Elucidating the Mechanism of Action of the Attributed Immunomodulatory Role of Eltrombopag in Primary Immune Thrombocytopenia: An In Silico Approach
Abstract
1. Introduction
2. Results
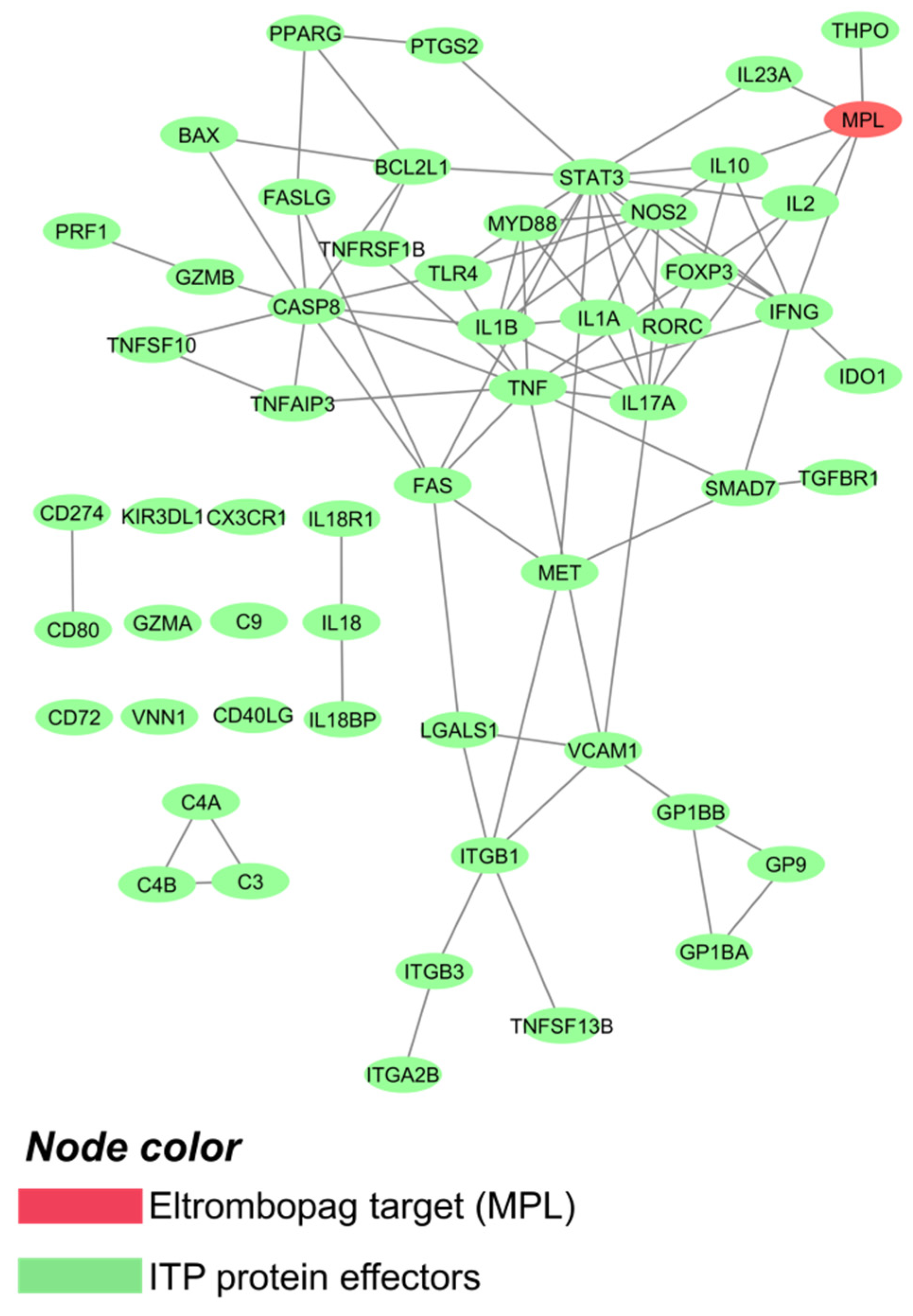
2.1. Primary Immune Thrombocytopenia Interactome
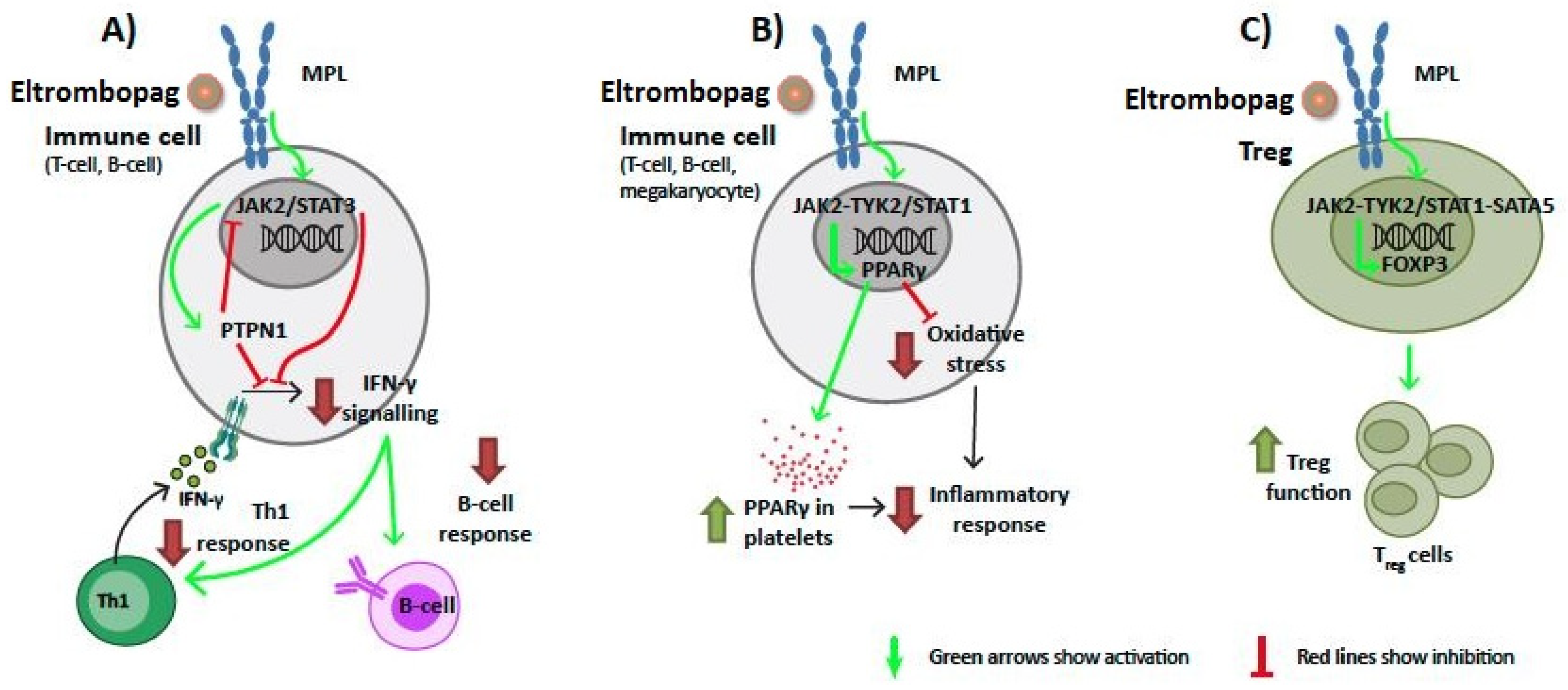
2.2. Eltrombopag Could Increase TGF-β Expression through MPL Signaling
2.3. Signaling of Eltrombopag through MPL on Immune Cells Could Affect IFN-γ, PPARγ, and FOXP3 Function
2.4. Eltrombopag Could Interact with the BCL2 Protein Family
3. Discussion
4. Materials and Methods
4.1. TPMS Technology: Systems Biology-Based Model Creation
4.2. Human Biological Network and Molecular Definition of Clinical Concepts
4.3. Human Physiological Rules and Mechanism Of Action Models
4.4. Chemical Similarity
4.5. Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Garrido, T. Advances in the pathophysiology of primary immune thrombocytopenia. Hematology 2017, 22, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Porras, J.R.; Bastida, J. Eltrombopag in immune thrombocytopenia: Efficacy review and update on drug safety. Adv. Drug Saf. 2018, 9, 263–285. [Google Scholar] [CrossRef]
- Samson, M.; Fraser, W.; Lebowitz, D. Treatments for Primary Immune Thrombocytopenia: A Review. Cureus 2019, 11, e5849. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef]
- Kuter, D. New thrombopoietic growth factors. Blood 2007, 109, 4607–4616. [Google Scholar] [CrossRef]
- European Medicines Agency. Revolade, INN Eltrombopag—Product information. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/revolade (accessed on 24 June 2021).
- De Graaf, C.A.; Metcalf, D. Thrombopoietin and hematopoietic stem cells. Cell Cycle 2011, 10, 1582–1589. [Google Scholar] [CrossRef]
- Leven, E.; Miller, A.; Boulad, N.; Haider, A.; Bussel, J.B. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012, 120, 1085. [Google Scholar] [CrossRef]
- Bussel, J.B.; Mahmud, S.N.; Brigstocke, S.L.; Torneten, S.M. Tapering eltrombopag in patients with chronic ITP: How successful is this and in whom does it work? Blood. 2015, 126, 1054. [Google Scholar] [CrossRef]
- Mahévas, M.; Fain, O.; Ebbo, M.; Roudot-Thoraval, F.; Limal, N.; Khellaf, M.; Schleinitz, N.; Bierling, P.; Languille, L.; Godeau, B.; et al. The temporary use of thrombopoietin-receptor agonists may induce a prolonged remission in adult chronic immune thrombocytopenia. Results of a French observational study. Br. J. Haematol. 2014, 165, 865–869. [Google Scholar] [CrossRef] [PubMed]
- González-López, T.J.; Pascual, C.; Álvarez-Román, M.T.; Fernández-Fuertes, F.; Sánchez-González, B.; Caparrós, I.; Jarque, I.; Mingot-Castellano, M.E.; Hernández-Rivas, J.A.; Martín-Salces, M.; et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am. J. Hematol. 2015, 90, E40–E43. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Wang, L.; Wang, J.Y.; Li, A. Successful discontinuation of eltrombopag in one child with refractory primary immune thrombocytopenia and literature review. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2019, 30, 71–74. [Google Scholar] [CrossRef]
- Červinek, L.; Mayer, J.; Doubek, M. Sustained remission of chronic immune thrombocytopenia after discontinuation of treatment with thrombopoietin-receptor agonists in adults. Int. J. Hematol. 2015, 102, 7–11. [Google Scholar] [CrossRef]
- Biagiotti, C.; Carrai, V.; Bacchiarri, F.; Di Gioia, M.; Raugei, G.; Bosi, A. Persisitent Remission of Chronic Immune Thrombocytopenia After Thrombopoieting Mimetics Discontinuation. Haematol Conf Publ. 2015, 100, P175. [Google Scholar]
- Mazza, P.; Minoia, C.; Melpignano, A.; Polimeno, G.; Cascavilla, N.; Di Renzo, N.; Specchia, G. The use of thrombopoietin-receptor agonists (TPO-RAs) in immune thrombocytopenia (ITP): A "real life" retrospective multicenter experience of the Rete Ematologica Pugliese (REP). Ann. Hematol. 2016, 95, 239–244. [Google Scholar] [CrossRef]
- Ghadaki, B.; Nazi, I.; Kelton, J.G.; Arnold, D. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion 2013, 53, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- González-López, T.J.; Sánchez-González, B.; Pascual, C.; Arefi, M.; de Cabo, E.; Alonso, A.; Martín-Salces, M.; Jiménez-Bárcenas, R.; Calbacho, M.; Galan, P.; et al. Sustained response after discontinuation of short-and medium-term treatment with eltrombopag in patients with immune thrombocytopenia. Platelets 2015, 26, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.D.; Routledge, D.J.M.; Kruse, A.; Connor, P.; Sibson, K.; Biss, T.T.; Bussel, J. Thrombopoietin Receptor Agonists in Paediatric ITP Patients: Long Term Follow up Data in 34 Patients. Blood. 2014, 124, 4206. [Google Scholar] [CrossRef]
- Santoro, C.; Volpicelli, P.; Baldacci, E.; Ferrara, G.; Di Rocco, A.; Ferretti, A.; Porrazzo, M.; Mazzucconi, M. Repeated successful use of eltrombopag in chronic primary immune thrombocytopenia: Description of an intriguing case. Clin. Case Rep. 2017, 5, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Etzl, M.M., Jr. Eltrombopag in long-term management of pediatric thrombocytopenia. Clin. Case Rep. 2019, 7, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Wang, X.; Lopez, A.; Eisen, M. Case study of remission in adults with immune thrombocytopenia following cessation of treatment with the thrombopoietin mimetic romiplostim. Hematol. 2016, 21, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, E.; Palandri, F.; Volpetti, S.; Vianelli, N.; Auteri, G.; Rossi, E.; Patriarca, A.; Carli, G.; Barcellini, W.; Celli, M.; et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: Results of a phase, II; multicentre, prospective study. Br. J. Haematol. 2021, 193, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bussel, J.B.; Heck, S.; He, W.; Karpoff, M.; Boulad, N.; Yazdanbakhsh, K. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010, 116, 4639–4645. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, H.; Bao, W.; Boulad, N.; Evangelista, J.; Haider, M.A.; Bussel, J.; Yazdanbakhsh, K. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012, 120, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T.; Numajiri, M.; Nakazaki, H.; Okazaki, Y.; Kuwana, M. Induction of immune tolerance to platelet antigen by short-term thrombopoietin treatment in a mouse model of immune thrombocytopenia. Int. J. Hematol. 2014, 100, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Miller, C.L.; Delorme, E.; Tian, S.S.; Hopson, C.B.; Landis, A.J.; Valoret, E.I.; Sellers, T.S.; Rosen, J.; Miller, S.G.; Luengo, J.I.; et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells 2009, 27, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Will, B.; Simkin, G.; Narayanagari, S.; Barreyro, L.; Bartholdy, B.; Tamari, R.; Mitsiades, C.S.; Verma, A.; Steidl, U. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood 2012, 120, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Raslova, H.; Vainchenker, W.; Plo, I. Eltrombopag, a potent stimulator of megakaryopoiesis. Haematologica 2016, 101, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Schifferli, A.; Kühne, T. Thrombopoietin receptor agonists: A new immune modulatory strategy in immune thrombocytopenia? Semin. Hematol. 2016, 53 (Suppl. 1), S31–S34. [Google Scholar] [CrossRef]
- Kao, Y.R.; Chen, J.; Narayanagari, S.R.; Todorova, T.I.; Aivalioti, M.M.; Ferreira, M.; Ramos, P.M.; Pallaud, C.; Mantzaris, I.; Shastri, A.; et al. Thrombopoietin receptor-independent stimulation of hematopoietic stem cells by eltrombopag. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Gao, W.; Chen, L.; Ma, Z.; Du, Z.; Zhao, Z.; Hu, Z.; Li, Q. Isolation and phenotypic characterization of colorectal cancer stem cells with organ-specific metastatic potential. Gastroenterology 2013, 145, 636–646.e5. [Google Scholar] [CrossRef]
- Wu, Z.; Wei, D.; Gao, W.; Xu, Y.; Hu, Z.; Ma, Z.; Gao, C.; Zhu, X.; Li, Q. TPO-Induced Metabolic Reprogramming Drives Liver Metastasis of Colorectal Cancer CD110+ Tumor-Initiating Cells. Cell Stem Cell 2015, 17, 47–59. [Google Scholar] [CrossRef]
- Neyaz, A.; Husain, N.; Gupta, S.; Kumari, S.; Arora, A.; Awasthi, N.P.; Malhotra, K.P.; Misra, S. Investigation of targetable predictive and prognostic markers in gallbladder carcinoma. J. Gastrointest. Oncol. 2018, 9, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ohuchida, K.; Zheng, B.; Okumura, T.; Takesue, S.; Nakayama, H.; Iwamoto, C.; Shindo, K.; Moriyama, T.; Nakata, K.; et al. CD110 promotes pancreatic cancer progression and its expression is correlated with poor prognosis. J. Cancer Res. Clin. Oncol. 2019, 145, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, B.J.; Singh, D.; Madrigal, A.; Valdovino-Gonzalez, A.G.; White, B.M.; Zapardiel-Gonzalo, J.; Ha, B.; Altay, G.; Greenbaum, J.A.; McVicker, G.; et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 2018, 175, 1701–1715.e16. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Lee, B.; Xu, W.; Mustafah, S.; Hwang, Y.Y.; Carré, C.; Burdin, N.; Visan, L.; Ceccarelli, M.; Poidinger, M.; et al. RNA-seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 2019, 26, 1627–1640.e7. [Google Scholar] [CrossRef]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef]
- Lorén, V.; Garcia-Jaraquemada, A.; Naves, J.E.; Carmona, X.; Mañosa, M.; Aransay, A.M.; Lavin, J.L.; Sánchez, I.; Cabré, E.; Manyé, J.; et al. ANP32E, a protein involved in steroid-refractoriness in ulcerative colitis, identified by a systems biology approach. J. Crohn’s Colitis 2019, 13, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.D.; Kumbale, C.M.; Zhang, Q.; Voit, E. Dynamical systems approaches to personalized medicine. Curr. Opin. Biotechnol. 2019, 58, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.; Heinzel, A.; Lukas, A.; Perco, P. Predictive biomarkers for linking disease pathology and drug effect. Curr. Pharm. Des. 2017, 23, 29–54. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Perez-Palacios, R.; Castillo, S.; Segú, C.; Sardón, T.; Mas, J.M.; Martín, A.C.; Arroyo, R. Biological Relationships between miRNAs used for colorectal cancer screening. J. Mol. Biomark. Diagn. 2018, 9. [Google Scholar] [CrossRef]
- Velez, G.; Bassuk, A.G.; Colgan, D.; Tsang, S.H.; Mahajan, V. Therapeutic drug repositioning using personalized proteomics of liquid biopsies. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Romeo-Guitart, D.; Forés, J.; Herrando-Grabulosa, M.; Valls, R.; Leiva-Rodríguez, T.; Galea, E.; González-Pérez, F.; Navarro, X.; Petegnief, V.; Bosch, A.; et al. Neuroprotective drug for nerve trauma revealed using artificial intelligence. Sci. Rep. 2018, 8, 1879. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bryant, S.H.; Cheng, T.; Wang, J.; Gindulyte, A.; Shoemaker, B.A.; Thiessen, P.A.; He, S.; Zhang, J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017, 45, D955–D963. [Google Scholar] [CrossRef]
- Gaulton, A.; Hersey, A.; Nowotka, M.; Bento, A.P.; Chambers, J.; Mendez, D.; Mutowo, P.; Atkinson, F.; Bellis, L.J.; Cibrián-Uhalte, E.; et al. The ChEMBL database in 2017. Nucleic Acids Res. 2017, 45, D945–D954. [Google Scholar] [CrossRef]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. Binding DB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.; Liang, C.; Jung, J.U.; Oh, B. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011, 21, 627–641. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, M.; Wu, J.; Shi, Y. Structural and biochemical analysis of Bcl-2 interaction with the hepatitis, B. virus protein HBx. Proc. Natl. Acad. Sci. USA 2016, 113, 2074–2079. [Google Scholar] [CrossRef]
- Muchmore, S.W.; Sattler, M.; Liang, H.; Meadows, R.P.; Harlan, J.E.; Yoon, H.S.; Nettesheim, D.; Chang, B.S.; Thompson, C.B.; Wong, S.L.; et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 1996, 381, 335–341. [Google Scholar] [CrossRef]
- Manion, M.K.; O’Neill, J.W.; Giedt, C.D.; Kim, K.M.; Zhang, K.Y.; Hockenbery, D. Bcl-XL mutations suppress cellular sensitivity to antimycin A. J. Biol. Chem. 2004, 279, 2159–2165. [Google Scholar] [CrossRef]
- Gavathiotis, E.; Suzuki, M.; Davis, M.L.; Pitter, K.; Bird, G.H.; Katz, S.G.; Tu, H.C.; Kim, H.; Cheng, E.H.; Tjandra, N.; et al. BAX activation is initiated at a novel interaction site. Nature 2008, 455, 1076–1081. [Google Scholar] [CrossRef]
- Ma, J.; Edlich, F.; Bermejo, G.A.; Norris, K.L.; Youle, R.J.; Tjandra, N. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl. Acad. Sci. USA 2012, 109, 20901–20906. [Google Scholar] [CrossRef]
- Pikora, M.; Gieldon, A. RASMOL AB—New functionalities in the program for structure analysis. Acta Biochim. Pol. 2015, 62, 629–631. [Google Scholar] [CrossRef]
- Di Buduo, C.A.; Currao, M.; Pecci, A.; Kaplan, D.L.; Balduini, C.L.; Balduini, A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica 2016, 101, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Miller, C.L.; DeLorme, E.; Tian, S.S.; Hopson, C.B.; Stark, K.; Giampa, L.; Valoret, E.I.; Duffy, K.J.; Luengo, J.L.; Rosen, J.; et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp. Hematol. 2005, 33, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Abbonante, V.; Di Buduo, C.A.; Gruppi, C.; Malara, A.; Gianelli, U.; Celesti, G.; Anselmo, A.; Laghi, L.; Vercellino, M.; Visai, L.; et al. Thrombopoietin/TGF-β1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells 2016, 34, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Liu, H.; Ouyang, X.; Li, Y.; Zeng, H.; Wang, X.; Xie, S.; Nie, D.; Xiao, J.; Wei, J.; Wu, Y.; et al. Involvement of levels of Toll like receptor-4 in monocytes, CD4+ T-lymphocyte subsets, and cytokines in patients with immune thrombocytopenic purpura. Thromb. Res. 2013, 132, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, B.M.; Guo, X.; Xu, L.; You, X.; West, R.B.; Bussel, J.B.; Zehnder, J. Blood transcriptome and clonal T-cell correlates of response and non-response to eltrombopag therapy in a cohort of patients with chronic immune thrombocytopenia. Haematologica 2020, 105, e129–e32. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Nakanishi, T.; Yoshimura, H.; Hotta, M.; Nakamichi, N.; Tamaki, T.; Ishii, K.; Ito, T.; Nomura, S. TGFβ (1) and sCTLA-4 levels are increased in eltrombopag-exposed patients with ITP. Thromb. Res. 2012, 130, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Frasch, S.C.; Thomas, S.M.; Bratton, D.L.; Henson, P. Induction of TGF-β1 synthesis by macrophages in response to apoptotic cells requires activation of the scavenger receptor CD36. PLoS ONE 2013, 8, e72772. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Tissue expression of MPL—Summary—The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000117400-MPL/tissue (accessed on 10 October 2019).
- Liu, X.G.; Liu, S.; Feng, Q.; Liu, X.N.; Li, G.S.; Sheng, Z.; Chen, P.; Liu, Y.; Wei, Y.; Dong, X.Y.; et al. Thrombopoietin receptor agonists shift the balance of Fcγ receptors toward inhibitory receptor IIb on monocytes in ITP. Blood 2016, 128, 852–861. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Schifferli, J. Ectosomes as immunomodulators. Semin. Immunopathol. 2011, 33, 487–495. [Google Scholar] [CrossRef]
- Sadallah, S.; Amicarella, F.; Eken, C.; Iezzi, G.; Schifferli, J. Ectosomes released by platelets induce differentiation of CD4+T cells into, T. regulatory cells. Thromb. Haemost. 2014, 112, 1219–1229. [Google Scholar]
- Yu, J.; Heck, S.; Patel, V.; Levan, J.; Yu, Y.; Bussel, J.B.; Yazdanbakhsh, K. Defective circulating CD25 regulatory, T. cells in patients with chronic immune thrombocytopenic purpura. Blood 2008, 112, 1325–1328. [Google Scholar] [CrossRef]
- Ouaked, N.; Mantel, P.Y.; Bassin, C.; Burgler, S.; Siegmund, K.; Akdis, C.A.; Schmidt-Weber, C. Regulation of the foxp3 gene by the Th1 cytokines: The role of IL-27-induced STAT1. J. Immunol. 2009, 182, 1041–1049. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, H.; Yang, R.C.; Han, Z. Multi-dysfunctional pathophysiology in ITP. Crit. Rev. Oncol. Hematol. 2005, 54, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R. Pathophysiology and therapeutic options in primary immune thrombocytopenia. Blood Transfus. Trasfus. Sangue 2011, 9, 262–273. [Google Scholar]
- Zufferey, A.; Kapur, R.; Semple, J. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J. Clin. Med. 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- McMillan, R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin. Hematol. 2007, 44, S3–S11. [Google Scholar] [CrossRef]
- Olsson, B.; Andersson, P.O.; Jernås, M.; Jacobsson, S.; Carlsson, B.; Carlsson, L.M.; Wadenvik, H. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nat. Med. 2003, 9, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Wong, W.; Jeng, M.; Zehnder, J. Gene expression profile of idiopathic thrombocytopenic purpura (ITP). Pediatric Blood Cancer 2006, 47, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, L.J.; Huntsman, H.D.; Cheng, H.; Townsley, D.M.; Winkler, T.; Feng, X.; Dunbar, C.E.; Young, N.S.; Larochelle, A. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood 2019, 133, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lo, C.; Shen, L.; Sood, R.; Jones, C.; Cusmano-Ozog, K.; Park-Snyder, S.; Wong, W.; Jeng, M.; Cowan, T.; et al. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood 2011, 117, 4569–4579. [Google Scholar] [CrossRef]
- Wolchinsky, R.; Hod-Marco, M.; Oved, K.; Shen-Orr, S.S.; Bendall, S.C.; Nolan, G.P.; Reiter, Y. Antigen-dependent integration of opposing proximal TCR-signaling cascades determines the functional fate of T. lymphocytes. J. Immunol. 2014, 192, 2109–2119. [Google Scholar] [CrossRef]
- Jernås, M.; Nookaew, I.; Wadenvik, H.; Olsson, B. MicroRNA regulate immunological pathways in T-cells in immune thrombocytopenia (ITP). Blood 2013, 121, 2095–2098. [Google Scholar] [CrossRef] [PubMed]
- Akbiyik, F.; Ray, D.M.; Gettings, K.F.; Blumberg, N.; Francis, C.W.; Phipps, R. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood 2004, 104, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Lannan, K.L.; Sahler, J.; Kim, N.; Spinelli, S.L.; Maggirwar, S.B.; Garraud, O.; Cognasse, F.; Blumberg, N.; Phipps, R. Breaking the mold: Transcription factors in the anucleate platelet and platelet-derived microparticles. Front. Immunol. 2015, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Sahler, J.; Woeller, C.; Spinelli, S.; Blumberg, N.; Phipps, R. A novel method for overexpression of peroxisome proliferator-activated receptor-γ in megakaryocyte and platelet microparticles achieves transcellular signaling. J. Thromb. Haemost. Jth 2012, 10, 2563–2572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iijima, K.; Sugita, K.; Inukai, T.; Goi, K.; Tezuka, T.; Uno, K.; Sato, H.; Kagami, K.; Nakazawa, S. Expression of thrombopoietin receptor and its functional role in human B-precursor leukemia cells with 11q23 translocation or Philadelphia chromosome. Leukemia 2000, 14, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Vlachodimitropoulou Koumoutsea, E.; Nichola, C.; Psaila, B.; Sola-Visner, M.; Porter, J. Eltrombopag mobilizes intracellular iron stores at concentrations lower than those required with other clinically available iron chelators. Blood. 2014, 21, 1353. [Google Scholar] [CrossRef]
- Vlachodimitropoulou, E.; Chen, Y.L.; Garbowski, M.; Koonyosying, P.; Psaila, B.; Sola-Visner, M.; Cooper, N.; Hider, R.; Porter, J. Eltrombopag: A powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood 2017, 130, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Spitz, A.Z.; Zacharioudakis, E.; Reyna, D.E.; Garner, T.P.; Gavathiotis, E. Eltrombopag directly inhibits BAX and prevents cell death. Nat. Commun. 2021, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.C.; Dowling, M.R.; Lebois, M.; Kile, B. The regulation of platelet life span. In Platelets; Michelson, A.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 51–65. [Google Scholar] [CrossRef]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef]
- Mitchell, W.B.; Pinheiro, M.P.; Boulad, N.; Kaplan, D.; Edison, M.N.; Psaila, B.; Karpoff, M.; White, M.J.; Josefsson, E.C.; Kile, B.T.; et al. Effect of thrombopoietin receptor agonists on the apoptotic profile of platelets in patients with chronic immune thrombocytopenia. Am. J. Hematol. 2014, 89, E228–E234. [Google Scholar] [CrossRef]
- Deng, G.; Yu, S.; Li, Q.; He, Y.; Liang, W.; Yu, L.; Xu, D.; Sun, T.; Zhang, R.; Li, Q. Investigation of platelet apoptosis in adult patients with chronic immune thrombocytopenia. Hematology 2017, 22, 155–161. [Google Scholar] [CrossRef]
- Justo Sanz, R.; Monzón Manzano, E.; Fernández Bello, I.; Teresa Álvarez Román, M.; Martín Salces, M.; Rivas Pollmar, M.I.; Jiménez Yuste, V.; Butta, N.V. Platelet apoptosis and PAI-1 are involved in the pro-coagulant state of immune thrombocytopaenia patients treated with thrombopoietin receptor agonists. Thromb. Haemost. 2019, 119, 645–659. [Google Scholar] [CrossRef]
- Monzón Manzano, E.; Álvarez Román, M.T.; Justo Sanz, R.; Fernández Bello, I.; Hernández, D.; Martín Salces, M.; Valor, L.; Rivas Pollmar, I.; Butta, N.V.; Jiménez Yuste, V. Platelet and immune characteristics of immune thrombocytopaenia patients non-responsive to therapy reveal severe immune dysregulation. Br. J. Haematol. 2020, 189, 943–953. [Google Scholar] [CrossRef]
- Jorba, G.; Aguirre-Plans, J.; Junet, V.; Segú-Vergés, C.; Ruiz, J.L.; Pujol, A.; Fernández-Fuentes, N.; Mas, J.M.; Oliva, B. In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan. PLoS ONE 2020, 15, e0228926. [Google Scholar] [CrossRef]
- Segú-Vergés, C.; Coma, M.; Kessel, C.; Smeets, S.; Foell, D.; Aldea, A. Application of systems biology-based in silico tools to optimize treatment strategy identification in Still’s disease. Arthritis Res. Ther. 2021, 23, 126. [Google Scholar] [CrossRef]
- Carcereny, E.; Fernández-Nistal, A.; López, A.; Montoto, C.; Naves, A.; Segú-Vergés, C.; Coma, M.; Jorba, G.; Oliva, B.; Mas, J. Head to head evaluation of second generation ALK inhibitors brigatinib and alectinib as first-line treatment for ALK+ NSCLC using an in silico systems biology-based approach. Oncotarget 2021, 12, 316–332. [Google Scholar] [CrossRef]
- Iborra-Egea, O.; Gálvez-Montón, C.; Roura, S.; Perea-Gil, I.; Prat-Vidal, C.; Soler-Botija, C.; Bayes-Genis, A. Mechanisms of action of sacubitril/valsartan on cardiac remodeling: A systems biology approach. Npj. Syst. Biol. Appl. 2017, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, D1102–D1109. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, R.; Consonni, V.; Xiang, H.; Holliday, J.; Buscema, M.; Willett, P. Similarity coefficients for binary chemoinformatics data: Overview and extended comparison using simulated and real data sets. J. Chem. Inf. Modeling 2012, 52, 2884–2901. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]

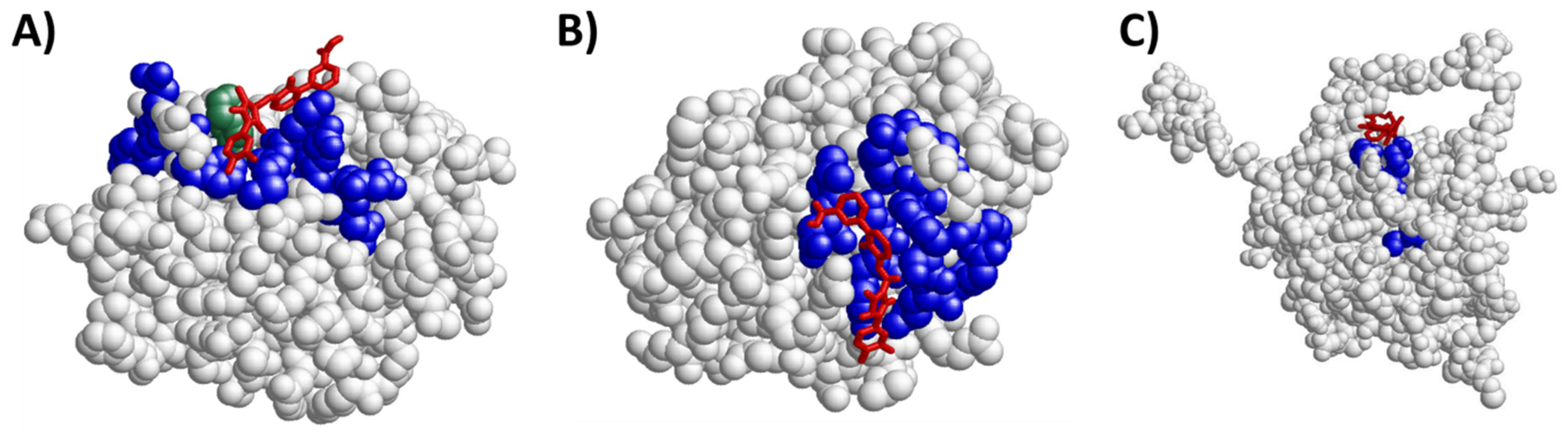

| Candidate Name | UniProt | PDB | Chain | Positions PDB | Pockets (Aminoacid Positions) | Type of Pocket |
|---|---|---|---|---|---|---|
| BCL2 | P10415 | 2XA0 [51] | A | 1–207 | 10;11;12;13;14;15;16;17;18;19;20;21;22;23;24;25;26;27;28;29;30 | BH4 domain (entire), individual BH4 amino acids, histidines BH4-domain |
| 3;20;94;184;186 | Individual histidines | |||||
| B | 1–207 | 10;11;12;13;14;15;16;17;18;19;20;21;22;23;24;25;26;27;28;29;30 | BH4 domain (entire), individual BH4 amino acids, histidines BH4-domain | |||
| 3;20;94;184;186 | Individual histidines | |||||
| 5FCG [52] | A | 1–207 | 12;13;14;15;16;17;18;19;20;21;22;23;24;25;26;27;28;29;30;31;32 | BH4 domain (entire), individual BH4 amino acids, histidines BH4-domain | ||
| 6;23;56;82;146;148 | Individual histidines | |||||
| BCL2L1 | Q07817 | 1MAZ [53] | A | 1–209 | 8;9;10;11;12;13;14;15;16;17;18;19;20;21;22;23;24;25;26;27;28 | BH4 domain (entire), individual BH4 amino acids |
| 62;75;117;181 | Individual histidines | |||||
| 1R2D [54] | A | 1–211 | 4;5;6;7;8;9;10;11;12;13;14;15;16;17;18;19;20;21;22;23;24 | BH4 domain (entire), individual BH4 amino acids | ||
| 58;71;113;117 | Individual histidines | |||||
| BAX | Q07812 | 2K7W [55] | A | 1–192 | 14;15;16;17;18;19;20;21;22;23;24;25;26;27;28;29;30;31;32;33;34;35;36;37;38 | Alpha helix-1, individual Alpha helix-1 amino acids |
| 59;60;61;62;63;64;65;66;67;68;69;70;71;72;73 | BH3 domain (entire), individual BH3 amino acids | |||||
| 2LR1 [56] | A | 1–192 | 14;15;16;17;18;19;20;21;22;23;24;25;26;27;28;29;30;31;32;33;34;35;36;37;38 | Alpha helix-1, individual Alpha helix-1 amino acids | ||
| 59;60;61;62;63;64;65;66;67;68;69;70;71;72;73 | BH3 domain (entire), individual BH3 amino acids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, M.L.; Segú-Vergés, C.; Coma, M.; Álvarez-Roman, M.T.; González-Porras, J.R.; Gutiérrez, L.; Valcárcel, D.; Butta, N. Elucidating the Mechanism of Action of the Attributed Immunomodulatory Role of Eltrombopag in Primary Immune Thrombocytopenia: An In Silico Approach. Int. J. Mol. Sci. 2021, 22, 6907. https://doi.org/10.3390/ijms22136907
Lozano ML, Segú-Vergés C, Coma M, Álvarez-Roman MT, González-Porras JR, Gutiérrez L, Valcárcel D, Butta N. Elucidating the Mechanism of Action of the Attributed Immunomodulatory Role of Eltrombopag in Primary Immune Thrombocytopenia: An In Silico Approach. International Journal of Molecular Sciences. 2021; 22(13):6907. https://doi.org/10.3390/ijms22136907
Chicago/Turabian StyleLozano, Maria L., Cristina Segú-Vergés, Mireia Coma, María T. Álvarez-Roman, José R. González-Porras, Laura Gutiérrez, David Valcárcel, and Nora Butta. 2021. "Elucidating the Mechanism of Action of the Attributed Immunomodulatory Role of Eltrombopag in Primary Immune Thrombocytopenia: An In Silico Approach" International Journal of Molecular Sciences 22, no. 13: 6907. https://doi.org/10.3390/ijms22136907
APA StyleLozano, M. L., Segú-Vergés, C., Coma, M., Álvarez-Roman, M. T., González-Porras, J. R., Gutiérrez, L., Valcárcel, D., & Butta, N. (2021). Elucidating the Mechanism of Action of the Attributed Immunomodulatory Role of Eltrombopag in Primary Immune Thrombocytopenia: An In Silico Approach. International Journal of Molecular Sciences, 22(13), 6907. https://doi.org/10.3390/ijms22136907






