Membrane Interactions Accelerate the Self-Aggregation of Huntingtin Exon 1 Fragments in a Polyglutamine Length-Dependent Manner
Abstract
:1. Introduction
2. Results
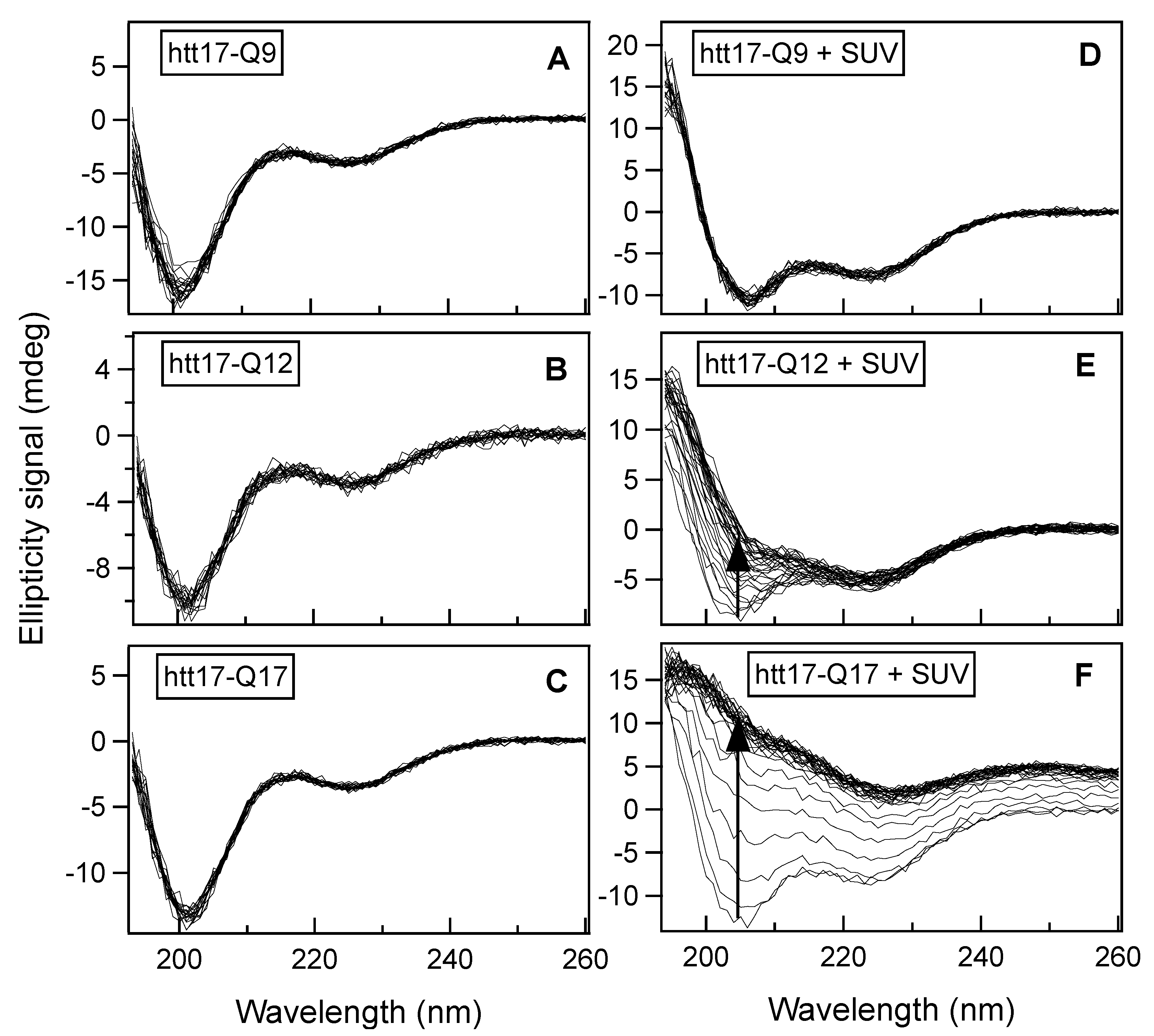
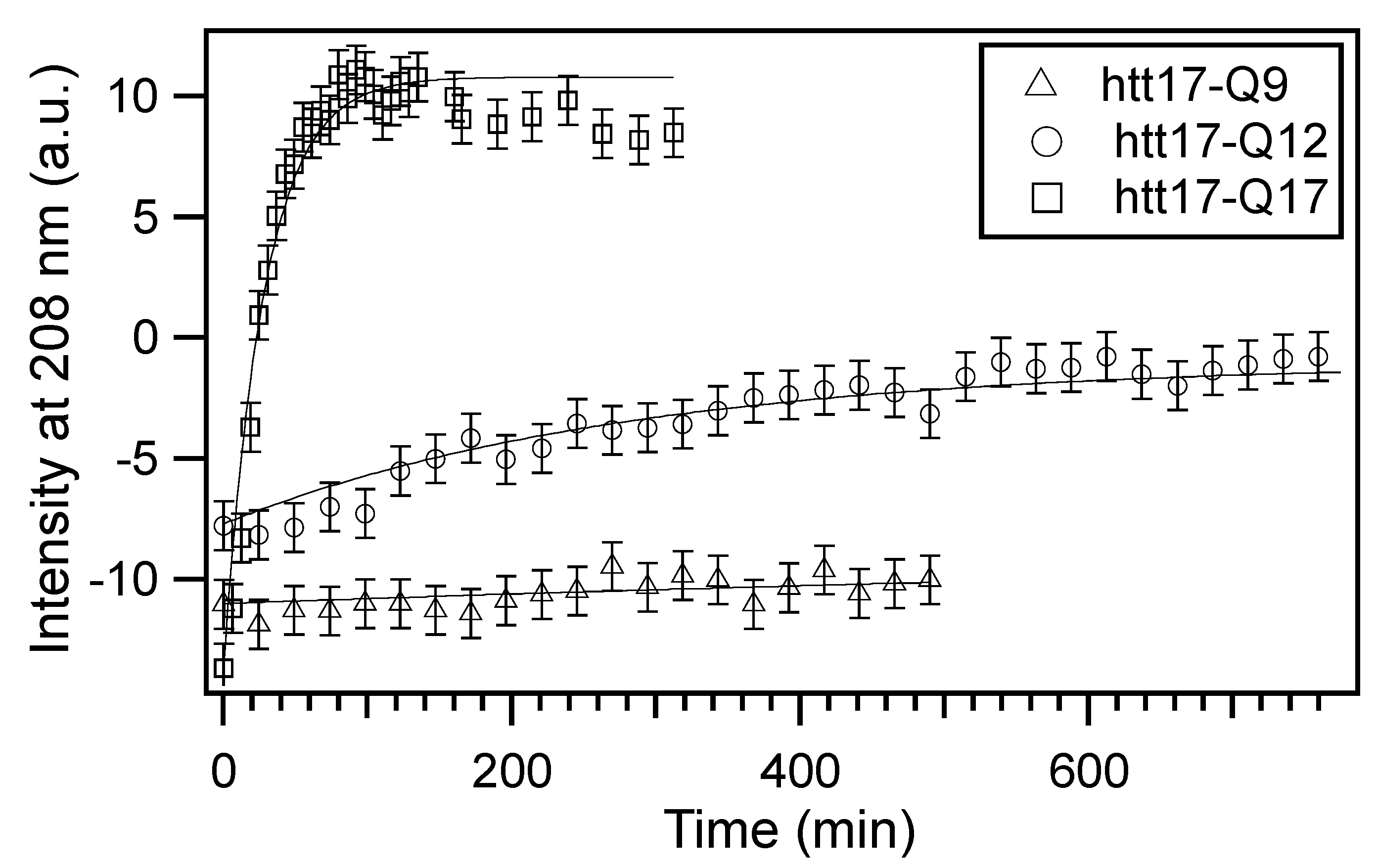
2.1. Circular Dichroism (CD) Spectroscopy
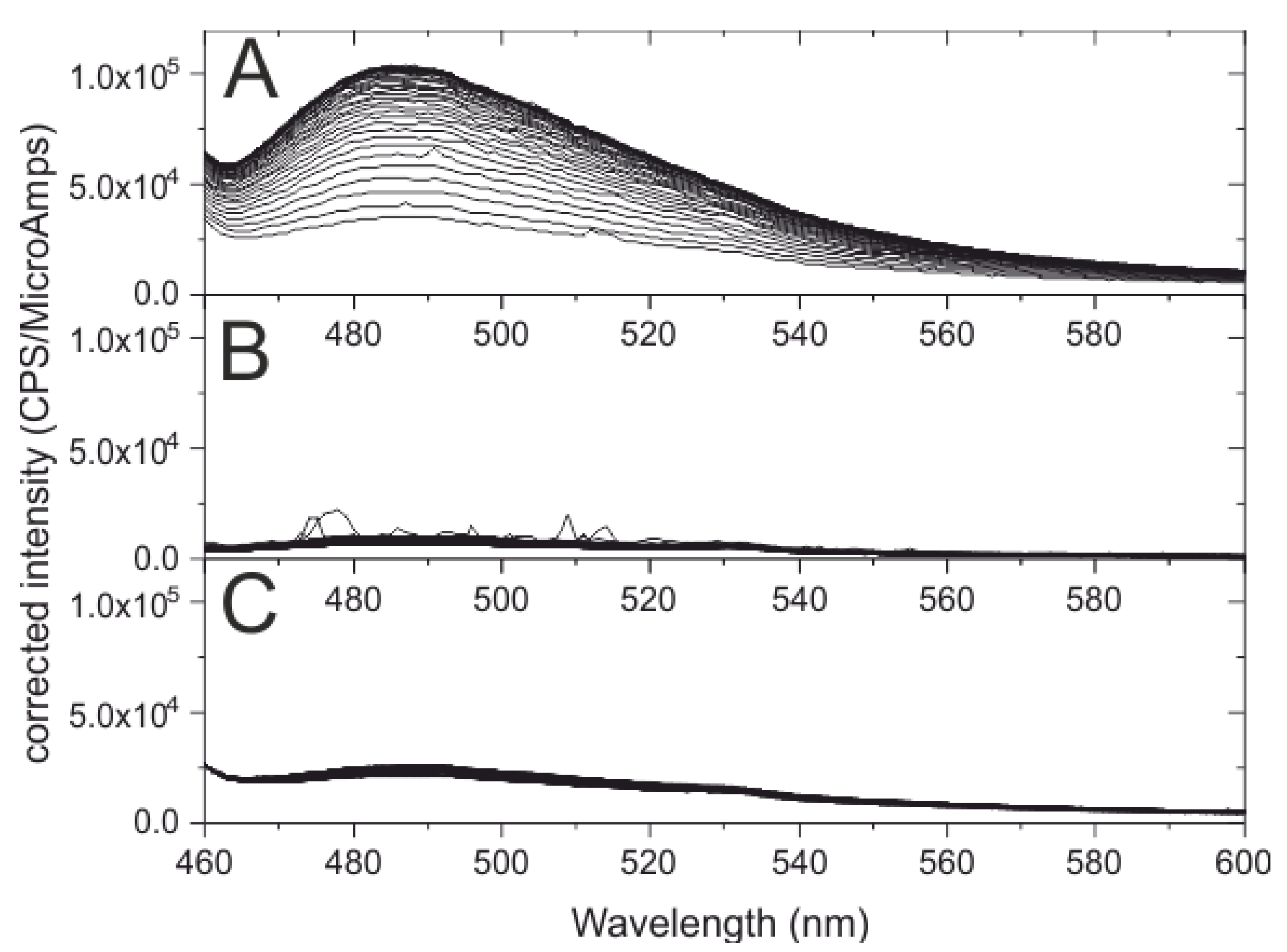
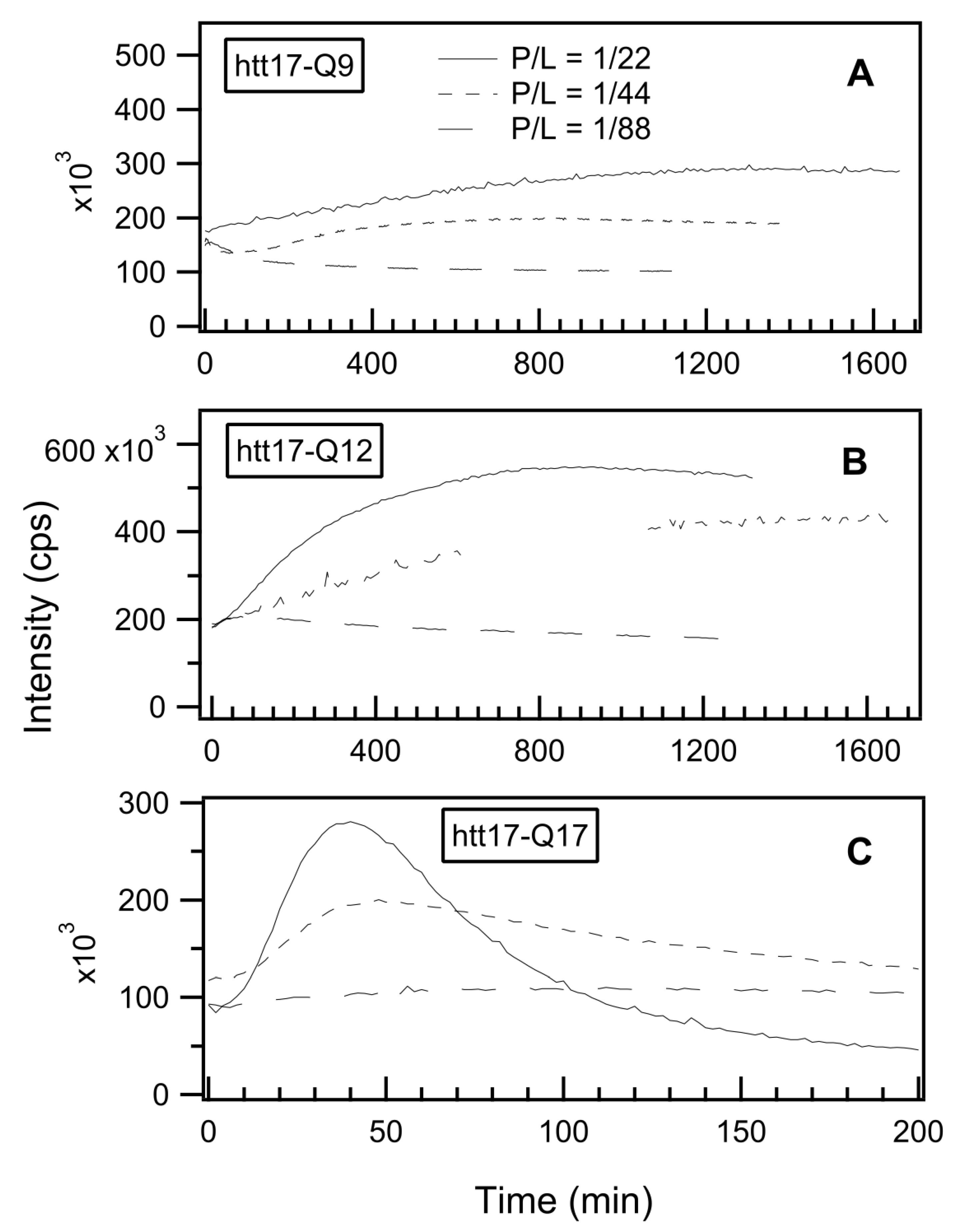
2.2. Thioflavin T Fluorescence
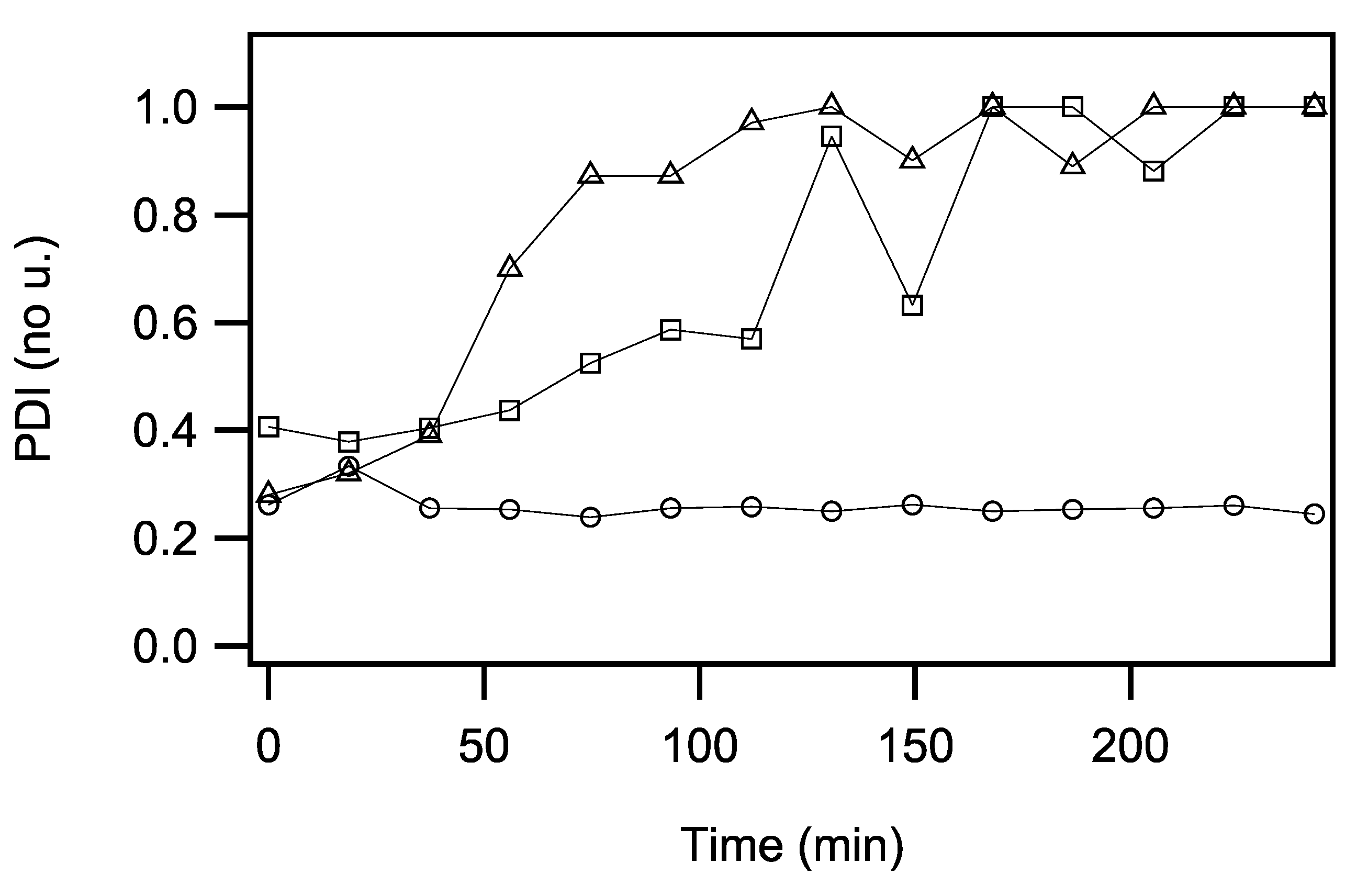
2.3. Dynamic Light Scattering
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Peptide Synthesis and Purification
4.3. Small Unilamellar Vesicles
4.4. Circular Dichroism Spectroscopy
4.5. Measurements of Thioflavin-T Fluorescence
4.6. Dynamic Light Scattering
4.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuiper, E.F.; de Mattos, E.P.; Jardim, L.B.; Kampinga, H.H.; Bergink, S. Chaperones in Polyglutamine Aggregation: Beyond the Q-Stretch. Front. Neurosci. 2017, 11, 145. [Google Scholar] [CrossRef] [Green Version]
- Aktar, F.; Burudpakdee, C.; Polanco, M.; Pei, S.; Swayne, T.C.; Lipke, P.N.; Emtage, L. The huntingtin inclusion is a dynamic phase-separated compartment. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [Green Version]
- Peskett, T.R.; Rau, F.; O’Driscoll, J.; Patani, R.; Lowe, A.R.; Saibil, H.R. A Liquid to Solid Phase Transition Underlying Pathological Huntingtin Exon1 Aggregation. Mol. Cell 2018, 70, 588–601.e586. [Google Scholar] [CrossRef]
- Pandey, M.; Rajamma, U. Huntington’s disease: The coming of age. J. Genet. 2018, 97, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Rubinsztein, D.C.; Leggo, J.; Coles, R.; Almqvist, E.; Biancalana, V.; Cassiman, J.J.; Chotai, K.; Connarty, M.; Crauford, D.; Curtis, A.; et al. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am. J. Hum. Genet. 1996, 59, 16–22. [Google Scholar] [PubMed]
- Andresen, J.M.; Gayan, J.; Djousse, L.; Roberts, S.; Brocklebank, D.; Cherny, S.S.; Cardon, L.R.; Gusella, J.F.; MacDonald, M.E.; Myers, R.H.; et al. The relationship between CAG repeat length and age of onset differs for Huntington’s disease patients with juvenile onset or adult onset. Ann. Hum. Genet. 2007, 71, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wanker, E.E.; Ast, A.; Schindler, F.; Trepte, P.; Schnoegl, S. The pathobiology of perturbed mutant huntingtin protein-protein interactions in Huntington’s disease. J. Neurochem. 2019, 151, 507–519. [Google Scholar] [CrossRef]
- Rockabrand, E.; Slepko, N.; Pantalone, A.; Nukala, V.N.; Kazantsev, A.; Marsh, J.L.; Sullivan, P.G.; Steffan, J.S.; Sensi, S.L.; Thompson, L.M. The first 17 amino acids of Huntingtin modulate its sub-cellular localization, aggregation and effects on calcium homeostasis. Hum. Mol. Genet. 2007, 16, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Monoi, H.; Futaki, S.; Kugimiya, S.; Minakata, H.; Yoshihara, K. Poly-L-glutamine forms cation channels: Relevance to the pathogenesis of the polyglutamine diseases. Biophys. J. 2000, 78, 2892–2899. [Google Scholar] [CrossRef] [Green Version]
- Terakawa, M.S.; Lin, Y.; Kinoshita, M.; Kanemura, S.; Itoh, D.; Sugiki, T.; Okumura, M.; Ramamoorthy, A.; Lee, Y.H. Impact of membrane curvature on amyloid aggregation. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1741–1764. [Google Scholar] [CrossRef] [PubMed]
- Relini, A.; Marano, N.; Gliozzi, A. Probing the interplay between amyloidogenic proteins and membranes using lipid monolayers and bilayers. Adv. Colloid. Interface Sci. 2014, 207, 81–92. [Google Scholar] [CrossRef]
- Kegel-Gleason, K.B. Huntingtin interactions with membrane phospholipids: Strategic targets for therapeutic intervention? J. Huntingt. Dis. 2013, 2, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Del Toro, D.; Alberch, J.; Lazaro-Dieguez, F.; Martin-Ibanez, R.; Xifro, X.; Egea, G.; Canals, J.M. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol. Biol. Cell 2009, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caviston, J.P.; Holzbaur, E.L. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009, 19, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwal, R.S.; Xia, J.; Pinchev, D.; Taylor, J.; Epand, R.M.; Truant, R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum. Mol. Genet. 2007, 16, 2600–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atwal, R.S.; Truant, R. A stress sensitive ER membrane-association domain in Huntingtin protein defines a potential role for Huntingtin in the regulation of autophagy. Autophagy 2008, 4, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Leitman, J.; Ulrich Hartl, F.; Lederkremer, G.Z. Soluble forms of polyQ-expanded huntingtin rather than large aggregates cause endoplasmic reticulum stress. Nat. Commun. 2013, 4, 2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flavin, W.P.; Bousset, L.; Green, Z.C.; Chu, Y.; Skarpathiotis, S.; Chaney, M.J.; Kordower, J.H.; Melki, R.; Campbell, E.M. Endocytic vesicle rupture is a conserved mechanism of cellular invasion by amyloid proteins. Acta Neuropathol. 2017, 134, 629–653. [Google Scholar] [CrossRef]
- Tanaka, Y.; Igarashi, S.; Nakamura, M.; Gafni, J.; Torcassi, C.; Schilling, G.; Crippen, D.; Wood, J.D.; Sawa, A.; Jenkins, N.A.; et al. Progressive phenotype and nuclear accumulation of an amino-terminal cleavage fragment in a transgenic mouse model with inducible expression of full-length mutant huntingtin. Neurobiol. Dis. 2006, 21, 381–391. [Google Scholar] [CrossRef]
- Hackam, A.S.; Hodgson, J.G.; Singaraja, R.; Zhang, T.Q.; Gan, L.; Gutekunst, C.A.; Hersch, S.M.; Hayden, M.R. Evidence for both the nucleus and cytoplasm as subcellular sites of pathogenesis in Huntington’s disease in cell culture and in transgenic mice expressing mutant huntingtin. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 1999, 354, 1047–1055. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Lee, D.H.; Taylor, J.; Vandelft, M.; Truant, R. Huntingtin contains a highly conserved nuclear export signal. Hum. Mol. Genet. 2003, 12, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Panov, A.V.; Gutekunst, C.A.; Leavitt, B.R.; Hayden, M.R.; Burke, J.R.; Strittmatter, W.J.; Greenamyre, J.T. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002, 5, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.S.; Johnson, G.V.; MacDonald, M.; Detloff, P.J.; Lesort, M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004, 13, 1407–1420. [Google Scholar] [CrossRef]
- Suopanki, J.; Gotz, C.; Lutsch, G.; Schiller, J.; Harjes, P.; Herrmann, A.; Wanker, E.E. Interaction of huntingtin fragments with brain membranes--clues to early dysfunction in Huntington’s disease. J. Neurochem. 2006, 96, 870–884. [Google Scholar] [CrossRef]
- Burke, K.A.; Yates, E.A.; Legleiter, J. Biophysical insights into how surfaces, including lipid membranes, modulate protein aggregation related to neurodegeneration. Front. Neurol. 2013, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Pandey, N.K.; Isas, J.M.; Rawat, A.; Lee, R.V.; Langen, J.; Pandey, P.; Langen, R. The 17-residue-long N terminus in huntingtin controls stepwise aggregation in solution and on membranes via different mechanisms. J. Biol. Chem. 2018, 293, 2597–2605. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, A.; Jawahery, S.; Matysiak, S. The effects of flanking sequences in the interaction of polyglutamine peptides with a membrane bilayer. J. Phys. Chem. B 2014, 118, 6368–6379. [Google Scholar] [CrossRef]
- Atwal, R.S.; Desmond, C.R.; Caron, N.; Maiuri, T.; Xia, J.R.; Sipione, S.; Truant, R. Kinase inhibitors modulate huntingtin cell localization and toxicity. Nat. Chem. Biol. 2011, 7, 453–460. [Google Scholar] [CrossRef]
- Arndt, J.R.; Chaibva, M.; Legleiter, J. The emerging role of the first 17 amino acids of huntingtin in Huntington’s disease. Biomol. Concepts 2015, 6, 33–46. [Google Scholar] [CrossRef]
- Chaibva, M.; Jawahery, S.; Pilkington, A.W.t.; Arndt, J.R.; Sarver, O.; Valentine, S.; Matysiak, S.; Legleiter, J. Acetylation within the First 17 Residues of Huntingtin Exon 1 Alters Aggregation and Lipid Binding. Biophys. J. 2016, 111, 349–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crick, S.L.; Ruff, K.M.; Garai, K.; Frieden, C.; Pappu, R.V. Unmasking the roles of N- and C-terminal flanking sequences from exon 1 of huntingtin as modulators of polyglutamine aggregation. Proc. Natl. Acad. Sci. USA 2013, 110, 20075–20080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, K.; Calamini, B.; Fauerbach, J.A.; Ma, B.; Shahmoradian, S.H.; Serrano Lachapel, I.L.; Chiu, W.; Lo, D.C.; Frydman, J. Control of the structural landscape and neuronal proteotoxicity of mutant Huntingtin by domains flanking the polyQ tract. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.F.; Greiner, E.R.; Mishra, R.; Kodali, R.; Osmand, A.; Finkbeiner, S.; Steffan, J.S.; Thompson, L.M.; Wetzel, R.; Yang, X.W. Serines 13 and 16 Are Critical Determinants of Full-Length Human Mutant Huntingtin Induced Disease Pathogenesis in HD Mice. Neuron 2009, 64, 828–840. [Google Scholar] [CrossRef] [Green Version]
- Thompson, L.M.; Aiken, C.T.; Kaltenbach, L.S.; Agrawal, N.; Illes, K.; Khoshnan, A.; Martinez-Vincente, M.; Arrasate, M.; O’Rourke, J.G.; Khashwji, H.; et al. IKK phosphorylates Huntingtin and targets it for degradation by the proteasome and lysosome. J. Cell Biol. 2009, 187, 1083–1099. [Google Scholar] [CrossRef]
- Aiken, C.T.; Steffan, J.S.; Guerrero, C.M.; Khashwji, H.; Lukacsovich, T.; Simmons, D.; Purcell, J.M.; Menhaji, K.; Zhu, Y.Z.; Green, K.; et al. Phosphorylation of threonine 3: Implications for Huntingtin aggregation and neurotoxicity. J. Biol. Chem. 2009, 284, 29427–29436. [Google Scholar] [CrossRef] [Green Version]
- Steffan, J.S.; Agrawal, N.; Pallos, J.; Rockabrand, E.; Trotman, L.C.; Slepko, N.; Illes, K.; Lukacsovich, T.; Zhu, Y.Z.; Cattaneo, E.; et al. SUMO modification of Huntingtin and Huntington’s disease pathology. Science 2004, 304, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.Q.; Li, A.M.; Holmes, B.B.; Marasa, J.C.; Diamond, M.I. An N-terminal Nuclear Export Signal Regulates Trafficking and Aggregation of Huntingtin (Htt) Protein Exon 1. J. Biol. Chem. 2013, 288, 6063–6071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedighi, F.; Adegbuyiro, A.; Legleiter, J. SUMOylation Prevents Huntingtin Fibrillization and Localization onto Lipid Membranes. ACS Chem. Neurosci. 2020, 11, 328–343. [Google Scholar] [CrossRef]
- Kelley, N.W.; Huang, X.; Tam, S.; Spiess, C.; Frydman, J.; Pande, V.S. The predicted structure of the headpiece of the Huntingtin protein and its implications on Huntingtin aggregation. J. Mol. Biol. 2009, 388, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.K.; Jayaraman, M.; Mishra, R.; Thakur, M.; Chellgren, V.M.; Byeon, I.J.; Anjum, D.H.; Kodali, R.; Creamer, T.P.; Conway, J.F.; et al. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat. Struct. Mol. Biol. 2009, 16, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Tam, S.; Spiess, C.; Auyeung, W.; Joachimiak, L.; Chen, B.; Poirier, M.A.; Frydman, J. The chaperonin TRiC blocks a huntingtin sequence element that promotes the conformational switch to aggregation. Nat. Struct. Mol. Biol. 2009, 16, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Boatz, J.C.; Piretra, T.; Lasorsa, A.; Matlahov, I.; Conway, J.F.; van der Wel, P.C.A. Protofilament Structure and Supramolecular Polymorphism of Aggregated Mutant Huntingtin Exon 1. J. Mol. Biol. 2020, 432, 4722–4744. [Google Scholar] [CrossRef] [PubMed]
- Michalek, M.; Salnikov, E.S.; Werten, S.; Bechinger, B. Membrane interactions of the amphipathic amino-terminus of huntingtin. Biochemistry 2013, 52, 847–858. [Google Scholar] [CrossRef]
- Tao, M.; Pandey, N.K.; Barnes, R.; Han, S.; Langen, R. Structure of Membrane-Bound Huntingtin Exon 1 Reveals Membrane Interaction and Aggregation Mechanisms. Structure 2019, 27, 1570–1580.e1574. [Google Scholar] [CrossRef]
- Levy, G.R.; Shen, K.; Gavrilov, Y.; Smith, P.E.S.; Levy, Y.; Chan, R.; Frydman, J.; Frydman, L. Huntingtin’s N-Terminus Rearrangements in the Presence of Membranes: A Joint Spectroscopic and Computational Perspective. ACS Chem. Neurosci. 2019, 10, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, A.; Clore, G.M.; Tugarinov, V. Decorrelating Kinetic and Relaxation Parameters in Exchange Saturation Transfer NMR: A Case Study of N-Terminal Huntingtin Peptides Binding to Unilamellar Lipid Vesicles. J. Phys. Chem. B 2018, 122, 11271–11278. [Google Scholar] [CrossRef] [PubMed]
- Karanji, A.K.; Beasley, M.; Sharif, D.; Ranjbaran, A.; Legleiter, J.; Valentine, S.J. Investigating the interactions of the first 17 amino acid residues of Huntingtin with lipid vesicles using mass spectrometry and molecular dynamics. J. Mass. Spectrom. 2020, 55, e4470. [Google Scholar] [CrossRef]
- Arndt, J.R.; Chaibva, M.; Beasley, M.; Kiani Karanji, A.; Ghassabi Kondalaji, S.; Khakinejad, M.; Sarver, O.; Legleiter, J.; Valentine, S.J. Nucleation Inhibition of Huntingtin Protein (htt) by Polyproline PPII Helices: A Potential Interaction with the N-Terminal alpha-Helical Region of Htt. Biochemistry 2020, 59, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Hoop, C.L.; Lin, H.K.; Kar, K.; Hou, Z.; Poirier, M.A.; Wetzel, R.; van der Wel, P.C. Polyglutamine amyloid core boundaries and flanking domain dynamics in huntingtin fragment fibrils determined by solid-state nuclear magnetic resonance. Biochemistry 2014, 53, 6653–6666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceccon, A.; Tugarinov, V.; Clore, G.M. Kinetics of Fast Tetramerization of the Huntingtin Exon 1 Protein Probed by Concentration-Dependent On-Resonance R1rho Measurements. J. Phys. Chem. Lett. 2020, 11, 5643–5648. [Google Scholar] [CrossRef]
- Kotler, S.A.; Tugarinov, V.; Schmidt, T.; Ceccon, A.; Libich, D.S.; Ghirlando, R.; Schwieters, C.D.; Clore, G.M. Probing initial transient oligomerization events facilitating Huntingtin fibril nucleation at atomic resolution by relaxation-based NMR. Proc. Natl. Acad. Sci. USA 2019, 116, 3562–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiumara, F.; Fioriti, L.; Kandel, E.R.; Hendrickson, W.A. Essential role of coiled coils for aggregation and activity of Q/N-rich prions and PolyQ proteins. Cell 2010, 143, 1121–1135. [Google Scholar] [CrossRef] [Green Version]
- Miettinen, M.S.; Monticelli, L.; Nedumpully-Govindan, P.; Knecht, V.; Ignatova, Z. Stable Polyglutamine Dimers Can Contain β-Hairpins with Interdigitated Side Chains—But Not α-Helices, β-Nanotubes, β-Pseudohelices, or Steric Zippers. Biophys. J. 2014, 106, 1721–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miettinen, M.S.; Knecht, V.; Monticelli, L.; Ignatova, Z. Assessing Polyglutamine Conformation in the Nucleating Event by Molecular Dynamics Simulations. J. Phys. Chem. B 2012, 116, 10259–10265. [Google Scholar] [CrossRef]
- Michalek, M.; Salnikov, E.; Werten, S.; Bechinger, B. Structure and topology of the huntingtin 1-17 membrane anchor by a combined solution and solid-state NMR approach. Biophys. J. 2013, 105, 699–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalek, M.; Aisenbrey, C.; Bechinger, B. Investigation of membrane penetration depth and interactions of the amino-terminal domain of huntingtin: Refined analysis by tryptophan fluorescence measurement. Eur. Biophys. J. 2014, 43, 347–360. [Google Scholar] [CrossRef]
- Bravo-Arredondo, J.M.; Kegulian, N.C.; Schmidt, T.; Pandey, N.K.; Situ, A.J.; Ulmer, T.S.; Langen, R. The folding equilibrium of huntingtin exon 1 monomer depends on its polyglutamine tract. J. Biol. Chem. 2018, 293, 19613–19623. [Google Scholar] [CrossRef] [Green Version]
- Kokona, B.; Rosenthal, Z.P.; Fairman, R. Role of the coiled-coil structural motif in polyglutamine aggregation. Biochemistry 2014, 53, 6738–6746. [Google Scholar] [CrossRef]
- Warner, J.B.t.; Ruff, K.M.; Tan, P.S.; Lemke, E.A.; Pappu, R.V.; Lashuel, H.A. Monomeric Huntingtin Exon 1 Has Similar Overall Structural Features for Wild-Type and Pathological Polyglutamine Lengths. J. Am. Chem. Soc. 2017, 139, 14456–14469. [Google Scholar] [CrossRef] [Green Version]
- Newcombe, E.A.; Ruff, K.M.; Sethi, A.; Ormsby, A.R.; Ramdzan, Y.M.; Fox, A.; Purcell, A.W.; Gooley, P.R.; Pappu, R.V.; Hatters, D.M. Tadpole-like Conformations of Huntingtin Exon 1 Are Characterized by Conformational Heterogeneity that Persists regardless of Polyglutamine Length. J. Mol. Biol. 2018, 430, 1442–1458. [Google Scholar] [CrossRef]
- Kokona, B.; Johnson, K.A.; Fairman, R. Effect of helical flanking sequences on the morphology of polyglutamine-containing fibrils. Biochemistry 2014, 53, 6747–6753. [Google Scholar] [CrossRef]
- Sahoo, B.; Arduini, I.; Drombosky, K.W.; Kodali, R.; Sanders, L.H.; Greenamyre, J.T.; Wetzel, R. Folding Landscape of Mutant Huntingtin Exon1: Diffusible Multimers, Oligomers and Fibrils, and No Detectable Monomer. PLoS ONE 2016, 11, e0155747. [Google Scholar] [CrossRef] [Green Version]
- Bulone, D.; Masino, L.; Thomas, D.J.; Biagio, P.L.S.; Pastore, A. The Interplay between PolyQ and Protein Context Delays Aggregation by Forming a Reservoir of Protofibrils. PLoS ONE 2006, 1, e111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatova, Z.; Thakur, A.K.; Wetzel, R.; Gierasch, L.M. In-cell aggregation of a polyglutamine-containing chimera is a multistep process initiated by the flanking sequence. J. Biol. Chem. 2007, 282, 36736–36743. [Google Scholar] [CrossRef] [Green Version]
- Beasley, M.; Stonebraker, A.R.; Hasan, I.; Kapp, K.L.; Liang, B.J.; Agarwal, G.; Groover, S.; Sedighi, F.; Legleiter, J. Lipid Membranes Influence the Ability of Small Molecules To Inhibit Huntingtin Fibrillization. Biochemistry 2019, 58, 4361–4373. [Google Scholar] [CrossRef] [PubMed]
- Chaibva, M.; Gao, X.; Jain, P.; Campbell, W.A.t.; Frey, S.L.; Legleiter, J. Sphingomyelin and GM1 Influence Huntingtin Binding to, Disruption of, and Aggregation on Lipid Membranes. ACS Omega 2018, 3, 273–285. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, E.I.; Lee, J.C. Interplay between alpha-synuclein amyloid formation and membrane structure. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Okubo, K.; Ikeda, K.; Yano, Y.; Hoshino, M.; Hayashi, Y.; Kiso, Y.; Itoh-Watanabe, H.; Naito, A.; Matsuzaki, K. Toxic Amyloid Tape: A Novel Mixed Antiparallel/Parallel beta-Sheet Structure Formed by Amyloid beta-Protein on GM1 Clusters. ACS Chem. Neurosci. 2019, 10, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Qiang, W.; Yau, W.M.; Lu, J.X.; Collinge, J.; Tycko, R. Structural variation in amyloid-beta fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Bungart, B.L.; Yang, X.; Zhumadilov, Z.; Lee, J.C.; Askarova, S. Role of membrane biophysics in Alzheimer’s-related cell pathways. Front. Neurosci. 2015, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Kotler, S.A.; Walsh, P.; Brender, J.R.; Ramamoorthy, A. Differences between amyloid-beta aggregation in solution and on the membrane: Insights into elucidation of the mechanistic details of Alzheimer’s disease. Chem. Soc. Rev. 2014, 43, 6692–6700. [Google Scholar] [CrossRef] [Green Version]
- Fusco, G.; De Simone, A.; Arosio, P.; Vendruscolo, M.; Veglia, G.; Dobson, C.M. Structural Ensembles of Membrane-bound alpha-Synuclein Reveal the Molecular Determinants of Synaptic Vesicle Affinity. Sci. Rep. 2016, 6, 27125. [Google Scholar] [CrossRef] [Green Version]
- Pieri, L.; Madiona, K.; Bousset, L.; Melki, R. Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys. J. 2012, 102, 2894–2905. [Google Scholar] [CrossRef] [Green Version]
- Monsellier, E.; Bousset, L.; Melki, R. alpha-Synuclein and huntingtin exon 1 amyloid fibrils bind laterally to the cellular membrane. Sci. Rep. 2016, 6, 19180. [Google Scholar] [CrossRef] [Green Version]
- Yushchenko, T.; Deuerling, E.; Hauser, K. Insights into the Aggregation Mechanism of PolyQ Proteins with Different Glutamine Repeat Lengths. Biophys. J. 2018, 114, 1847–1857. [Google Scholar] [CrossRef] [Green Version]
- Deleage, G.; Geourjon, C. An interactive graphic program for calculating the secondary structure content of proteins from circular dichroism spectrum. Comput. Appl. Biosci. 1993, 2, 197–199. [Google Scholar] [CrossRef]
- Greenfield, N.J. Methods to estimate the conformation of proteins and polypeptides from circular dichroism data. Anal. Biochem. 1996, 235, 1–10. [Google Scholar] [CrossRef]
- Vermeer, L.S.; Marquette, A.; Schoup, M.; Fenard, D.; Galy, A.; Bechinger, B. Simultaneous Analysis of Secondary Structure and Light Scattering from Circular Dichroism Titrations: Application to Vectofusin-1. Sci. Rep. 2016, 6, 39450. [Google Scholar] [CrossRef] [Green Version]
- Streets, A.M.; Sourigues, Y.; Kopito, R.R.; Melki, R.; Quake, S.R. Simultaneous measurement of amyloid fibril formation by dynamic light scattering and fluorescence reveals complex aggregation kinetics. PLoS ONE 2013, 8, e54541. [Google Scholar] [CrossRef] [Green Version]
- LeVine, H.; 3rd. Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzym. 1999, 309, 274–284. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 2010, 1804, 1405–1412. [Google Scholar] [CrossRef] [Green Version]
- Marquette, A.; Lorber, B.; Bechinger, B. Reversible liposome association induced by LAH4: A peptide with potent antimicrobial and nucleic acid transfection activities. Biophys. J. 2010, 98, 2544–2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaraman, M.; Kodali, R.; Sahoo, B.; Thakur, A.K.; Mayasundari, A.; Mishra, R.; Peterson, C.B.; Wetzel, R. Slow amyloid nucleation via alpha-helix-rich oligomeric intermediates in short polyglutamine-containing huntingtin fragments. J. Mol. Biol. 2012, 415, 881–899. [Google Scholar] [CrossRef] [Green Version]
- Ceccon, A.; Tugarinov, V.; Ghirlando, R.; Clore, G.M. Abrogation of prenucleation, transient oligomerization of the Huntingtin exon 1 protein by human profilin I. Proc. Natl. Acad. Sci. USA 2020, 117, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.; Roy, I. Stabilization of elongated polyglutamine tracts by a helical peptide derived from N-terminal huntingtin. IUBMB Life 2020. [Google Scholar] [CrossRef]
- Smith, D.M. Could a Common Mechanism of Protein Degradation Impairment Underlie Many Neurodegenerative Diseases? J. Exp. Neurosci. 2018, 12, 1179069518794675. [Google Scholar] [CrossRef] [PubMed]
- Juhl, D.W.; Glattard, E.; Lointier, M.; Bampilis, P.; Bechinger, B. The antimicrobial and synergistic activities of PGLa and magainin 2 fibrils. Front. Cell. Infect. Microbiol. 2020, 10, 526459. [Google Scholar] [CrossRef]
- Gao, X.; Campbell, W.A.t.; Chaibva, M.; Jain, P.; Leslie, A.E.; Frey, S.L.; Legleiter, J. Cholesterol Modifies Huntingtin Binding to, Disruption of, and Aggregation on Lipid Membranes. Biochemistry 2016, 55, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Cote, S.; Binette, V.; Salnikov, E.S.; Bechinger, B.; Mousseau, N. Probing the Huntingtin 1-17 membrane anchor on a phospholipid bilayer by using all-atom simulations. Biophys. J. 2015, 108, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- Sivanandam, V.N.; Jayaraman, M.; Hoop, C.L.; Kodali, R.; Wetzel, R.; van der Wel, P.C. The aggregation-enhancing huntingtin N-terminus is helical in amyloid fibrils. J. Am. Chem. Soc. 2011, 133, 4558–4566. [Google Scholar] [CrossRef] [Green Version]
- Scherzinger, E.; Sittler, A.; Schweiger, K.; Heiser, V.; Lurz, R.; Hasenbank, R.; Bates, G.P.; Lehrach, H.; Wanker, E.E. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: Implications for Huntington’s disease pathology. Proc. Natl. Acad. Sci. USA 1999, 96, 4604–4609. [Google Scholar] [CrossRef] [Green Version]
- Nucifora, L.G.; Burke, K.A.; Feng, X.; Arbez, N.; Zhu, S.; Miller, J.; Yang, G.; Ratovitski, T.; Delannoy, M.; Muchowski, P.J.; et al. Identification of novel potentially toxic oligomers formed in vitro from mammalian-derived expanded huntingtin exon-1 protein. J. Biol. Chem. 2012, 287, 16017–16028. [Google Scholar] [CrossRef] [Green Version]
- Arndt, J.R.; Brown, R.J.; Burke, K.A.; Legleiter, J.; Valentine, S.J. Lysine residues in the N-terminal huntingtin amphipathic alpha-helix play a key role in peptide aggregation. J. Mass. Spectrom. 2015, 50, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Ganglioside-Mediated Assembly of Amyloid beta-Protein: Roles in Alzheimer’s Disease. Prog. Mol. Biol. Transl. Sci. 2018, 156, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Kobayashi, T.; Menon, A.K. Transbilayer lipid asymmetry. Curr. Biol. 2018, 28, R386–R391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| htt17-Q17 | MATLEKLMKAFESLKSF QQQ QQQ QQQ QQQ QQQ QQ |
| htt17-Q12 | MATLEKLMKAFESLKSF QQQ QQQ QQQ QQQ |
| htt17-Q9 | MATLEKLMKAFESLKSFQQQ QQQ QQQ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquette, A.; Aisenbrey, C.; Bechinger, B. Membrane Interactions Accelerate the Self-Aggregation of Huntingtin Exon 1 Fragments in a Polyglutamine Length-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 6725. https://doi.org/10.3390/ijms22136725
Marquette A, Aisenbrey C, Bechinger B. Membrane Interactions Accelerate the Self-Aggregation of Huntingtin Exon 1 Fragments in a Polyglutamine Length-Dependent Manner. International Journal of Molecular Sciences. 2021; 22(13):6725. https://doi.org/10.3390/ijms22136725
Chicago/Turabian StyleMarquette, Arnaud, Christopher Aisenbrey, and Burkhard Bechinger. 2021. "Membrane Interactions Accelerate the Self-Aggregation of Huntingtin Exon 1 Fragments in a Polyglutamine Length-Dependent Manner" International Journal of Molecular Sciences 22, no. 13: 6725. https://doi.org/10.3390/ijms22136725
APA StyleMarquette, A., Aisenbrey, C., & Bechinger, B. (2021). Membrane Interactions Accelerate the Self-Aggregation of Huntingtin Exon 1 Fragments in a Polyglutamine Length-Dependent Manner. International Journal of Molecular Sciences, 22(13), 6725. https://doi.org/10.3390/ijms22136725






