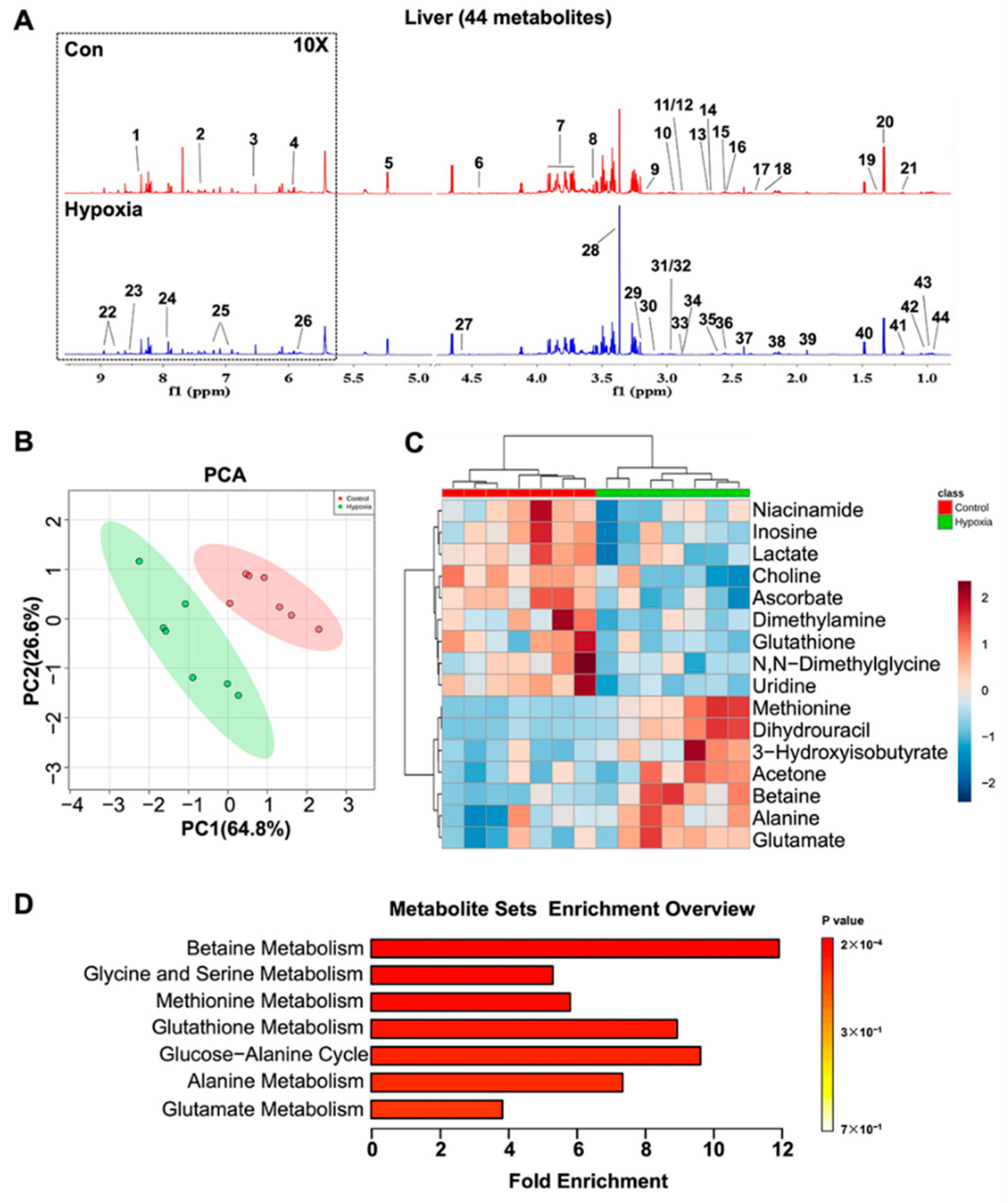
2.4. Metabolic Features in the Liver
2.5. Metabolic Features in the Kidney
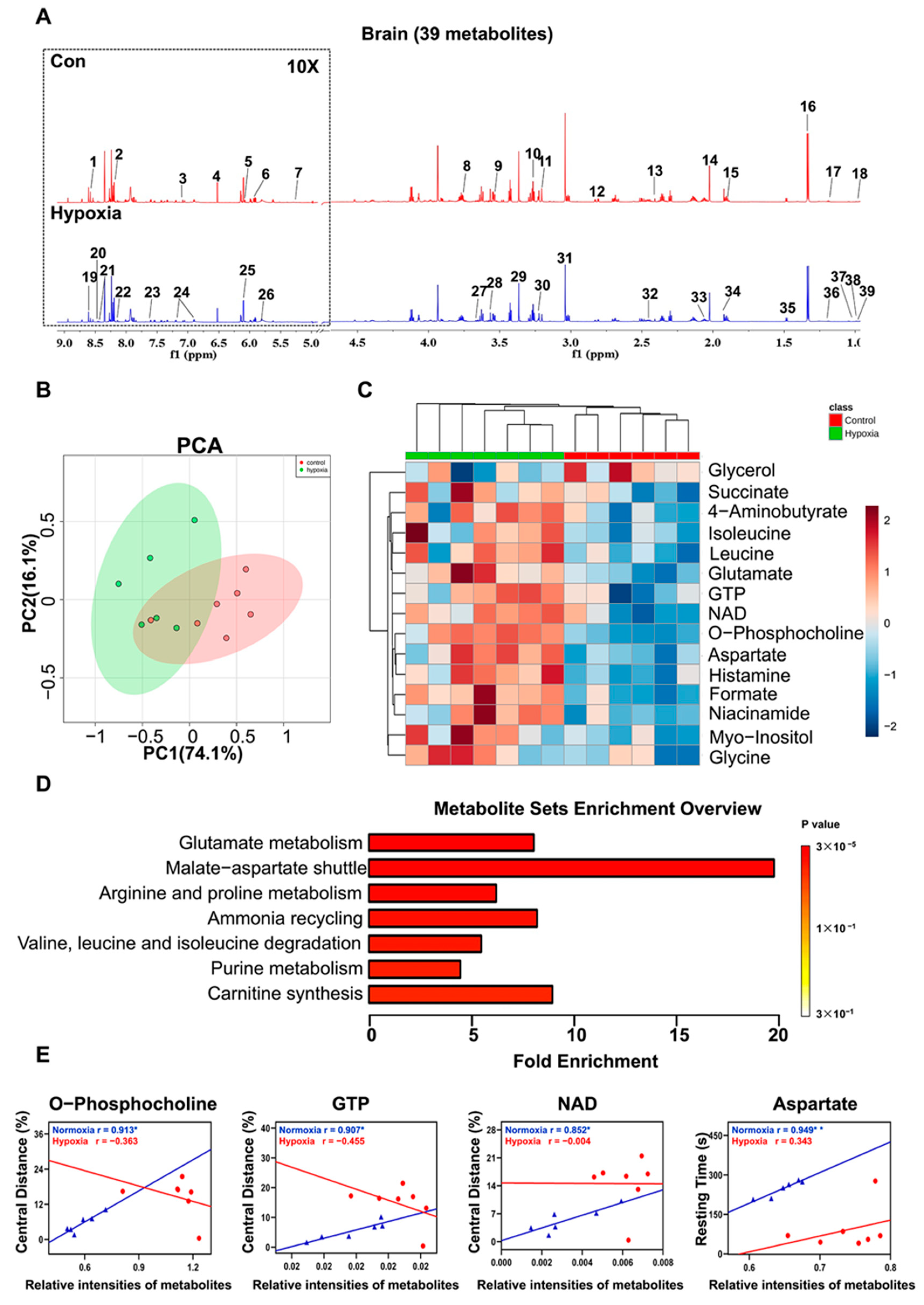
2.6. Metabolic Features in the Brain
3. Discussion
4. Materials and Methods
4.1. Animals and Patterns of Hypoxic Exposure
4.2. Physiological Measurements
4.3. Open Field Test
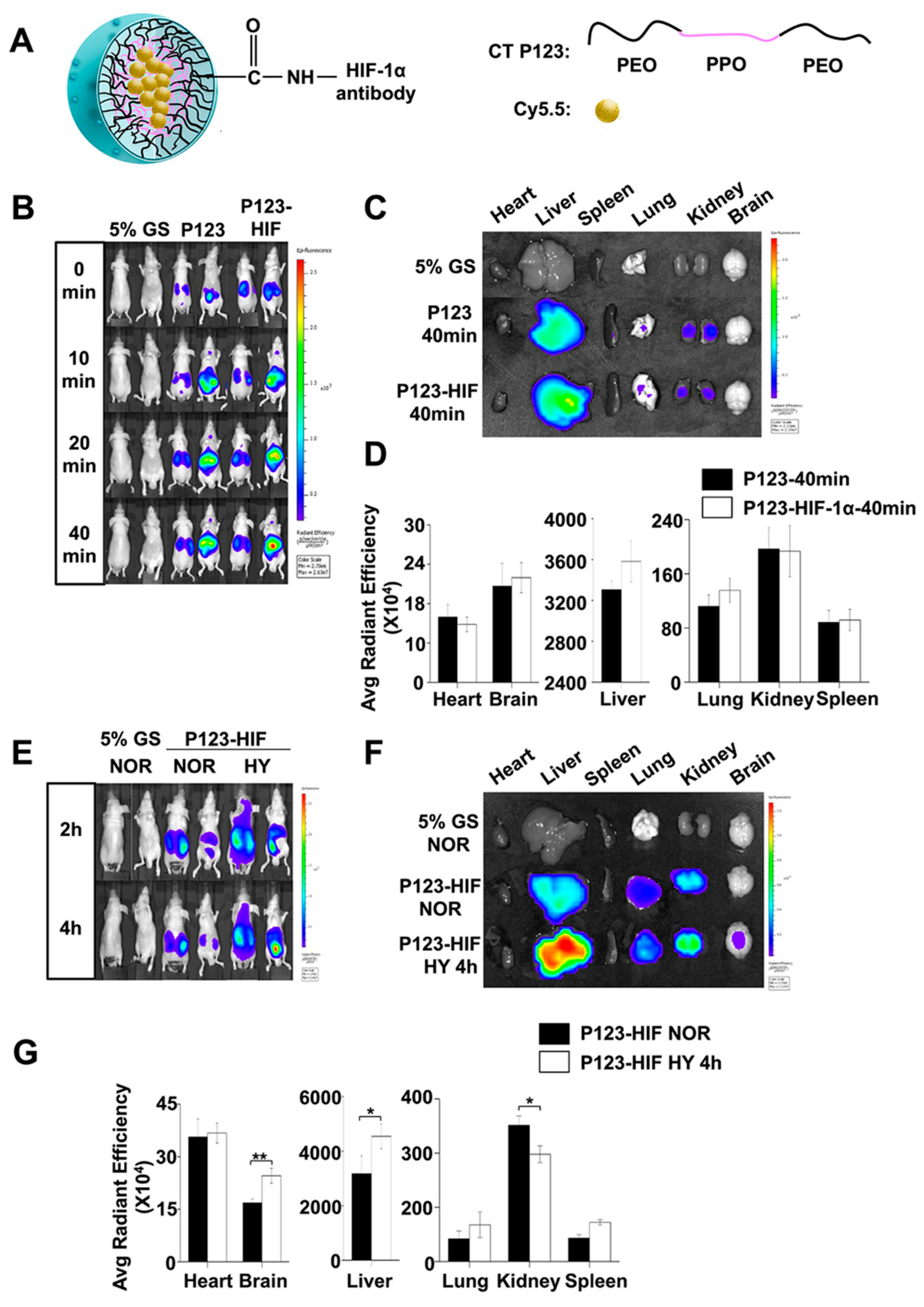
4.4. In Vivo and In Vitro Imaging of HIF-1α Accumulation
4.4.1. Preparation of Anti-HIF-1α Antibody-Conjugated Pluronic P123 Micelles
4.4.2. Biodistribution of HIF-1α Protein Using In Vivo Imaging
4.5. Western Blot
4.6. Immunofluorescence Staining
4.7. NMR Sample Preparation and Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, R.O.; Weaver, L.K.; Pope, D.; Orme, J.F.; Bigler, E.D.; Larson-Lohr, V. Neuropsychological Sequelae and Impaired Health Status in Survivors of Severe Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 1999, 160, 50–56. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen Sensing, Hypoxia-Inducible Factors, and Disease Pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Kennel, K.B.; Burmeister, J.; Schneider, M.; Taylor, C.T. The PHD1 oxygen sensor in health and disease. J. Physiol. 2018, 596, 3899–3913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, M.R.; Ghofrani, H.-A.; Weissmann, N.; Aldashev, A.; Zhao, L. Pathophysiology and Treatment of High-Altitude Pulmonary Vascular Disease. Circulation 2015, 131, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Savransky, V.; Nanayakkara, A.; Vivero, A.; Li, J.; Bevans, S.; Smith, P.L.; Torbenson, M.S.; Polotsky, V.Y. Chronic intermittent hypoxia predisposes to liver injury. Hepatology 2007, 45, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, V.; Anand, A.S.; Baidya, A.K.; Malik, S.M.; Kohli, E.; Reddy, M.P.K. Hypobaric hypoxia induced renal damage is mediated by altering redox pathway. PLoS ONE 2018, 13, e0195701. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial pressure of oxygen in the human body: A general review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Johannes, T.; Mik, E.G.; Nohé, B.; Unertl, K.E.; Ince, C. Acute decrease in renal microvascular Po2 during acute normovolemic hemodilution. Am. J. Physiol. Physiol. 2007, 292, F796–F803. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S. Cardiogenic Shock: Failure of Oxygen Delivery and Oxygen Utilization. Clin. Cardiol. 2016, 39, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Shohet, R.V.; Garcia, J.A. Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia. J. Mol. Med. 2007, 85, 1309–1315. [Google Scholar] [CrossRef] [Green Version]
- Sano, M.; Minamino, T.; Toko, H.; Miyauchi, H.; Orimo, M.; Qin, Y.; Akazawa, H.; Tateno, K.; Kayama, Y.; Harada, M.; et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007, 446, 444–448. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Dass, S.; Suttie, J.; Sever, E.; Birks, J.; Holloway, C.J.; Robson, M.D.; Jerosch-Herold, M.; Watkins, H.; Neubauer, S. Blunted myocardial oxygenation response during vasodilator stress in patients with hypertrophic cardio-myopathy. J. Am. Coll. Cardiol. 2013, 61, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, M.; Francis, J.M.; Pal, N.; Lewis, A.; Dass, S.; De Silva, R.; Petrou, M.; Sayeed, R.; Westaby, S.; Robson, M.D.; et al. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 2014, 16, 29. [Google Scholar] [CrossRef] [Green Version]
- Nakada, Y.; Canseco, D.C.; Thet, S.; Abdisalaam, S.; Asaithamby, A.; Santos, C.X.; Shah, A.M.; Zhang, H.; Faber, J.E.; Kinter, M.T.; et al. Hypoxia induces heart regeneration in adult mice. Nature 2017, 541, 222–227. [Google Scholar] [CrossRef]
- Zauner, A.; Daugherty, W.P.; Bullock, M.R.; Warner, D.S. Brain oxygenation and energy metabolism: Part I-biological function and pathophysiology. Neurosurgery 2002, 51, 289–301. [Google Scholar] [PubMed]
- Weaver, J.; Liu, K.J. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med. Gas Res. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taher, A.; Pilehvari, Z.; Poorolajal, J.; Aghajanloo, M. Effects of Normobaric Hyperoxia in Traumatic Brain Injury: A Randomized Controlled Clinical Trial. Trauma Mon. 2016, 21, 26772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terraneo, L.; Paroni, R.; Bianciardi, P.; Giallongo, T.; Carelli, S.; Gorio, A.; Samaja, M. Brain adaptation to hypoxia and hyperoxia in mice. Redox Biol. 2017, 11, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhang, H.; Li, J.; Chen, J.; Han, Q.; Lin, J.; Yang, T.; Fan, M. Adaptive Modulation of Adult Brain Gray and White Matter to High Altitude: Structural MRI Studies. PLoS ONE 2013, 8, e68621. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, J.; Sun, Y.; Huang, Y.; Ye, H.; Wang, X.; Yang, T.; Jiang, X.; Zhang, J. Compromised white matter micro-structural integrity after mountain climbing: Evidence from diffusion tensor imaging. High Alt. Med. Biol. 2012, 13, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, J.; Fan, C.; Li, J.; Lin, J.; Yang, T.; Fan, M. Alteration of Spontaneous Brain Activity After Hypoxia-Reoxygenation: A Resting-State fMRI Study. High Alt. Med. Biol. 2017, 18, 20–26. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Gough, A.; A Vernetti, L.; Taylor, D.L.; Monga, S.P. Pre-clinical and clinical investigations of metabolic zonation in liver diseases: The potential of microphysiology systems. Exp. Biol. Med. 2017, 242, 1605–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, T.; Shinjo, S.; Arai, T.; Kanai, M.; Goda, N. Hypoxia and fatty liver. World J. Gastroenterol. 2014, 20, 15087–15097. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, T.; Dimova, E.; Flügel, D.; Scharf, J.-G. Oxygen: Modulator of Physiological and Pathophysiological Processes in the Liver. Z. Gastroenterol. 2006, 44, 67–76. [Google Scholar] [CrossRef]
- Zhi, F.; Shao, N.; Xue, L.; Xu, Y.; Kang, X.; Yang, Y.; Xia, Y. Characteristic MicroRNA Expression Induced by δ-Opioid Receptor Activation in the Rat Liver Under Prolonged Hypoxia. Cell. Physiol. Biochem. 2017, 44, 2296–2309. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Wang, Y.; Zheng, M.; Liu, Z.; Cai, J.; Tang, C.; Dong, Z. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Arestegui, A.; Plata-Cornejo, R.; Cornejo, A.; Mas, G.; Carbajal, L.; Sharma, S.; Swenson, E.R.; Johnson, R.J.; Pando, J. Higher prevalence of unrecognized kidney disease at high altitude. J. Nephrol. 2017, 31, 263–269. [Google Scholar] [CrossRef]
- Goldfarb-Rumyantzev, A.S.; Alper, S.L. Short-term responses of the kidney to high altitude in mountain climbers. Nephrol. Dial. Transplant. 2014, 29, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Luks, A.M.; Johnson, R.J.; Swenson, E.R. Chronic Kidney Disease at High Altitude. J. Am. Soc. Nephrol. 2008, 19, 2262–2271. [Google Scholar] [CrossRef] [Green Version]
- Michiels, C. Physiological and Pathological Responses to Hypoxia. Am. J. Pathol. 2004, 164, 1875–1882. [Google Scholar] [CrossRef] [Green Version]
- Stroka, D.M.; Burkhardt, T.; Desbaillets, I.; Wenger, R.H.; Neil, D.A.H.; Bauer, C.; Gassmann, M.; Candinas, D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001, 15, 2445–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianciardi, P.; Fantacci, M.; Caretti, A.; Ronchi, R.; Milano, G.; Morel, S.; Von Segesser, L.; Corno, A.; Samaja, M. Chronic in vivo hypoxia in various organs: Hypoxia-inducible factor-1α and apoptosis. Biochem. Biophys. Res. Commun. 2006, 342, 875–880. [Google Scholar] [CrossRef]
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physio-logically relevant range of O2 tension. Am. J. Physiol. 1996, 271, C1172–C1180. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.K.; Das, D.K. Oxygen Sensing, Cardiac Ischemia, HIF-1α and Some Emerging Concepts. Curr. Cardiol. Rev. 2010, 6, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Ratcliffe, P.J. Oxygen sensing and hypoxia-induced responses. Essays Biochem. 2007, 43, 1–16. [Google Scholar] [CrossRef]
- Lee, K.E.; Simon, M.C. SnapShot: Hypoxia-Inducible Factors. Cell 2015, 163, 1288.e1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Fu, Z.; Linke, S.; Chicher, J.; Gorman, J.J.; Visk, D.; Haddad, G.G.; Poellinger, L.; Peet, D.J.; Powell, F.; et al. The Asparaginyl Hydroxylase Factor Inhibiting HIF-1α Is an Essential Regulator of Metabolism. Cell Metab. 2010, 11, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Mansfield, K.D.; Bertozzi, C.C.; Rudenko, V.; Chan, D.A.; Giaccia, A.J.; Simon, M.C. Multiple Factors Affecting Cellular Redox Status and Energy Metabolism Modulate Hypoxia-Inducible Factor Prolyl Hydroxylase Activity In Vivo and In Vitro. Mol. Cell. Biol. 2006, 27, 912–925. [Google Scholar] [CrossRef] [Green Version]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Girgis, C.M.; Cheng, K.; Scott, C.H.; Gunton, J.E. Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol. Metab. 2012, 23, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jiang, L.; Zhang, H.; Shimoda, L.A.; DeBerardinis, R.J.; Semenza, G.L. Analysis of Hypoxia-Induced Metabolic Reprogramming. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 542, pp. 425–455. ISBN 9780124166189. [Google Scholar]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, I.; Cairns, R.A.; Fontana, L.; Lim, A.L.; Denko, N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006, 3, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassavaugh, J.; Lounsbury, K.M. Hypoxia-mediated biological control. J. Cell. Biochem. 2011, 112, 735–744. [Google Scholar] [CrossRef]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012, 481, 380–384. [Google Scholar] [CrossRef] [Green Version]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nat. Cell Biol. 2011, 481, 385–388. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; He, R.; Wang, K.; Ruan, J.; Bao, C.; Li, N.; Ji, J.; Cui, D. Anti-HIF-1α antibody-conjugated pluronic triblock copolymers encapsulated with Paclitaxel for tumor targeting therapy. Biomaterials 2010, 31, 2302–2312. [Google Scholar] [CrossRef]
- Wenger, R.H.; Rolfs, A.; Spielmann, P.; Zimmermann, D.R.; Gassmann, M. Mouse hypoxia-inducible factor-1alpha is encoded by two different mRNA isoforms: Expression from a tissue-specific and a housekeeping-type promoter. Blood 1998, 91, 3471–3480. [Google Scholar] [CrossRef] [Green Version]
- Willam, C.; Maxwell, P.H.; Nichols, L.; Lygate, C.; Tian, Y.M.; Bernhardt, W.; Wiesener, M.; Ratcliffe, P.; Eckardt, K.-U.; Pugh, C.W. HIF prolyl hydroxylases in the rat; organ distribution and changes in expression following hypoxia and coronary artery ligation. J. Mol. Cell. Cardiol. 2006, 41, 68–77. [Google Scholar] [CrossRef]
- Jungermann, K.; Kietzmann, T. Zonation of parenchymal and nonparenchymal metabolism in liver. Annu. Rev. Nutr. 1996, 16, 179–203. [Google Scholar] [CrossRef]
- Wang, X.L.; Suzuki, R.; Lee, K.; Tran, T. Gunton, J.E.; Saha, A.K.; Patti, M.E.; Goldfine, A.; Ruderman, N.B.; Gonzalez, F.J.; et al. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009, 9, 428–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetology 2016, 59, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, T.; Goda, N.; Fujiki, N.; Hishiki, T.; Nishiyama, Y.; Senoo-Matsuda, N.; Shimazu, M.; Soga, T.; Yoshimura, Y.; Johnson, R.S.; et al. HIF-1α is necessary to support gluconeogenesis during liver regeneration. Biochem. Biophys. Res. Commun. 2009, 387, 789–794. [Google Scholar] [CrossRef]
- Bartels, H.; Vogt, B.; Jungermann, K. Glycogen synthesis from pyruvate in the periportal and from glucose in the perivenous zone in perfused livers from fasted rats. FEBS Lett. 1987, 221, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Ribas, V.; Garcia-Ruiz, C.; Fernãndez-Checa, J.C. Glutathione and mitochondria. Front. Pharmacol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhang, H.; Li, H.; Lai, F.; Li, X.; Tang, Y.; Min, T.; Wu, H. Antioxidant Mechanism of Betaine without Free Radical Scavenging Ability. J. Agric. Food Chem. 2016, 64, 7921–7930. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.-H.; Howe, J.C.; Holden, J.M. Concentrations of Choline-Containing Compounds and Betaine in Common Foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- HoleČek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, G.; Chen, D.; Chen, J.; Yuan, Z.B.; Zhang, E.L.; Gao, Y.X.; Xu, G.; Sun, B.D.; Liao, W.; et al. UPLC-QTOFMS-based metabolomic analysis of the serum of hypoxic preconditioning mice. Mol. Med. Rep. 2017, 16, 6828–6836. [Google Scholar] [CrossRef] [Green Version]
- Kempson, S.A.; Vovor-Dassu, K.; Day, C. Betaine Transport in Kidney and Liver: Use of Betaine in Liver Injury. Cell. Physiol. Biochem. 2013, 32, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olli, K. Lahtinen, S.; Rautonen, N.; Tiihonen, K. Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br. J. Nutr. 2013, 109, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chipot, C.; Dehez, F.; Schnell, J.R.; Zitzmann, N.; Pebay-Peyroula, E.; Catoire, L.J.; Miroux, B.; Kunji, E.R.S.; Veglia, G.; Cross, T.A.; et al. Perturbations of Native Membrane Protein Structure in Alkyl Phosphocholine Detergents: A Critical Assessment of NMR and Biophysical Studies. Chem. Rev. 2018, 118, 3559–3607. [Google Scholar] [CrossRef] [Green Version]
- Marklund, N.; Östman, B.; Nalmo, L.; Persson, L.; Hillered, L. Hypoxanthine, uric acid and allantoin as indicators of in vivo free radical reactions. Description of a HPLC method and human brain microdialysis data. Acta Neurochir. 2000, 142, 1135–1142. [Google Scholar] [CrossRef]
- Qaid, E.; Zakaria, R.; Sulaiman, S.F.; Yusof, N.M.; Shafin, N.; Othman, Z.; Ahmad, A.H.; Aziz, C.A. Insight into potential mechanisms of hypobaric hypoxia–induced learning and memory deficit—Lessons from rat studies. Hum. Exp. Toxicol. 2017, 36, 1315–1325. [Google Scholar] [CrossRef]
- Bouak, F.; Vartanian, O.; Hofer, K.; Cheung, B. Acute Mild Hypoxic Hypoxia Effects on Cognitive and Simulated Aircraft Pilot Performance. Aerosp. Med. Hum. Perform. 2018, 89, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Walsh, B.H.; Boylan, G.B.; Sykes, B.D.; Kenny, L.C.; Murray, D.M.; Broadhurst, D.I. 1H NMR Derived Metabolomic Profile of Neonatal Asphyxia in Umbilical Cord Serum: Implications for Hypoxic Ischemic Encephalopathy. J. Proteome Res. 2013, 12, 4230–4239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Sun, M.; Li, M.; Zhang, D.; Han, F.; Wu, J.C.; Fukunaga, K.; Chen, Z.; Qin, Z.-H. Combination of NAD+ and NADPH Offers Greater Neuroprotection in Ischemic Stroke Models by Relieving Metabolic Stress. Mol. Neurobiol. 2017, 55, 6063–6075. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, F.; Ma, X.L.; He, C.C.; Tian, K.; Wang, H.G.; An, D.; Heng, B.; Xie, L.H.; Liu, Y.Q. Oxygen-glucose deprivation enhancement of cell death/apoptosis in PC12 cells and hippocampal neurons correlates with changes in neuronal excitatory amino acid neurotransmitter signaling and potassium currents. Neuroreport 2016, 27, 617–626. [Google Scholar] [CrossRef]
- Sperringer, J.E.; Addington, A.; Hutson, S.M. Branched-Chain Amino Acids and Brain Metabolism. Neurochem. Res. 2017, 42, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Jouhten, P.; Sarajärvi, T.; Haapasalo, A.; Hiltunen, M. Hypoxia and GABA shunt activation in the pathogenesis of Alzheimer’s disease. Neurochem. Int. 2016, 92, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.; Alves, O.L.; Reinert, M.; Doppenberg, E.; Zauner, A.; Bullock, R. Association between elevated brain tissue glycerol levels and poor outcome following severe traumatic brain injury. J. Neurosurg. 2005, 103, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, S.; Castaldo, P.; Macrì, M.L.; Amoroso, S.; Magi, S. Glutamate as a potential “survival factor” in an in vitro model of neuronal hypoxia/reoxygenation injury: Leading role of the Na+/Ca2+ exchanger. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maiolino, M.; Castaldo, P.; Lariccia, V.; Piccirillo, S.; Amoroso, S.; Magi, S. Essential role of the Na+-Ca2+ exchanger (NCX) in glutamate-enhanced cell survival in cardiac cells exposed to hypoxia/reoxygenation. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014, 121, 799–817. [Google Scholar] [CrossRef] [Green Version]
- Saijo, K.; Glass, C.K. Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 2011, 11, 775–787. [Google Scholar] [CrossRef]
- Hanisch, U.-K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Kanai, Y.; Hediger, M.A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 1992, 360, 467–471. [Google Scholar] [CrossRef]
- Kobayashi, S.; Millhorn, D.E. Hypoxia regulates glutamate metabolism and membrane transport in rat PC12 cells. J. Neurochem. 2001, 76, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Cassim, S.; Raymond, V.-A.; Gottschalk, S.; Merlen, G.; Zwingmann, C.; Lapierre, P.; Darby, P.; Mazer, C.D.; Bilodeau, M. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS ONE 2018, 13, e0199177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Ham, S.; Lee, S.-E.; Lee, Y.; Lee, G.H. Hypoxia regulates the level of glutamic acid decarboxylase enzymes and interrupts inhibitory synapse stability in primary cultured neurons. Neurotoxicology 2018, 65, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Shao, W.; Huang, C.; Wu, C.-J.; Jiang, B.; Lin, D. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet. Muscle 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Song, H.; Qiu, Q.; Jiang, T.; Ge, P.; Su, Z.; Ma, W.; Zhang, R.; Huang, C.; Li, S.; et al. Different Expressions of HIF-1α and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice. Int. J. Mol. Sci. 2021, 22, 6705. https://doi.org/10.3390/ijms22136705
Xu L, Song H, Qiu Q, Jiang T, Ge P, Su Z, Ma W, Zhang R, Huang C, Li S, et al. Different Expressions of HIF-1α and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice. International Journal of Molecular Sciences. 2021; 22(13):6705. https://doi.org/10.3390/ijms22136705
Chicago/Turabian StyleXu, Lu, Hua Song, Qi Qiu, Ting Jiang, Pingyun Ge, Zaiji Su, Wenhui Ma, Ran Zhang, Caihua Huang, Shanhua Li, and et al. 2021. "Different Expressions of HIF-1α and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice" International Journal of Molecular Sciences 22, no. 13: 6705. https://doi.org/10.3390/ijms22136705
APA StyleXu, L., Song, H., Qiu, Q., Jiang, T., Ge, P., Su, Z., Ma, W., Zhang, R., Huang, C., Li, S., Lin, D., & Zhang, J. (2021). Different Expressions of HIF-1α and Metabolism in Brain and Major Visceral Organs of Acute Hypoxic Mice. International Journal of Molecular Sciences, 22(13), 6705. https://doi.org/10.3390/ijms22136705






