Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome
Abstract
:1. Introduction
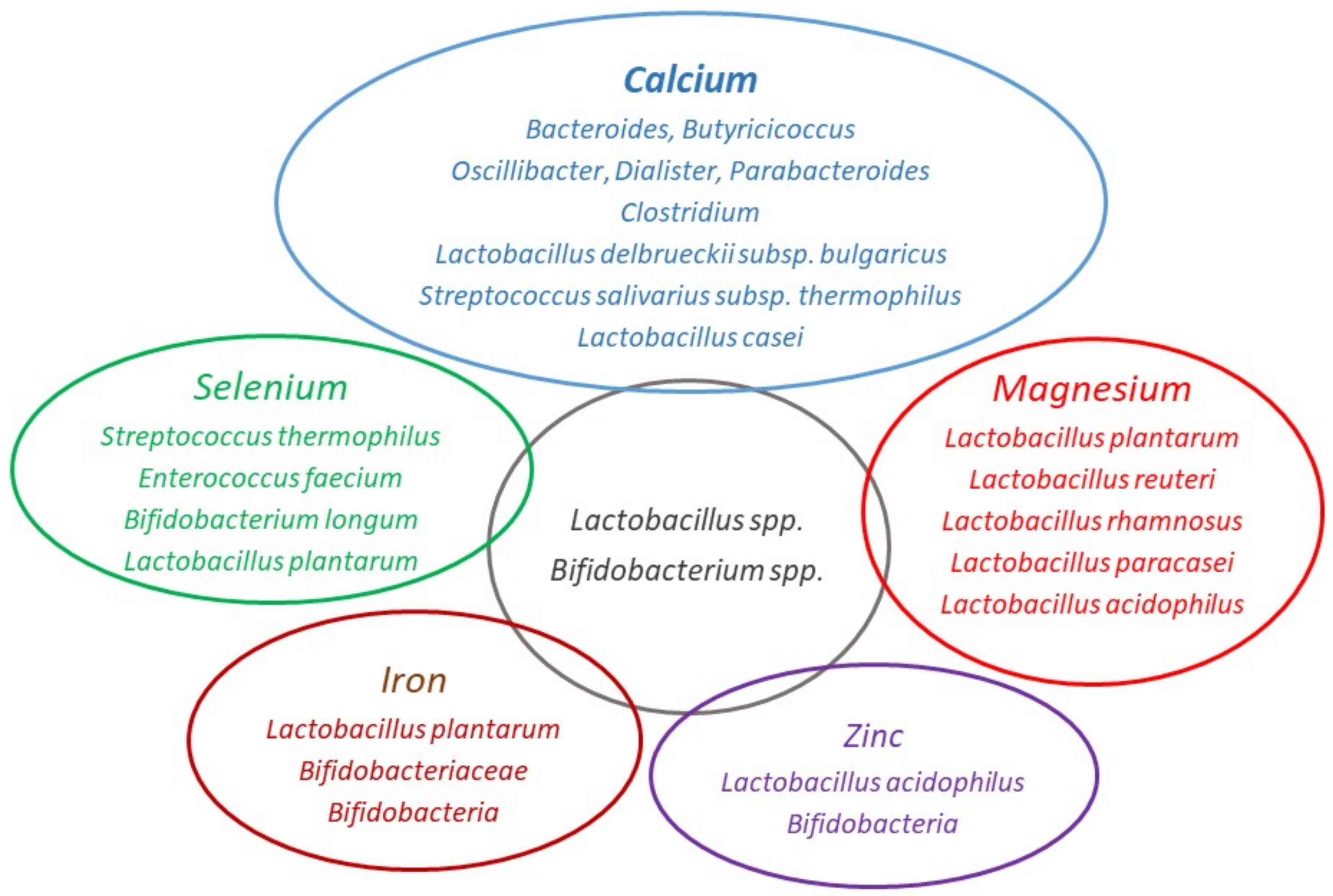
2. Gut Microbiota, Mineral Availability, and Function
2.1. Calcium
2.1.1. Effect of Prebiotics on Calcium Absorption
2.1.2. Effect of Probiotics on Calcium Absorption
2.2. Magnesium
Interaction between Magnesium and Intestinal Microbiota
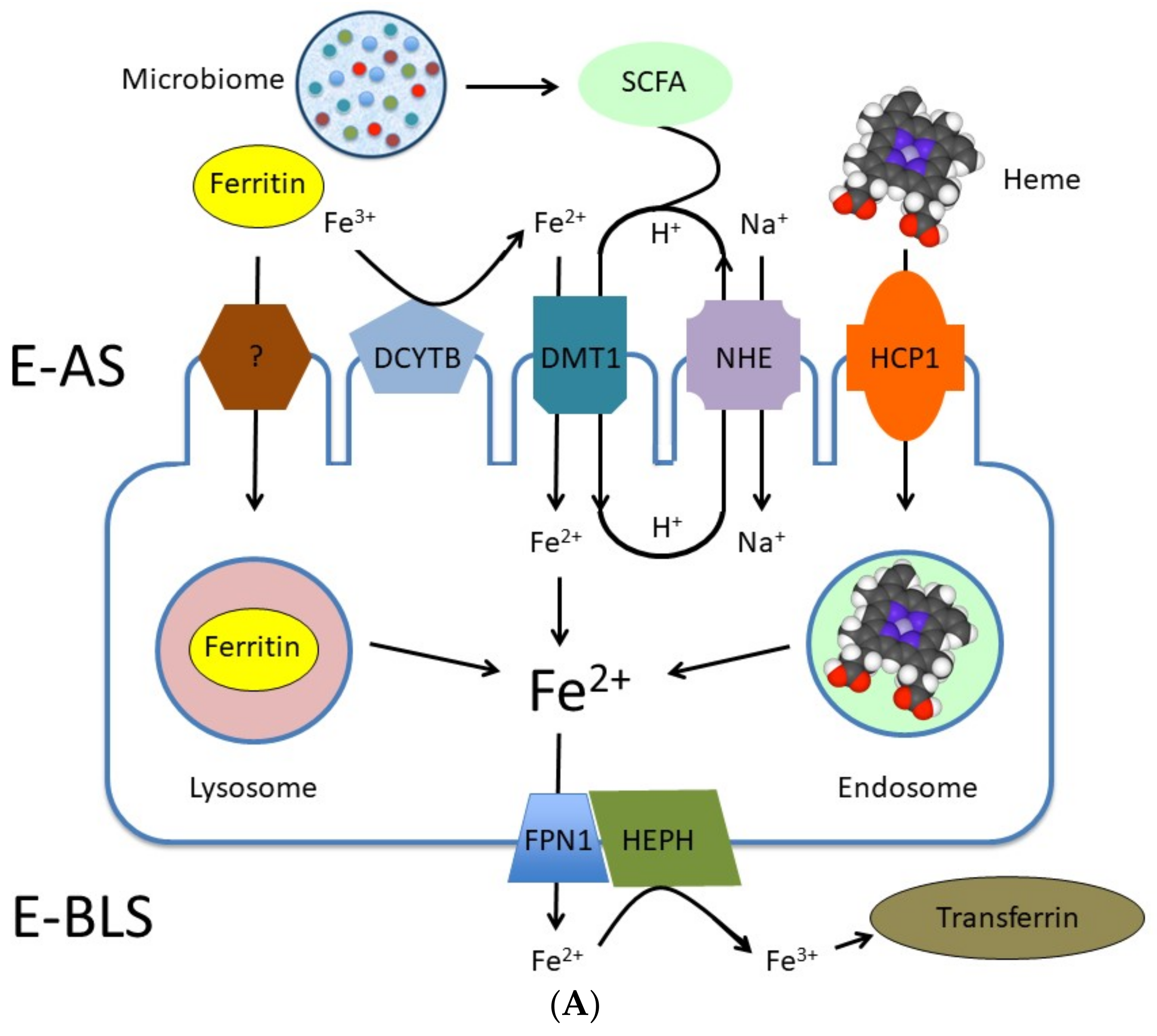
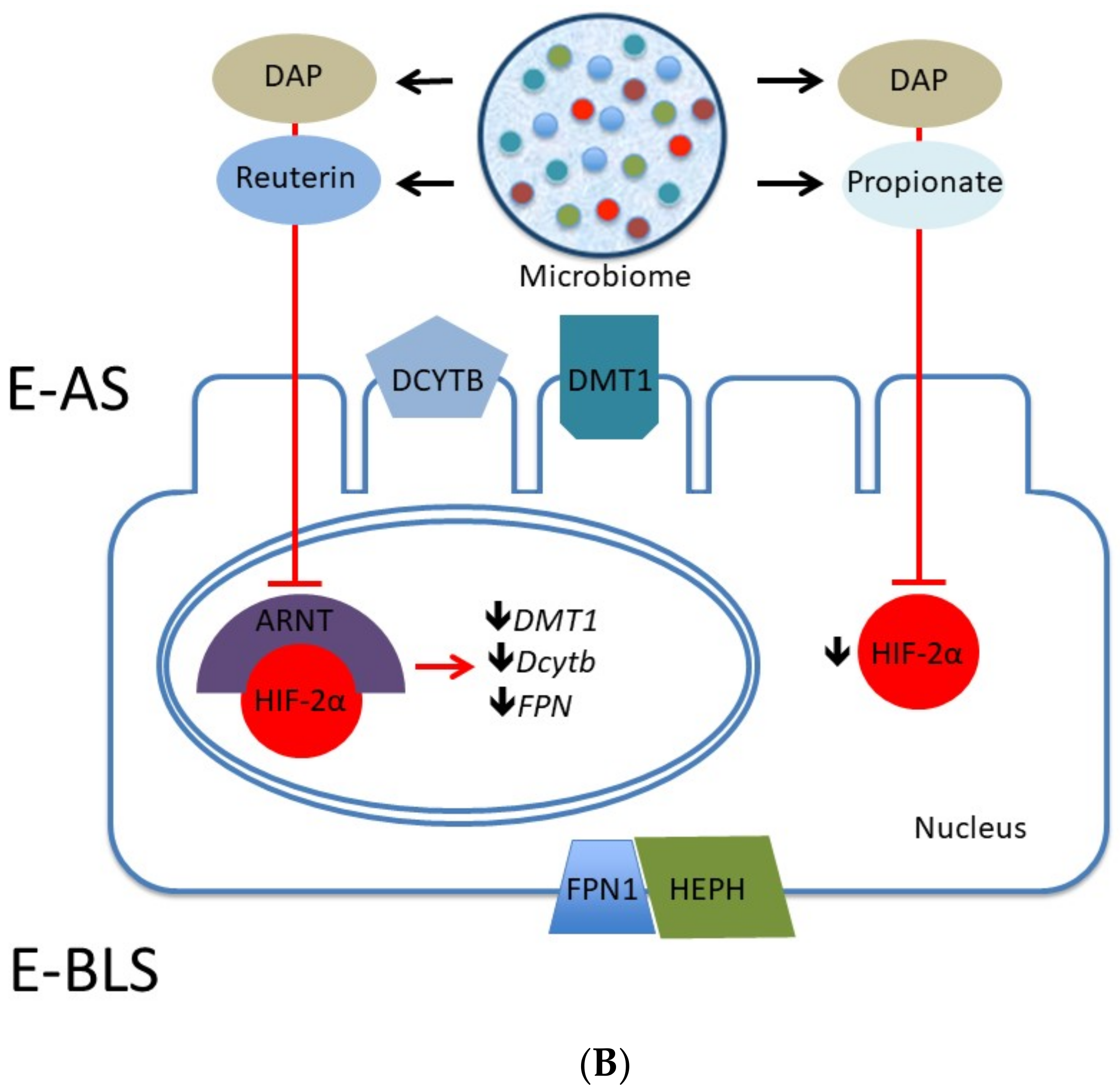
2.3. Iron
Fermentation and Iron Bioavailability
2.4. Zinc
Association between Zinc and Gut Microbiota
| Intervention | Study Population | Main Outcome | References |
|---|---|---|---|
| Soluble maize fiber | 24 adolescent children (12–15 years) | Fractional Ca2+ absorption was 12% higher after treatment. Phylum Bacteroidetes was significantly greater | Whissner et al. [19] |
| Mg2+ oxide | 60 young children with functional constipation (>6 month to <6 years) | Decrease in stool consistency and suppressed presence of the genus Dialister | Kubota et al. [77] |
| Iron sulfate | 53 patients with IBD | Decreased abundances of Ruminococcus bromii, Dorea sp., Faecalibacterium prausnitzii and Collinsella aerofaciens | Lee at a. [93] |
| Zn-biofortified wheat diet | Animal model (Gallus gallus) | Increased β-microbial diversity and increased Zn-dependent bacterial metabolic pathways | Reed at al. [129] |
| Se- and Zn-enriched Lactobacillus plantarum | Animal model (Mus musculus) | Increased antioxidant activity and blood Se level | Kang et al. [135] |
2.5. Selenium
Interaction of Selenium and Probiotics
| Prebiotic/Probiotics | Mechanisms | Main Outcome | References |
|---|---|---|---|
| Lactobacillus salivarius and Bifidobacterium infantis | Transepithelial calcium transport | Enhanced intestinal calcium uptake | Gilman and Cashman [55] |
| Se-enriched Bifidobacterium longum | Biotransformation of inorganic Se into bioactive organic Se | High bioaccessibility of selenomethionine and 98% enteric absorbtion | Zhu et al. [151]; Wastney et al. [152] |
| Prebiotic fiber Acacia | Increased Lactobacillus and Bifidobacterium spp in the gut | Higher Zn concentrations in the femur of Wistar rats | Massot-Cladera et al. [26] |
| Lactobacillus plantarum | Microbial metabolite production, enhanced mucin production and immunomodulation | Increased non-heme dietary Fe absorption | Vonderheid et al. [26] |
| Soluble corn fiber, Parabacteroides and Clostridium | Acidification and SCFA production | Increased mineral solubility and calcium absorption | Trinidad et al. [26]; Cashman [44] |
| Fermented soymilk with various lactic acid bacteria | Reducing the content of phytic acid | Increasing the bioavailability of magnesium, calcium, iron and zinc | Rekha and Vijayalakshmi [79] |
| Fermented goats’ milks with Lactobacillus plantarum | Not totally clear | Increased magnesium and calcium bioavailability | Bergillos-Meca et al. [23] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maughan, R.J. Role of micronutrients in sport and physical activity. Br. Med. Bull. 1999, 55, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, N.R.; Di Marco, N.M.; Langley, S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2009, 41, 709–731. [Google Scholar] [PubMed]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casazza, G.A.; Tovar, A.P.; Richardson, C.E.; Cortez, A.N.; Davis, B.A. Energy Availability, Macronutrient Intake, and Nutritional Supplementation for Improving Exercise Performance in Endurance Athletes. Curr. Sports Med. Rep. 2018, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Kinkorová, I.; Kokstejn, J.; Petr, M. Macronutrient Intake in Soccer Players—A Meta-Analysis. Nutrients 2019, 11, 1305. [Google Scholar] [CrossRef] [Green Version]
- Black, K.E.; Black, A.D.; Baker, D.F. Macronutrient Intakes of Male Rugby Union Players: A Review. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Storey, A.; Smith, H.K. Unique aspects of competitive weightlifting: Performance, training and physiology. Sports Med. 2012, 42, 769–790. [Google Scholar] [CrossRef]
- Slater, G.J.; Sygo, J.; Jorgensen, M. SPRINTING… Dietary Approaches to Optimize Training Adaptation and Performance. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 85–94. [Google Scholar] [CrossRef]
- Close, G.L.; Sale, C.; Baar, K.; Bermon, S. Nutrition for the Prevention and Treatment of Injuries in Track and Field Athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Heydenreich, J.; Melzer, K.; Flury, C.; Kayser, B. Low Energy Turnover of Physically Inactive Participants as a Determinant of Insufficient Mineral and Vitamin Intake in NHANES. Nutrients 2017, 9, 754. [Google Scholar] [CrossRef]
- Hannon, M.P.; Flueck, J.L.; Gremeaux, V.; Place, N.; Kayser, B.; Donnelly, C. Key Nutritional Considerations for Youth Winter Sports Athletes to Optimize Growth, Maturation and Sporting Development. Front. Sports Act. Living 2021, 3. [Google Scholar] [CrossRef]
- Volpe, S.L. Micronutrient Requirements for Athletes. Clin. Sports Med. 2007, 26, 119–130. [Google Scholar] [CrossRef]
- Maughan, R.J.; Noakes, T.D. Fluid replacement and exercise stress. A brief review of studies on fluid replacement and some guidelines for the athlete. Sports Med. 1991, 12, 16–31. [Google Scholar] [CrossRef]
- Baker, L.B.; De Chavez, P.J.D.; Ungaro, C.T.; Sopeña, B.C.; Nuccio, R.P.; Reimel, A.J.; Barnes, K.A. Exercise intensity effects on total sweat electrolyte losses and regional vs. whole-body sweat [Na+], [Cl−], and [K+]. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 361–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godek, S.F.; Peduzzi, C.; Burkholder, R.; Condon, S.; Dorshimer, G.; Bartolozzi, A.R. Sweat Rates, Sweat Sodium Concentrations, and Sodium Losses in 3 Groups of Professional Football Players. J. Athl. Train. 2010, 45, 364–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [Green Version]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations Between Body Mass Index and the Prevalence of Low Micronutrient Levels Among US Adults. MedGenMed Medscape Gen. Med. 2006, 8, 59. [Google Scholar] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; McCabe, G.P.; McCabe, L.D.; Peacock, M.; Weaver, C.M. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: A randomised controlled trial using dual stable isotopic tracers. Br. J. Nutr. 2014, 112, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; A Story, J.; Macdonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble Corn Fiber Increases Calcium Absorption Associated with Shifts in the Gut Microbiome: A Randomized Dose-Response Trial in Free-Living Pubertal Females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Aljewicz, M.; Siemianowska, E.; Cichosz, G.; Tońska, E. The effect of probiotics (Lactobacillus rhamnosus HN001, Lactobacillus paracasei LPC-37, and Lactobacillus acidophilus NCFM) on the availability of minerals from Dutch-type cheese. J. Dairy Sci. 2014, 97, 4824–4831. [Google Scholar] [CrossRef] [Green Version]
- Bergillos-Meca, T.; Cabrera-Vique, C.; Artacho, R.; Moreno-Montoro, M.; Navarro-Alarcón, M.; Olalla, M.; Giménez, R.; Seiquer, I.; Ruiz-López, M.D. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats’ milk? Food Chem. 2015, 187, 314–321. [Google Scholar] [CrossRef]
- Skrypnik, K.; Suliburska, J. Association between the gut microbiota and mineral metabolism. J. Sci. Food Agric. 2018, 98, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Lidbeck, A.; E Nord, C.; Gustafsson, J.-A.; Rafter, J. Lactobacilli, anticarcinogenic activities and human intestinal microflora. Eur. J. Cancer Prev. 1992, 1, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Azagra-Boronat, I.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Gut Health-Promoting Benefits of a Dietary Supplement of Vitamins with Inulin and Acacia Fibers in Rats. Nutrients 2020, 12, 2196. [Google Scholar] [CrossRef] [PubMed]
- Krausova, G.; Kana, A.; Vecka, M.; Hyrslova, I.; Stankova, B.; Kantorova, V.; Mrvikova, I.; Huttl, M.; Malinska, H. In Vivo Bioavailability of Selenium in Selenium-Enriched Streptococcus thermophilus and Enterococcus faecium in CD IGS Rats. Antioxidants 2021, 10, 463. [Google Scholar] [CrossRef]
- Malyar, R.M.; Li, H.; Enayatullah, H.; Hou, L.; Farid, R.A.; Liu, D. Zinc-enriched probiotics enhanced growth performance, antioxidant status, immune function, gene expression, and morphological characteristics of Wistar rats raised under high ambient temperature. 3 Biotech. 2019, 9, 291. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, H.; Qi, Y.; Wu, C.; Zhang, J.; Shao, L.; Tan, J.; Chen, D. Absorption and Distribution of Selenium Following Oral Administration of Selenium-Enriched Bifidobacterium longum DD98, Selenized Yeast, or Sodium Selenite in Rats. Biol. Trace Element Res. 2019, 197, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, H.; Karczemna, A.; Włodarek, D. Nutrition for Female Soccer Players—Recommendations. Medicina 2020, 56, 28. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Berges, G.; Matute-Llorente, Á.; González-Agüero, A.; Gómez-Bruton, A.; Gómez-Cabello, A.; Vicente-Rodríguez, G.; Casajús, J.A. Soccer helps build strong bones during growth: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2017, 177, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.; Marques, E.; Brito, J.; Krustrup, P.; Abreu, S.; Oliveira, J.; Rego, C.; Mota, J.; Rebelo, A. Muscle strength and soccer practice as major determinants of bone mineral density in adolescents. Jt. Bone Spine 2012, 79, 403–408. [Google Scholar] [CrossRef]
- Goolsby, M.A.; Boniquit, N. Bone Health in Athletes. Sports Health Multidiscip. Approach 2017, 9, 108–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thein-Nissenbaum, J.; Hammer, E. Treatment strategies for the female athlete triad in the adolescent athlete: Current perspectives. Open Access J. Sports Med. 2017, 8, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Sale, C.; Elliott-Sale, K.J. Nutrition and Athlete Bone Health. Sports Med. 2019, 49, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorgiou, M.; Dolan, E.; Elliott-Sale, K.J.; Sale, C. Reduced energy availability: Implications for bone health in physically active populations. Eur. J. Nutr. 2018, 57, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Elliott-Sale, K.J.; Parsons, A.; Tang, J.C.; Greeves, J.P.; Fraser, W.D.; Sale, C. Effects of reduced energy availability on bone metabolism in women and men. Bone 2017, 105, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Diaz de Barboza, G.; Guizzardi, S.; Tolosa de Talamoni, N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Peng, J.-B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.; Kozak, J.A.; Putney, J.W. TRPV5 and TRPV6 Calcium-Selective Channels. In Calcium Entry Channels in Non-Excitable Cells; Informa UK Limited: London, UK, 2017; pp. 241–274. [Google Scholar]
- Liao, Q.-S.; Du, Q.; Lou, J.; Xu, J.-Y.; Xie, R. Roles of Na+/Ca2+ exchanger 1 in digestive system physiology and pathophysiology. World J. Gastroenterol. 2019, 25, 287–299. [Google Scholar] [CrossRef]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef]
- Weaver, C.M. Diet, Gut Microbiome, and Bone Health. Curr. Osteoporos. Rep. 2015, 13, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Trinidad, T.P.; Wolever, T.M.; Thompson, L.U. Effect of acetate and propionate on calcium absorption from the rectum and distal colon of humans. Am. J. Clin. Nutr. 1996, 63, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Cashman, K. Prebiotics and calcium bioavailability. Curr. Issues Intest. Microbiol. 2003, 4, 21–32. [Google Scholar] [PubMed]
- Chaplin, A.; Parra, P.; Laraichi, S.; Serra, F.; Palou, A. Calcium supplementation modulates gut microbiota in a prebiotic manner in dietary obese mice. Mol. Nutr. Food Res. 2015, 60, 468–480. [Google Scholar] [CrossRef]
- Salque, M.; Bogucki, P.I.; Pyzel, J.; Sobkowiak-Tabaka, I.; Grygiel, R.; Szmyt, M.; Evershed, R.P. Earliest evidence for cheese making in the sixth millennium bc in northern Europe. Nat. Cell Biol. 2012, 493, 522–525. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Effects of Fermented Milk Products on Bone. Calcif. Tissue Int. 2018, 102, 489–500. [Google Scholar] [CrossRef]
- Rizzoli, R.; Biver, E. Are Probiotics the New Calcium and Vitamin D for Bone Health? Curr. Osteoporos. Rep. 2020, 18, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.; Molloy, A.M.; McNulty, H.; Ward, M.; McCarroll, K.; Hoey, L.; Hughes, C.F.; Cunningham, C.; Strain, J.J.; Casey, M.C. Greater yogurt consumption is associated with increased bone mineral density and physical function in older adults. Osteoporos. Int. 2017, 28, 2409–2419. [Google Scholar] [CrossRef]
- Biver, E.; Durosier-Izart, C.; Merminod, F.; Chevalley, T.; Van Rietbergen, B.; Ferrari, S.; Rizzoli, R. Fermented dairy products consumption is associated with attenuated cortical bone loss independently of total calcium, protein, and energy intakes in healthy postmenopausal women. Osteoporos. Int. 2018, 29, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Tucker, K.; Kiel, D.; Quach, L.; Casey, V.A.; Hannan, M.T. Milk and yogurt consumption are linked with higher bone mineral density but not with hip fracture: The Framingham Offspring Study. Arch. Osteoporos. 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Burton, K.J.; Rosikiewicz, M.; Pimentel, G.; Bütikofer, U.; Von Ah, U.; Voirol, M.-J.; Croxatto, A.; Aeby, S.; Drai, J.; McTernan, P.G.; et al. Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. Br. J. Nutr. 2017, 117, 1312–1322. [Google Scholar] [CrossRef] [Green Version]
- García-Albiach, R.; José, M.; De Felipe, M.J.P.; Angulo, S.; Morosini, M.-I.; Bravo, D.; Baquero, F.; Del Campo, R. Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am. J. Clin. Nutr. 2008, 87, 91–96. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Prabu, S.M.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Gilman, J.; Cashman, K.D. The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like Caco-2 cells. Curr. issues Intest. Microbiol. 2006, 7, 1–5. [Google Scholar] [PubMed]
- Terashima, A.; Takayanagi, H. Overview of Osteoimmunology. Calcif. Tissue Int. 2018, 102, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk; The National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.; Monteiro, C.P.; Vasco, A.M.; Baptista, F.; Sardinha, L.; Laires, M.J.; Silva, A. Magnesium intake mediates the association between bone mineral density and lean soft tissue in elite swimmers. Magnes. Res. 2012, 25, 120–125. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, E.F.; Da Costa, T.H.M.; Nogueira, J.A.D.; Vivaldi, L.J. Assessment of nutrient and water intake among adolescents from sports federations in the Federal District, Brazil. Br. J. Nutr. 2008, 99, 1275–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalcman, I.; Guarita, H.V.; Juzwiak, C.R.; Crispim, C.A.; Antunes, H.K.M.; Edwards, B.; Tufik, S.; de Mello, M.T. Nutritional status of adventure racers. Nutrition 2007, 23, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Juzwiak, C.R.; Amancio, O.M.S.; Vitalle, M.S.S.; Pinheiro, M.M.; Szejnfeld, V.L. Body composition and nutritional profile of male adolescent tennis players. J. Sports Sci. 2008, 26, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Wierniuk, A.; Włodarek, D. Estimation of energy and nutritional intake of young men practicing aerobic sports. Roczniki Państwowego Zakładu Higieny 2013, 64, 143–148. [Google Scholar]
- Silva, M.-R.G.; Paiva, T. Low energy availability and low body fat of female gymnasts before an international competition. Eur. J. Sport Sci. 2014, 15, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar] [PubMed]
- Wang, R.; Chen, C.; Liu, W.; Zhou, T.; Xun, P.; He, K.; Chen, P. The effect of magnesium supplementation on muscle fitness: A meta-analysis and systematic review. Magnes. Res. 2017, 30, 120–132. [Google Scholar] [CrossRef]
- Mittermeier, L.; Demirkhanyan, L.; Stadlbauer, B.; Breit, A.; Recordati, C.; Hilgendorff, A.; Matsushita, M.; Braun, A.; Simmons, D.G.; Zakharian, E.; et al. TRPM7 is the central gatekeeper of intestinal mineral absorption essential for postnatal survival. Proc. Natl. Acad. Sci. USA 2019, 116, 4706–4715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luongo, F.; Pietropaolo, G.; Gautier, M.; Dhennin-Duthille, I.; Ouadid-Ahidouch, H.; Wolf, F.I.; Trapani, V. TRPM6 is Essential for Magnesium Uptake and Epithelial Cell Function in the Colon. Nutrients 2018, 10, 784. [Google Scholar] [CrossRef] [Green Version]
- Funato, Y.; Yamazaki, D.; Mizukami, S.; Du, L.; Kikuchi, K.; Miki, H. Membrane protein CNNM4–dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014, 124, 5398–5410. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ Extrusion via CNNM4 Mediates Transcellular Mg2+ Transport across Epithelia: A Mouse Model. PLoS Genet. 2013, 9, e1003983. [Google Scholar] [CrossRef] [Green Version]
- Kolisek, M.; Sponder, G.; Pilchova, I.; Cibulka, M.; Tatarkova, Z.; Werner, T. Magnesium Extravaganza: A Critical Compendium of Current Research into Cellular Mg(2+) Transporters Other than TRPM6/7. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2019; Volume 176, pp. 65–105. [Google Scholar]
- Pyndt Jørgensen, B.; Winther, G.; Kihl, P.; Nielsen, D.S.; Wegener, G.; Hansen, A.K. Dietary magnesium deficiency affects gut microbiota and anxiety-like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015, 27, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.M.; Ederveen, T.; van der Wijst, J.; Bos, C.; Kortman, G.A.M.; Boekhorst, J.; Bindels, R.J.M.; de Baaij, J.; Hoenderop, J.G.J. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI-induced hypomagnesemia. FASEB J. 2019, 33, 11235–11246. [Google Scholar] [CrossRef] [Green Version]
- Zimowska, W.; Girardeau, J.P.; Kuryszko, J.; Bayle, D.; Rayssiguier, Y.; Mazur, A.; Zimowska, W.; Girardeau, J.P.; Kuryszko, J.; Bayle, D.; et al. Morphological and immune response alterations in the intestinal mucosa of the mouse after short periods on a low-magnesium diet. Br. J. Nutr. 2002, 88, 515–522. [Google Scholar] [CrossRef] [PubMed]
- García-Legorreta, A.; Soriano-Pérez, L.A.; Flores-Buendía, A.M.; Medina-Campos, O.N.; Noriega, L.G.; Granados-Portillo, O.; Nambo-Venegas, R.; Tovar, A.R.; Mendoza-Vargas, A.; Barrera-Oviedo, D.; et al. Effect of Dietary Magnesium Content on Intestinal Microbiota of Rats. Nutrients 2020, 12, 2889. [Google Scholar] [CrossRef]
- Kubota, M.; Ito, K.; Tomimoto, K.; Kanazaki, M.; Tsukiyama, K.; Kubota, A.; Kuroki, H.; Fujita, M.; Vandenplas, Y. Lactobacillus reuteri DSM 17938 and Magnesium Oxide in Children with Functional Chronic Constipation: A Double-Blind and Randomized Clinical Trial. Nutrients 2020, 12, 225. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekha, C.R.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Piuri, G.; Zocchi, M.; Della Porta, M.; Ficara, V.; Manoni, M.; Zuccotti, G.V.; Pinotti, L.; Maier, J.A.; Cazzola, R. Magnesium in Obesity, Metabolic Syndrome, and Type 2 Diabetes. Nutrients 2021, 13, 320. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Dahlerup, J.; Lindgren, S.; Moum, B. Iron deficiency and iron deficiency anemia are global health problems. Lakartidningen 2015, 112. [Google Scholar] [PubMed]
- Yilmaz, B.; Li, H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, N. The iron transporter DMT1. Int. J. Biochem. Cell Biol. 1999, 31, 991–994. [Google Scholar] [CrossRef]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta BBA Bioenerg. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, N.U.; I Cercamondi, C.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef]
- Olivares, M.; Pizarro, F.; De Romaña, D.L. Effect of Zinc Sulfate Fortificant on Iron Absorption from Low Extraction Wheat Flour Co-Fortified with Ferrous Sulfate. Biol. Trace Element Res. 2012, 151, 471–475. [Google Scholar] [CrossRef]
- Auerbach, M.; Schrier, S. Treatment of iron deficiency is getting trendy. Lancet Haematol. 2017, 4, e500–e501. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef] [Green Version]
- Kortman, G.A.M.; Reijnders, D.; Swinkels, D.W. Oral iron supplementation: Potential implications for the gut microbiome and metabolome in patients with CKD. Hemodial. Int. 2017, 21, S28–S36. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2016, 66, 863–871. [Google Scholar] [CrossRef]
- Ng, O. Iron, microbiota and colorectal cancer. Wien. Med. Wochenschr. 2016, 166, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Mary, R.R.; Chittaranjan, S.; Jancy, H.; Devi, R.S.; Ramakrishna, B. Low levels of faecal lactobacilli in women with iron-deficiency anaemia in south India. Br. J. Nutr. 2010, 104, 931–934. [Google Scholar] [CrossRef] [Green Version]
- Bouglé, D.; Vaghefi-Vaezzadeh, N.; Roland, N.; Bouvard, G.; Arhan, P.; Bureau, F.; Neuville, D.; Maubois, J.-L. Influence of Short-Chain Fatty Acids on Iron Absorption by Proximal Colon. Scand. J. Gastroenterol. 2002, 37, 1008–1011. [Google Scholar] [CrossRef]
- Das, N.K.; Schwartz, A.J.; Barthel, G.; Inohara, N.; Liu, Q.; Sankar, A.; Hill, D.R.; Ma, X.; Lamberg, O.; Schnizlein, M.K.; et al. Microbial Metabolite Signaling Is Required for Systemic Iron Homeostasis. Cell Metab. 2020, 31, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A. An Introduction to the Traditional Fermented Foods and Beverages of Turkey. Crit. Rev. Food Sci. Nutr. 2011, 51, 248–260. [Google Scholar] [CrossRef]
- Blandino, A.; Al-Aseeri, M.; Pandiella, S.; Cantero, D.; Webb, C. Cereal-based fermented foods and beverages. Food Res. Int. 2003, 36, 527–543. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Bioavailability of iron, zinc, phytate and phytase activity during soaking and germination of white sorghum varieties. PLoS ONE 2011, 6, e25512. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637–652.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, X.; Chen, X.; Yin, N.; Du, H.; Sun., G.; Wang, L. Estimation of the bioaccessibility and bioavailability of Fe, Mn, Cu, and Zn in Chinese vegetables using the in vitro digestion/Caco-2 cell model: The influence of gut microbiota. Food Funct. 2017, 8, 4592–4600. [Google Scholar] [CrossRef]
- Verma, C.; Tapadia, K.; Soni, A.B. Determination of iron (III) in food, biological and environmental samples. Food Chem. 2017, 221, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.R.; Bae, D.-H.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Duodenal Cytochrome b (DCYTB) in Iron Metabolism: An Update on Function and Regulation. Nutrients 2015, 7, 2274–2296. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Zhang, Z.; Cai, X.; Du, H.; Sun, G.; Cui, Y. In Vitro Method To Assess Soil Arsenic Metabolism by Human Gut Microbiota: Arsenic Speciation and Distribution. Environ. Sci. Technol. 2015, 49, 10675–10681. [Google Scholar] [CrossRef]
- Luu, Y.-S.; Ramsay, J.A. Review: Microbial mechanisms of accessing insoluble Fe(III) as an energy source. World J. Microbiol. Biotechnol. 2003, 19, 215–225. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; Hemphill, N.O.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Camacho, J.D.; Vicente-García, C.; Parsons, D.S.; Navas-Enamorado, I. Zinc at the crossroads of exercise and proteostasis. Redox Biol. 2020, 35, 101529. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Yan, G.; Guan, M. Zinc Homeostasis in Bone: Zinc Transporters and Bone Diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef] [Green Version]
- Jurowski, K.; Szewczyk, B.; Nowak, G.; Piekoszewski, W. Biological consequences of zinc deficiency in the pathomechanisms of selected diseases. J. Biol. Inorg. Chem. JBIC 2014, 19, 1069–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, M. Immunological aspects of sport nutrition. Immunol. Cell Biol. 2015, 94, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Micheletti, A.; Rossi, R.; Rufini, S. Zinc status in athletes: Relation to diet and exercise. Sports Med. 2001, 31, 577–582. [Google Scholar] [CrossRef]
- Baranauskas, M.; Stukas, R.; Tubelis, L.; Žagminas, K.; Šurkienė, G.; Švedas, E.; Giedraitis, V.R.; Dobrovolskij, V.; Abaravičius, J.A. Nutritional habits among high-performance endurance athletes. Medicina 2015, 51, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Economos, C.D.; Bortz, S.S.; Nelson, M.E. Nutritional Practices of Elite Athletes. Sports Med. 1993, 16, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Schüpbach, R.; Wegmuller, R.; Berguerand, C.; Bui, M.; Herteraeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur. J. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Nebl, J.; Schuchardt, J.P.; Ströhle, A.; Wasserfurth, P.; Haufe, S.; Eigendorf, J.; Tegtbur, U.; Hahn, A. Micronutrient Status of Recreational Runners with Vegetarian or Non-Vegetarian Dietary Patterns. Nutrients 2019, 11, 1146. [Google Scholar] [CrossRef] [Green Version]
- Heffernan, S.M.; Horner, K.; De Vito, G.; Conway, G.E. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef] [Green Version]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Sodunke, T.E.; Abubakar, A.R.; Jahan, I.; Sharma, P.; Islam, S.; Dutta, S.; Haque, M. The Implications of Zinc Therapy in Combating the COVID-19 Global Pandemic. J. Inflamm. Res. 2021, 14, 527–550. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Absorption Mechanisms of Iron, Copper, and Zinc: An Overview. J. Nutr. Sci. Vitaminol. 2018, 64, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umair, M.; Alfadhel, M. Genetic Disorders Associated with Metal Metabolism. Cells 2019, 8, 1598. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, S.; Küry, S.; Giraud, M.; Dréno, B.; Kharfi, M.; Bézieau, S. An update on mutations of theSLC39A4gene in acrodermatitis enteropathica. Hum. Mutat. 2009, 30, 926–933. [Google Scholar] [CrossRef]
- Shusterman, E.; Beharier, O.; Shiri, L.; Zarivach, R.; Etzion, Y.; Campbell, C.R.; Lee, I.-H.; Okabayashi, K.; Dinudom, A.; Cook, D.I.; et al. ZnT-1 extrudes zinc from mammalian cells functioning as a Zn2+/H+exchanger. Metallomics 2014, 6, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, J.; Starchl, C.; Berisha, A.T.; Amrein, K. Thyroid-Gut-Axis: How Does the Microbiota Influence Thyroid Function? Nutrients 2020, 12, 1769. [Google Scholar] [CrossRef]
- Yin, N.; Cai, X.; Chen, X.; Du, H.; Xu, J.; Wang, L.; Sun, G.; Cui, Y. Investigation of bioaccessibility of Cu, Fe, Mn, and Zn in market vegetables in the colon using PBET combined with SHIME. Sci. Rep. 2017, 7, 17578. [Google Scholar] [CrossRef] [Green Version]
- Intawongse, M.; Dean, J.R. Use of the physiologically-based extraction test to assess the oral bioaccessibility of metals in vegetable plants grown in contaminated soil. Environ. Pollut. 2008, 152, 60–72. [Google Scholar] [CrossRef]
- Reed, S.; Knez, M.; Uzan, A.; Stangoulis, J.; Glahn, R.P.; Koren, O.; Tako, E. Alterations in the Gut (Gallus gallus) Microbiota Following the Consumption of Zinc Biofortified Wheat (Triticum aestivum)-Based Diet. J. Agric. Food Chem. 2018, 66, 6291–6299. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency During Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef]
- Celis, A.I.; Relman, D.A. Competitors versus Collaborators: Micronutrient Processing by Pathogenic and Commensal Human-Associated Gut Bacteria. Mol. Cell 2020, 78, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Calame, W.; Weseler, A.R.; Viebke, C.; Flynn, C.; Siemensma, A.D. Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br. J. Nutr. 2008, 100, 1269–1275. [Google Scholar] [CrossRef]
- Zackular, J.P.; Skaar, E.P. The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microbes. 2018, 9, 469–476. [Google Scholar] [CrossRef]
- Kang, S.; Li, R.; Jin, H.; You, H.J.; Ji, G.E. Effects of Selenium- and Zinc-Enriched Lactobacillus plantarum SeZi on Antioxidant Capacities and Gut Microbiome in an ICR Mouse Model. Antioxidants 2020, 9, 1028. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooij, B.; Mensink, M.; Witkamp, R.; De Vries, J. Micronutrient Intakes in 553 Dutch Elite and Sub-Elite Athletes: Prevalence of Low and High Intakes in Users and Non-Users of Nutritional Supplements. Nutrients 2017, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gøbel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- McSwiney, F.; Doyle, L. Low-Carbohydrate Ketogenic Diets in Male Endurance Athletes Demonstrate Different Micronutrient Contents and Changes in Corpuscular Haemoglobin over 12 Weeks. Sports 2019, 7, 201. [Google Scholar] [CrossRef] [Green Version]
- Tessier, F.; Margaritis, I.; Richard, M.J.; Moynot, C.; Marconnet, P. Selenium and training effects on the glutathione system and aerobic performance. Med. Sci. Sports Exerc. 1995, 27, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, I.; Tessier, F.; Prou, E.; Marconnet, P.; Marini, J.-F. Effects of Endurance Training on Skeletal Muscle Oxidative Capacities with and without Selenium Supplementation. J. Trace Elements Med. Biol. 1997, 11, 37–43. [Google Scholar] [CrossRef]
- Shafiei Neek, L.; Gaeini, A.A.; Choobineh, S. Effect of zinc and selenium supplementation on serum testosterone and plasma lactate in cyclist after an exhaustive exercise bout. Biol. Trace Elem. Res. 2011, 144, 454–462. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Córdova Martínez, A.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764. [Google Scholar] [CrossRef]
- Hrdina, J.; Banning, A.; Kipp, A.; Loh, G.; Blaut, M.; Brigelius-Flohé, R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J. Nutr. Biochem. 2009, 20, 638–648. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Effect of gut microflora on nutritional availability of selenium. Food Chem. 2020, 319, 126537. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.; Qi, Y.; Ji, R.; Zhang, J.; Qian, Z.; Wu, C.; Tan, J.; Shao, L.; Chen, D. Preparation and characterization of selenium enriched-Bifidobacterium longum DD98, and its repairing effects on antibiotic-induced intestinal dysbacteriosis in mice. Food Funct. 2019, 10, 4975–4984. [Google Scholar] [CrossRef]
- Wastney, M.E.; Combs, G.F.; Canfield, W.K.; Taylor, P.R.; Patterson, K.Y.; Hill, A.D.; E Moler, J.; Patterson, B.H. A Human Model of Selenium that Integrates Metabolism from Selenite and Selenomethionine. J. Nutr. 2011, 141, 708–717. [Google Scholar] [CrossRef] [Green Version]
- Mogna, L.; Nicola, S.; Pane, M.; Lorenzini, P.; Strozzi, G.; Mogna, G. Selenium and zinc internalized by Lactobacillus buchneri Lb26 (DSM 16341) and Bifidobacterium lactis Bb1 (DSM 17850): Improved bioavailability using a new biological approach. J. Clin. Gastroenterol. 2012, 46, S41–S45. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. https://doi.org/10.3390/ijms22136803
Bielik V, Kolisek M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. International Journal of Molecular Sciences. 2021; 22(13):6803. https://doi.org/10.3390/ijms22136803
Chicago/Turabian StyleBielik, Viktor, and Martin Kolisek. 2021. "Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome" International Journal of Molecular Sciences 22, no. 13: 6803. https://doi.org/10.3390/ijms22136803
APA StyleBielik, V., & Kolisek, M. (2021). Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. International Journal of Molecular Sciences, 22(13), 6803. https://doi.org/10.3390/ijms22136803