Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation
Abstract
1. Introduction
2. Results
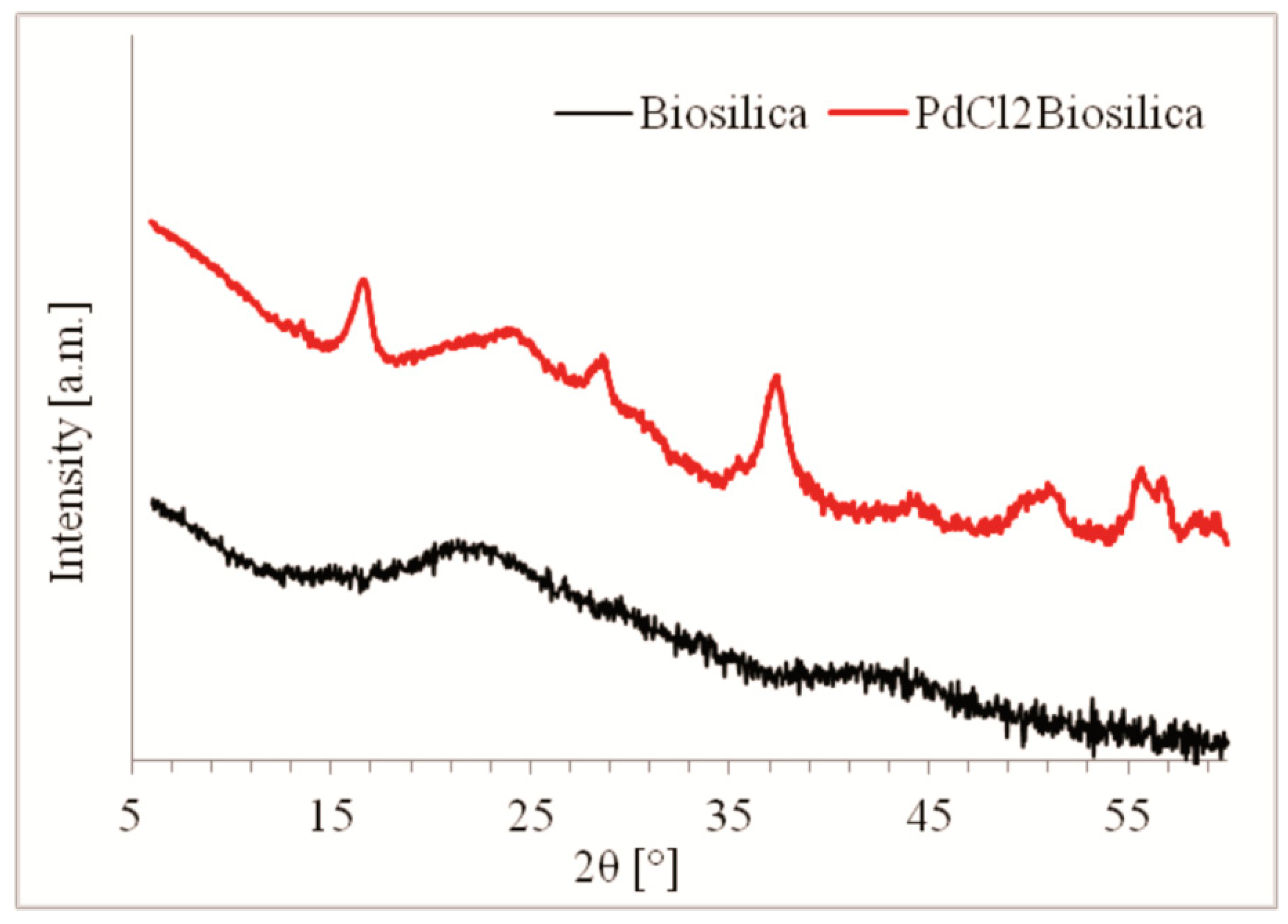
2.1. Photocatalyst Characterization
2.2. Photocatalytic Activity
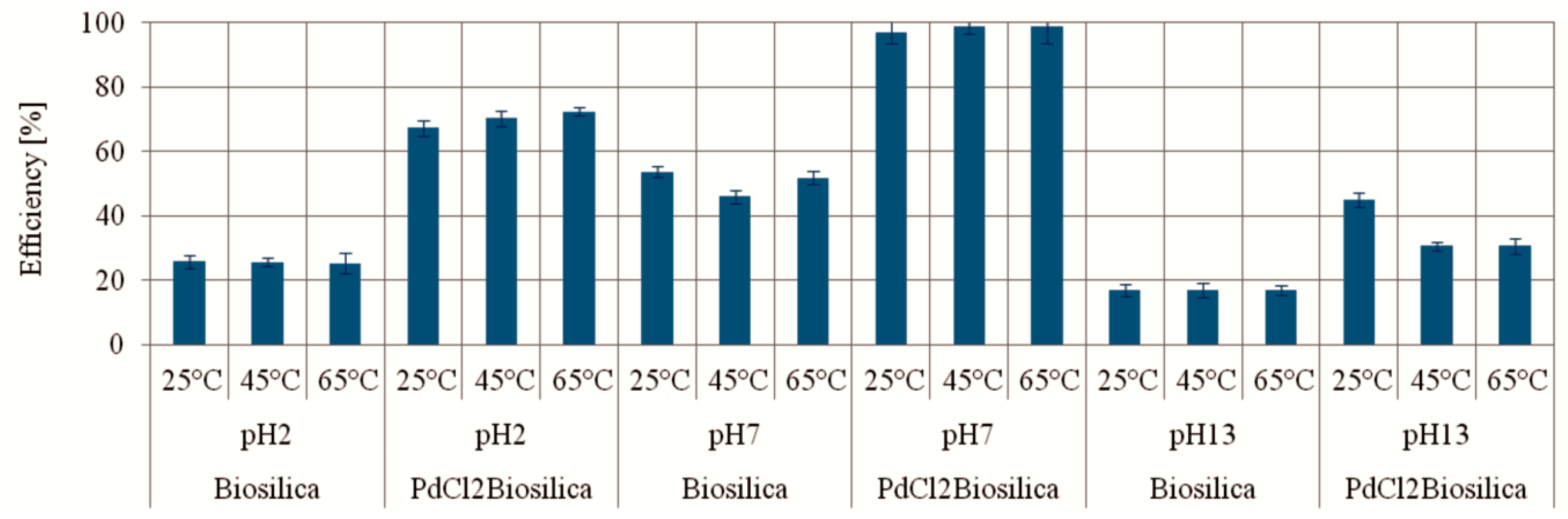
2.2.1. MO Photocatalytic Degradation Depending on the pH Value
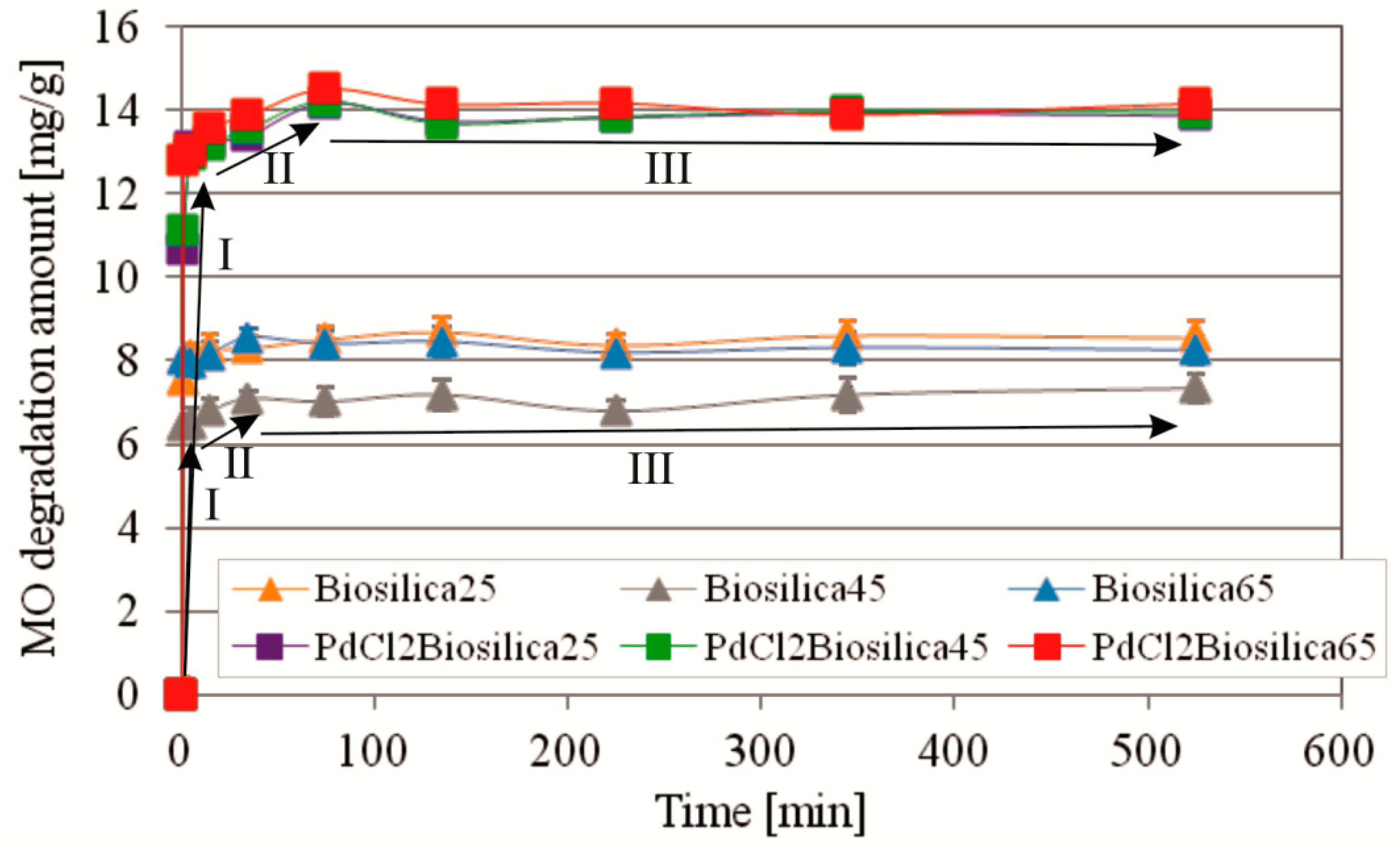
2.2.2. Kinetics of MO Photocatalytic Degradation at Different Temperatures
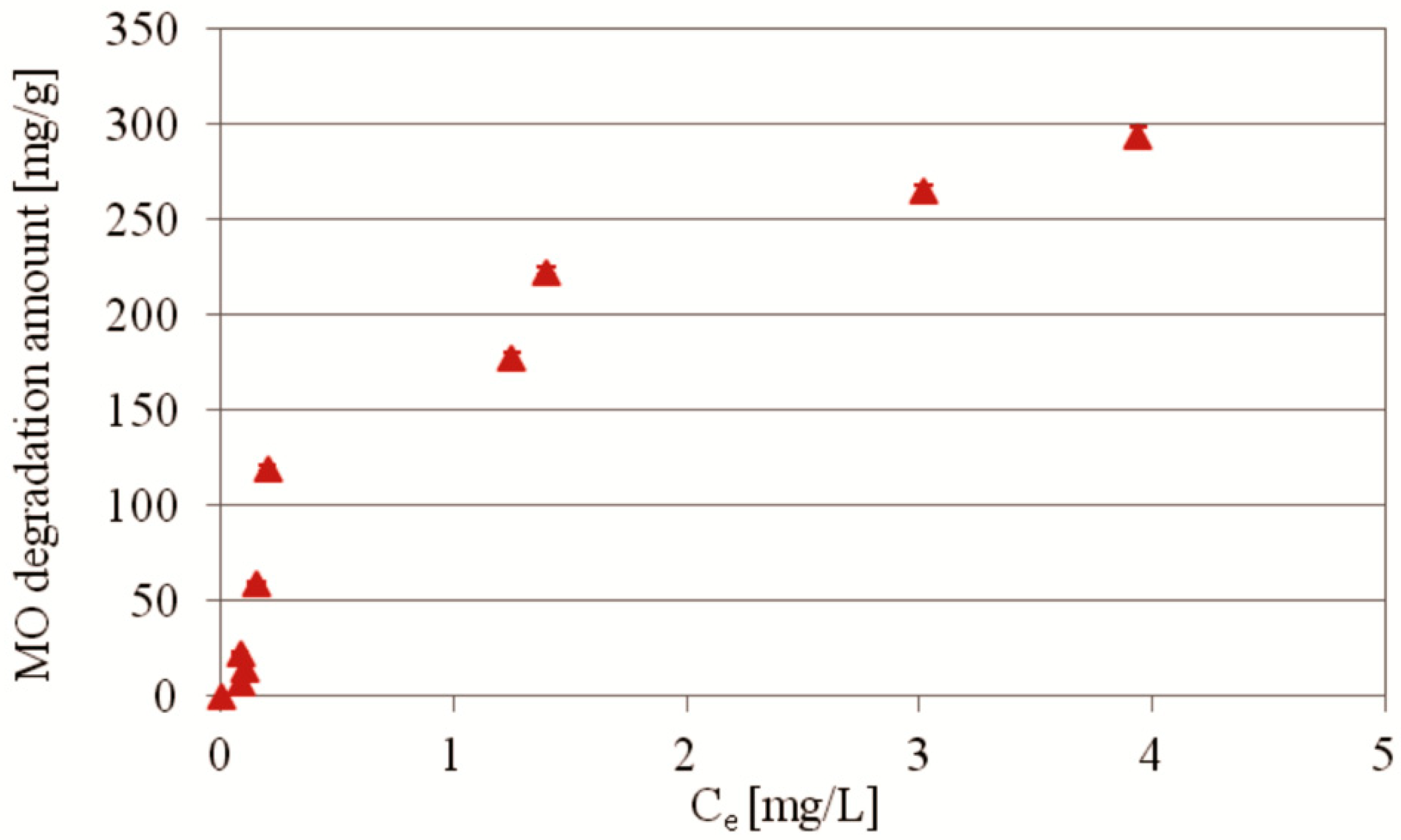
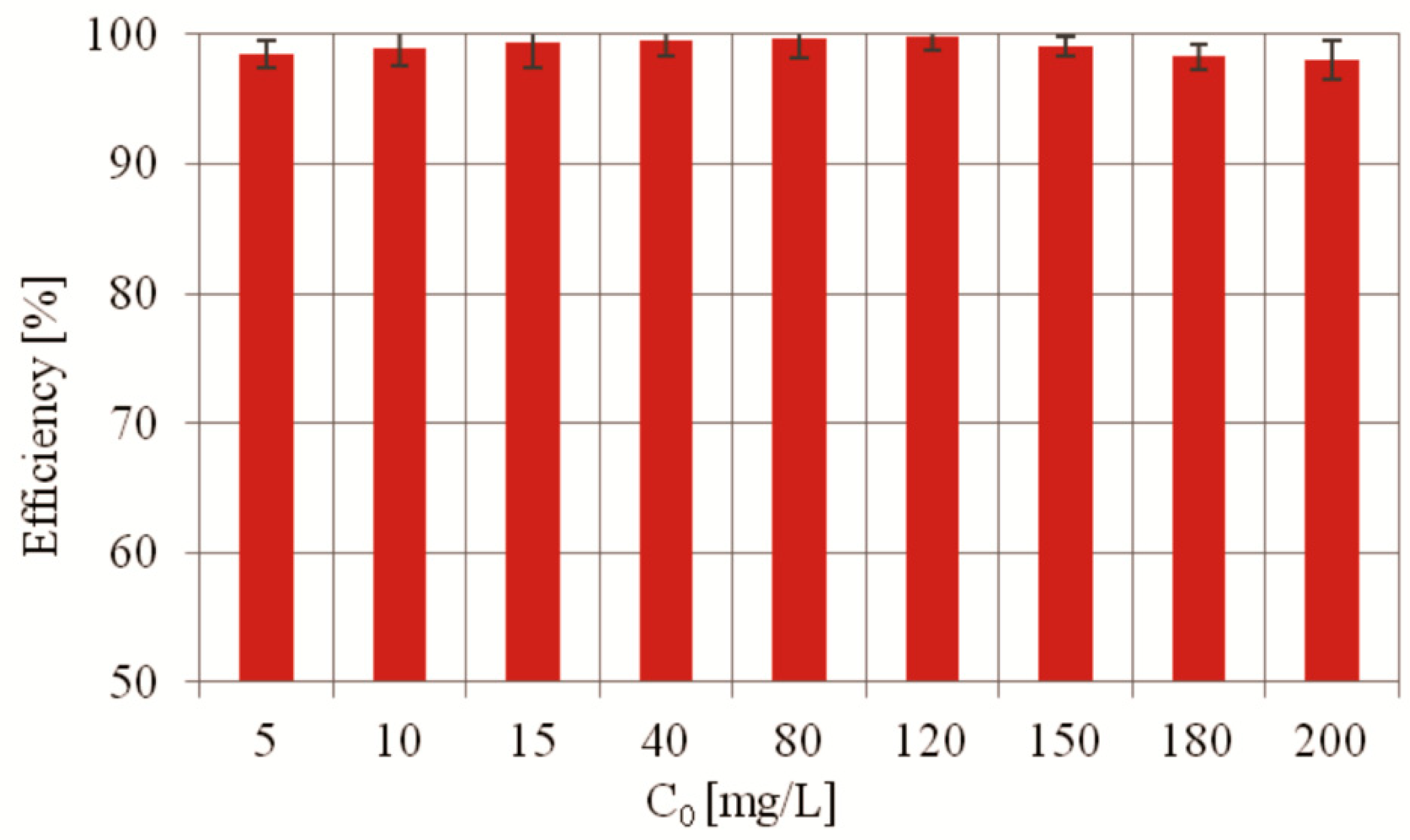
2.2.3. MO Photocatalytic Degradation Depending on the MO Initial Concentration
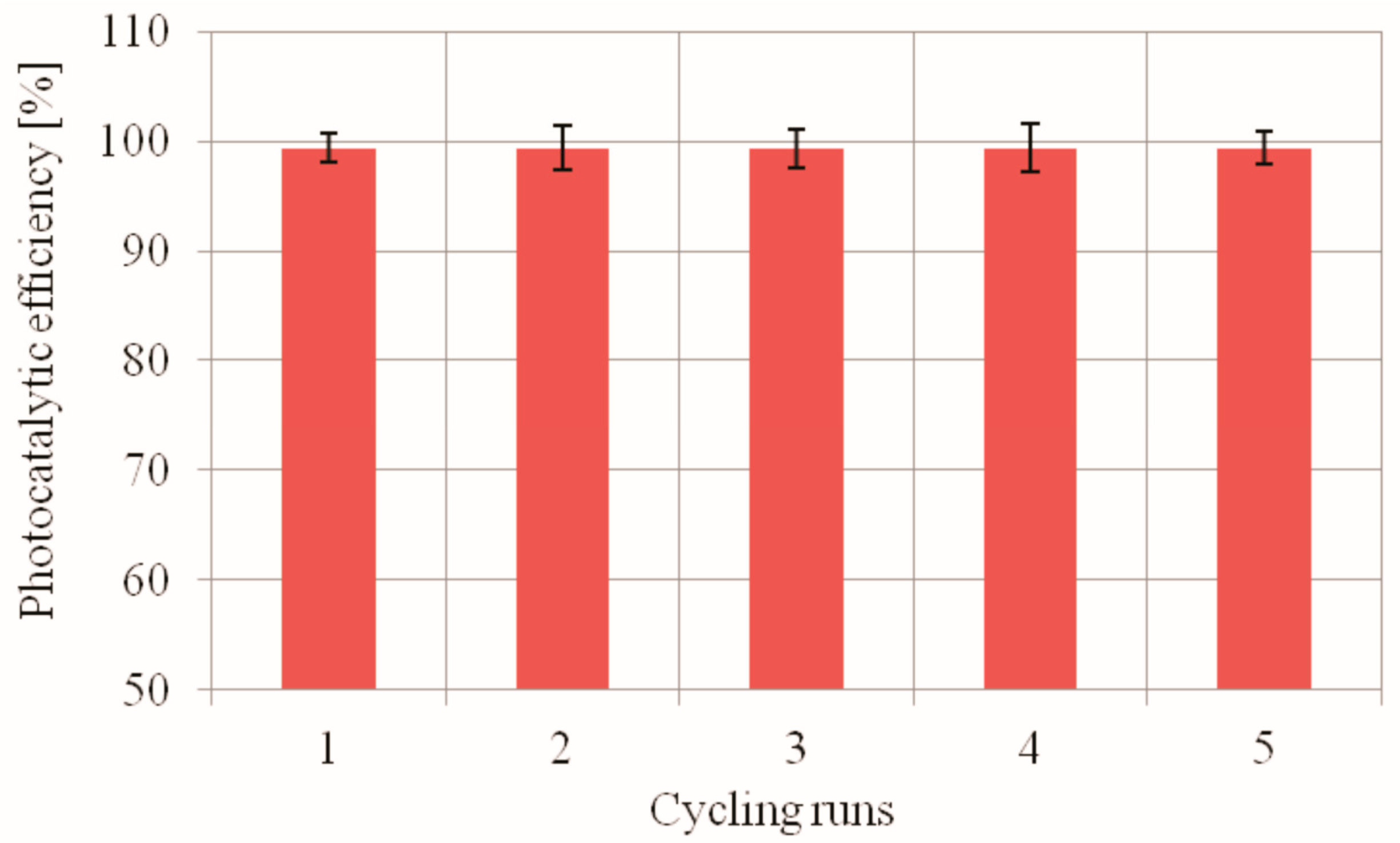
2.2.4. The Recyclability of the Used PdCl2 Biosilica Photocatalyst
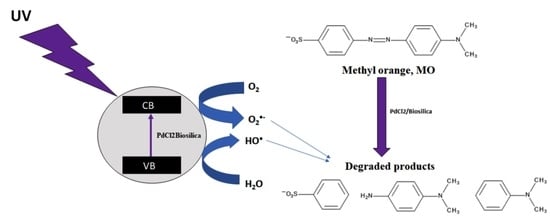
2.3. Methyl Orange Degradation Products
3. Materials and Methods
3.1. Preparation of the Photocatalyst
3.1.1. Diatom Biosilica
3.1.2. Palladium(II) Chloride-Doped Diatom Biosilica/Commercial Amorphous Silica
3.2. Photocatalyst Characterization
3.3. Photocatalytic Activity
The Stability of the Used Photocatalysts
3.4. Electrospray Ionization–High-Resolution Mass Spectrometry (ESI-HRMS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Telke, A.A.; Kalyani, D.C.; Govindwar, S.P. Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J. Hazard. Mater. 2010, 176, 503–509. [Google Scholar] [CrossRef]
- Vitor, V.; Corso, C.R. Decolorization of textile dye by Candida albicans isolated from industrial effluents. J. Ind. Microbiol. Biotechnol. 2008, 35, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Saroj, S.; Kumar, K.; Pareek, V.; Prasad, R.; Singh, R.P. Biodegradation of azo dyes Acid Red 183, Direct Blue 15 and Direct Red 75 by the isolate Penicillium oxalicum SAR-3. Chemosphere 2014, 107, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, T.; Yang, L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: A review. Bioresour. Technol. 2012, 107, 10–18. [Google Scholar] [CrossRef]
- Boucherit, N.; Abouseoud, M.; Adour, L. Degradation of direct azo dye by Cucurbita pepo free and immobilized peroxidase. J. Environ. Sci. 2013, 25, 1235–1244. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water: Investigation of the effect of operational parameters. J. Photochem. Photobiol. A Chem. 2003, 157, 111–116. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Coalesced chitosan activated carbon composite for batch and fixed-bed adsorption of cationic and anionic dyes. Colloids Surf. B Biointerfaces 2013, 105, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, F.; Ghaedi, M.; Kamali, K.; Sharifpour, E.; Sahraie, R.; Purkait, M.K. Comparison of nickel and/or zinc selenide nanoparticle loaded on activated carbon as efficient adsorbents for kinetic and equilibrium study of removal of Arsenazo (ΙΙΙ) dye. Powder Technol. 2013, 245, 217–226. [Google Scholar] [CrossRef]
- Koupaie, E.H.; Moghaddam, M.R.A.; Hashemi, S.H. Successful treatment of high azo dye concentration wastewater using combined anaerobic/aerobic granular activated carbon-sequencing batch biofilm reactor (GAC-SBBR): Simultaneous adsorption and biodegradation processes. Water Sci. Technol. 2013, 67, 1816–1821. [Google Scholar] [CrossRef]
- Tabatabaee, M.; Ghotbifar, A.; Mozafari, A.A. Photocatalytic degradation and kinetic study of textile azo dyes direct blue 71 and black 19 in ZnO-suspended solution. Fresenius Environ. Bull. 2012, 21, 1468–1473. [Google Scholar]
- Chatzisymeon, E.; Petrou, C.; Mantzavinos, D. Photocatalytic treatment of textile dyehouse effluents with simulated and natural solar light. Glob. Nest J. 2013, 15, 21–28. [Google Scholar]
- Pang, Y.L.; Abdullah, A.Z. Fe3+ doped TiO2 nanotubes for combined adsorption-sonocatalytic degradation of real textile wastewater. Appl. Catal. B Environ. 2013, 129, 473–481. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Shi, R.; Lin, J.; Wang, Y.J.; Xu, J.; Zhu, Y.F. Visible-Light Photocatalytic Degradation of BiTaO4 Photocatalyst and Mechanism of Photocorrosion Suppression. J. Phys. Chem. C 2010, 114, 6472–6477. [Google Scholar] [CrossRef]
- Ge, L.; Han, C.; Liu, J. Novel visible light-induced g-C3N4/Bi2WO6 composite photocatalysts for efficient degradation of methyl orange. Appl. Catal. B Environ. 2011, 108–109, 100–107. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.-H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Fan, W.; Leung, M.K.H. Recent Development of Plasmonic Resonance-Based Photocatalysis and Photovoltaics for Solar Utilization. Molecules 2016, 21, 180. [Google Scholar] [CrossRef]
- Lv, K.; Perriman, A.W.; Mann, S. Photocatalytic multiphase micro-droplet reactors based on complex coacervation. Chem. Commun. 2015, 51, 8600–8602. [Google Scholar] [CrossRef]
- Chen, L.; Tian, L.; Zhao, X.; Hu, Z.; Fan, J.; Lv, K. SPR effect of Au nanoparticles on the visible photocatalytic RhB degradation and NO oxidation over TiO2 hollow nanoboxes. Arab. J. Chem. 2020, 13, 4404–4416. [Google Scholar] [CrossRef]
- Ge, L.; Xu, M.X.; Fang, H.B. Synthesis of novel photocatalytic InVO4-TiO2 thin films with visible light photoactivity. Mater. Lett. 2007, 61, 63–66. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A Novel Aqueous Process for Preparation of Crystal Form-Controlled and Highly Crystalline BiVO4 Powder from Layered Vanadates at Room Temperature and Its Photocatalytic and Photophysical Properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chi, C.F.; Liau, S.Y. CdS/CdSe Co-Sensitized TiO2 Photoelectrode for Efficient Hydrogen Generation in a Photoelectrochemical Cell. Chem. Mater. 2010, 22, 922–927. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.Z.; Sun, S.M. Enhanced photocatalytic activity of Bi2WO6 loaded with Ag nanoparticles under visible light irradiation. Appl. Catal. B Environ. 2009, 92, 50–55. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Chen, X.F.; Takanabe, K.; Domen, K.; Hou, Y.D.; Fu, X.Z.; Antonietti, M. Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Lv, K.; Li, Q.; Ye, H.; Du, D.; Li, M. Effect of acid on the photocatalytic degradation of rhodamine B over g-C3N4. Appl. Surf. Sci. 2015, 358, 336–342. [Google Scholar] [CrossRef]
- Fang, S.; Xia, Y.; Lv, K.; Li, Q.; Sun, J.; Li, M. Effect of carbon-dots modification on the structure and photocatalytic activity of g-C3N4. Appl. Catal. B 2016, 185, 225–232. [Google Scholar] [CrossRef]
- Huo, Y.; Jin, Y.; Zhang, Y. Citric acid assisted solvothermal synthesis of BiFeO3 microspheres with high visible-light photocatalytic activity. J. Mol. Catal. A Chem. 2010, 331, 15–20. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Gordon, R.; Drum, R.W. The chemical basis of diatom morphogenesis. Int. Rev. Cytol. 1994, 150, 243–372. [Google Scholar]
- Zhang, X.Y.; Suna, X.W. Diatom Nanotechnology: Progress and Emerging Applications. In Nanoscience & Nanotechnology, 4th ed.; Dusan, L., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 175–200. [Google Scholar]
- Ghobara, M.M.; Mazumder, N.; Vinayak, V.; Reissig, L.; Gebeshuber, I.C.; Tiffany, M.A.; Gordon, R. Diatoms: Fundamentals and Applications. In On Light and Diatoms: A Photonics and Photobiology Review; Joseph, S., Richard, G., Eds.; Wiley-Scrivener: Beverly, MA, USA, 2019; pp. 129–190. [Google Scholar]
- Rorrer, G.L. Functionalization of Frustules from Diatom Cell Culture for Optoelectronic Properties. In Nanoscience & Nanotechnology, 4th ed.; Dusan, L., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 79–110. [Google Scholar]
- Gordon, R.; Losic, D.; Tiffany, M.A.; Nagy, S.S.; Sterrenburg, F.A.S. The Glass Menagerie: Diatoms for novel applications in nanotechnology. Trends Biotechnol. 2009, 27, 116–127. [Google Scholar] [CrossRef]
- Umernura, K.; Gao, Y.; Nishikawa, T. Preparation of Photocatalyst Using Diatom Frustules by Liquid Phase Deposition Method. J. Nanosci. Nanotechnol. 2010, 10, 4883–4888. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally organic functionalized 3D biosilica from diatom microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Schmid, G. Large clusters and colloids. Metals in the embryonic state. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Bonnemann, H.; Brijoux, W.; Brinkmann, R.; Fretzen, R.; Joussen, T.; Koeppler, R.; Korall, B.; Neiteler, P. Richter Preparation, characterization, and application of fine metal particles and metal colloids using hydrotriorganoborates. J. Mol. Catal. 1994, 86, 129–177. [Google Scholar] [CrossRef]
- Li, F.; Zhang, B.; Dong, S.; Wang, E. A novel method of electrodepositing highly dispersed nano palladium particles on glassy carbon electrode. Electrochim. Acta 1997, 42, 2563–2568. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, H.; Cheng, S.; Ren, Z.; Hu, Z.; Wang, Z. Lewis Acid-Promoted Suzuki Reaction using Palladium Chloride Anchored on a Polymer as a Catalyst. Aust. J. Chem. 2008, 61, 610–614. [Google Scholar] [CrossRef]
- Aoki, K.; Zhao, Y.; Chen, J. Colloidal submicron-palladium particles stabilized with acetate. Electrochim. Acta 2007, 52, 2485–2491. [Google Scholar] [CrossRef][Green Version]
- Van Eynde, E.; Tytgat, T.; Smits, M.; Verbruggen, S.W.; Hauchecorne, B.; Lenaerts, S. Biotemplated diatom silica-titania materials for air purification. Photochem. Photobiol. Sci. 2013, 12, 690–695. [Google Scholar] [CrossRef]
- Ouwehand, J.; Van Eynde, E.; De Canck, E.; Lenaerts, S.; Verberckmoes, A.; Van Der Voort, P. Titania-functionalized diatom frustules as photocatalyst for indoor air purification. Appl. Catal. B Environ. 2018, 226, 303–310. [Google Scholar] [CrossRef]
- He, J.; Chen, D.M.; Li, Y.L.; Shao, J.L.; Xie, J.; Sun, Y.J.; Yan, Z.Y.; Wang, J.Q. Diatom template TiO2 with enhanced photocatalytic activity: Biomimetics of photonic crystals. Appl. Phys. A 2013, 113, 327–332. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Haq, E.U.; Licciulli, A. Nanocrystalline TiO2-diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhu, S.; Zhang, D.; Chen, Z.; Chen, C. Sonochemical fabrication of mesoporous TiO2 inside diatom frustules for photocatalyst. Ultrason. Sonochem. 2014, 21, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Van Eynde, E.; Hu, Z.-Y.; Tytgat, T.; Verbruggen, S.W.; Watté, J.; Van Tendeloo, G.; Van Driessche, I.; Blust, R.; Lenaerts, S. Diatom silica–titania photocatalysts for air purification by bio-accumulation of different titanium sources. Environ. Sci. Nano 2016, 3, 1052–1061. [Google Scholar] [CrossRef]
- Kumari, S.; Min, K.H.; Kanth, B.K.; Jang, E.K.; Pack, S.P. Production of TiO2-deposited Diatoms and Their Applications for Photo-catalytic Degradation of Aqueous Pollutants. Biotechnol. Bioprocess Eng. 2020, 25, 758–765. [Google Scholar] [CrossRef]
- Gannavarapu, K.P.; Thakkar, M.; Veerapaga, S.; Wei, L.; Dandamudi, R.B.; Mitra, S. Novel diatom-FeOx composite as highly active catalyst in photodegradation of Rhodamine-6G. Nanotechnol. Rev. 2018, 7, 247–255. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Kigga, M.; Sriram, G.; Ajeya, K.V.; Jung, H.-Y.; Neelgund, G.M.; Kurkuri, M.D. Facile green synthetic approach of bio inspired polydopamine coated diatoms as a drug vehicle for controlled drug release and active catalyst for dye degradation. Microporous Mesoporous Mater. 2019, 288, 109572. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thommes, M. Physical Adsorption Characterization of Nanoporous Materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of large pore MCM-41 molecular sieves to improve pore size analysis using nitrogen adsorption measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Al-Qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Trabelsi, H.; Atheba, G.P.; Hentati, O.; Mariette, Y.D.; Robert, D.; Drogui, P.; Ksibi, M. Solar Photocatalytic Decolorization and Degradation of Methyl Orange Using Supported TiO2. J. Adv. Oxid. Technol. 2016, 19, 79–84. [Google Scholar] [CrossRef][Green Version]
- Peng, H.H.; Chen, J.; Jiang, D.Y.; Li, M.; Feng, L.; Losic, D.; Dong, F.; Zhang, Y.X. Synergistic effect of manganese dioxide and diatomite for fast decolorization and high removal capacity of methyl orange. J. Colloid Interface Sci. 2016, 484, 1–9. [Google Scholar] [CrossRef]
- Alabbad, E.A. Efficient Removal of Methyl Orange from Wastewater by Polymeric Chitosan-iso-vanillin. Open Chem. J. 2020, 7, 16–25. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsuda, K.; Tanaka, M.; Kuboyama, S.; Uto, K.; Wada, N.; Kawazumi, H.; Tsuji, T.; Ago, H.; Hayashi, J. Enhanced Photocatalytic Degradation of Methyl Orange by Au/TiO2 Nanoparticles under Neutral and Acidic Solutions. ChemistrySelect 2018, 3, 1432–1438. [Google Scholar] [CrossRef]
- Soto, A.I.; Valencia, R.N.A.; Vásquez, A.F.L. Effect of pH and Temperature on Photocatalytic Oxidation of Methyl Orange using Black Sand as Photocatalyst. Rev. Mutis 2018, 8, 43–54. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, R.; Wang, J. Adsorption of methyl orange from aqueous solution using chitosan/diatomite composite. Water Sci. Technol. 2017, 75, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Szeto, W.; Kan, C.W.; Yuen, C.W.M.; Chan, S.-W.; Lam, K.H. Effective Photodegradation of Methyl Orange Using Fluidized Bed Reactor Loaded with Cross-Linked Chitosan Embedded Nano-CdS Photocatalyst. Int. J. Chem. Eng. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Xie, S.; Huang, P.; Kruzic, J.J.; Zeng, X.; Qian, H. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947–21957. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N.; Eltaher, M.A.; Abdou, A.N.A. Adsorption and photocatalysis for methyl orange and Cd removal from wastewater using TiO2/sewage sludge-based activated carbon nanocomposites. R. Soc. Open Sci. 2017, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.; Sharma, T.R. Photo catalytic degradation of two commercial dyes in aqueous phase using photo catalyst TiO2. Adv. Appl. Sci. Res. 2012, 3, 849–853. [Google Scholar]
- He, H.Y.; Huang, J.F.; Cao, L.Y.; Wu, J.P. Photodegradation of methyl orange aqueous on MnWO4 powder under different light resources and initial pH. Desalination 2010, 252, 66–70. [Google Scholar] [CrossRef]
- Smith, Y.R.; Kar, A.; Subramanian, V.R. Investigation of Physicochemical Parameters That Influence Photocatalytic Degradation of Methyl Orange over TiO2 Nanotubes. Ind. Eng. Chem. Res. 2009, 48, 10268–10276. [Google Scholar] [CrossRef]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. The effect of pH on the photonic efficiency of the destruction of methyl orange under controlled periodic illumination with UV-LED sources. Chem. Eng. J. 2009, 246, 337–342. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Zhang, L.; Xu, J. Bi2WO6/SiO2 photonic crystal film with high photocatalytic activity under visible light irradiation. Appl. Catal. B Environ. 2012, 125, 144–148. [Google Scholar] [CrossRef]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic Crystals for Plasmonic Photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Huang, M.; Xu, C.; Wu, Z.; Huang, Y.; Lin, J.; Wu, J. Photocatalytic discolorization of methyl orange solution by Pt modified TiO2 loaded on natural zeolite. Dye. Pigment. 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Li, M.; Guan, R.; Li, J.; Zhao, Z.; Zhang, J.; Qi, Y.; Zhai, H.; Wan, L. Photocatalytic Performance and Mechanism Research of Ag/HSTiO2 on Degradation of Methyl Orange. ACS Omega 2020, 5, 21451–21457. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Zhang, W. Adsorption and Photocatalytic Degradation of Methyl Orange on TiO2 and CuZSM-5 Mixture. Appl. Mech. Mater. 2011, 48–49, 149–152. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Nasseri, S.; Azizi, N.; Shahmoradi, B.; Khosravi, T. Optimization of photocatalytic degradation of methyl orange using immobilized scoria-Ni/TiO2 nanoparticles. J. Nanostruct. Chem. 2020, 10, 143–159. [Google Scholar] [CrossRef]
- Zha, R.; Nadimicherla, R.; Guo, X. Ultraviolet photocatalytic degradation of methyl orange by nanostructured TiO2/ZnO heterojunctions. J. Mater. Chem. A 2015, 3, 6565–6574. [Google Scholar] [CrossRef]
- Lin, C.; Gao, Y.; Zhang, J.; Xue, D.; Fang, H.; Tian, J.; Zhou, C.; Zhang, C.; Li, Y.; Li, H. GO/TiO2 composites as a highly active photocatalyst for the degradation of methyl orange. J. Mater. Res. 2020, 35, 1307–1315. [Google Scholar] [CrossRef]
- Fu, M.; Li, Y.; Wu, S.; Lu, P.; Liu, J.; Dong, F. Sol-gel preparation and enhanced photocatalytic performance of Cu-doped ZnO nanoparticles. Appl. Surf. Sci. 2011, 258, 1587–1591. [Google Scholar] [CrossRef]
- Dhanalakshmi, J.; Padiyan, D.P. Photocatalytic degradation of methyl orange and bromophenol blue dyes in water using sol-gel synthesized TiO2 nanoparticles. Mater. Res. Express 2017, 4, 1–9. [Google Scholar] [CrossRef]
- Al-Abdallat, Y.; Jum’h, I.; Bsoul, A.A.; Jumah, R.; Telfah, A. Photocatalytic Degradation Dynamics of Methyl Orange Using Coprecipitation Synthesized Fe3O4 Nanoparticles. Water Air Soil Pollut. 2019, 230, 277–293. [Google Scholar] [CrossRef]
- Zhou, L.; Han, X.; Zhang, W.; He, H. Characterization of SiO2-TiO2 and photocatalytic degradation of methyl orange. Asian J. Chem. 2013, 26, 3837–3839. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Gao, Y.; Wu, Y.; Liu, Q.; Zhu, X. Synthesis and characterization of cubic Ag/TiO2 nanocomposites for the photocatalytic degradation of methyl orange in aqueous solutions. Inorg. Chem. Commun. 2019, 110, 107589–107596. [Google Scholar] [CrossRef]
- Raliya, R.; Avery, C.; Chakrabarti, S.; Biswas, P. Photocatalytic degradation of methyl orange dye by pristine titanium dioxide, zinc oxide, and graphene oxide nanostructures and their composites under visible light irradiation. Appl. Nanosci. 2017, 7, 253–259. [Google Scholar] [CrossRef]
- Baiocchi, C.; Brussino, M.C.; Pramauro, E.; Prevot, A.B.; Palmisano, L.; Marcí, G. Characterization of methyl orange and its photocatalytic degradation products by HPLC/UV–VIS diode array and atmospheric pressure ionization quadrupole ion trap mass spectrometry. Int. J. Mass Spectrom. 2002, 214, 247–256. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, Y.; Lin, J.M.; Chen, G. Study on the photocatalytic degradation of methyl orange in water using Ag/ZnO as catalyst by liquid chromatography electrospray ionization ion-trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 997–1003. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, C.; Wang, C.; Sun, J.; Wang, X.; Li, X. A TiO2 modified abiotic–biotic process for the degradation of the azo dye methyl orange. RSC Adv. 2015, 5, 58704–58712. [Google Scholar] [CrossRef]
- Nowak, A.P.; Sprynskyy, M.; Brzozowska, W.; Lisowska-Oleksiak, A. Electrochemical behavior of a composite material containing 3D-structured diatom biosilica. Algal Res. 2019, 41, 101538–101544. [Google Scholar] [CrossRef]




| Sample | Specific Surface Area [m2 g−1] | Pore Volume [cm3 g−1] | Average Pore Diameter [nm] |
|---|---|---|---|
| Biosilica | 30 | 0.43 | 3.93 |
| PdCl2Biosilica | 40 | 0.28 | 4.45 |
| Kinetics Model | Equation | Parameters | Value | R2 |
|---|---|---|---|---|
| PdCl2Biosilica25 | ||||
| Langmuir | Ce/qe = (1/qmKL) + Ce/qm | KL (L/mg) qm (mg/g) | 0.5442 434.8 | 0.5047 |
| Freundlich | logqe = logKF + 1/n logCe | KF (L/g) 1/n | 137.27 0.767 | 0.7707 |
| Temkin | qe = BTlnAT + BTlnCe | BT (J/mol) AT (L/g) | 69.0 15.203 | 0.9634 |
| m/z | Compound | ESI Mode | Molecular Formula | Symbolic Formula |
|---|---|---|---|---|
| 304.0760 | MO standard | (−) | C14H14N3SO3 | MMO |
| 306.0918 | MO protonated standard fragmentation product | (+) and (+) + FA | C14H16N3SO3 | MMOH2 |
| 156.9966 | Benzenesulphonic acid radical anion standard | (−) | C6H5O3S | MBA |
| 159.0107 | Benzenesulphonic acid protonated standard | (+) and (+) + FA | C6H5O3SH2 | MBAH2 |
| 135.0882 | N,N-dimethyl-p-phenylenediamine radical anion standard | (−) | C8H11N2H | MD |
| 137.1072 | N,N-dimethyl-p-phenylenediamine radical cation standard | (+) and (+) + FA | C8H11N2H2 | MDH |
| 120.0439 | N,N-dimethylbenzenamine anion radical standard | (−) | C8H10N | MDB |
| 122.0969 | N,N-dimethylbenzenamine cation radical standard | (+) and (+) + FA | C8H10NH2 | MDBH2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprynskyy, M.; Szczyglewska, P.; Wojtczak, I.; Nowak, I.; Witkowski, A.; Buszewski, B.; Feliczak-Guzik, A. Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation. Int. J. Mol. Sci. 2021, 22, 6734. https://doi.org/10.3390/ijms22136734
Sprynskyy M, Szczyglewska P, Wojtczak I, Nowak I, Witkowski A, Buszewski B, Feliczak-Guzik A. Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation. International Journal of Molecular Sciences. 2021; 22(13):6734. https://doi.org/10.3390/ijms22136734
Chicago/Turabian StyleSprynskyy, Myroslav, Paulina Szczyglewska, Izabela Wojtczak, Izabela Nowak, Andrzej Witkowski, Bogusław Buszewski, and Agnieszka Feliczak-Guzik. 2021. "Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation" International Journal of Molecular Sciences 22, no. 13: 6734. https://doi.org/10.3390/ijms22136734
APA StyleSprynskyy, M., Szczyglewska, P., Wojtczak, I., Nowak, I., Witkowski, A., Buszewski, B., & Feliczak-Guzik, A. (2021). Diatom Biosilica Doped with Palladium(II) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation. International Journal of Molecular Sciences, 22(13), 6734. https://doi.org/10.3390/ijms22136734