Image-Based In Vitro Screening Reveals the Trypanostatic Activity of Hydroxymethylnitrofurazone against Trypanosoma cruzi
Abstract
:1. Introduction
2. Results
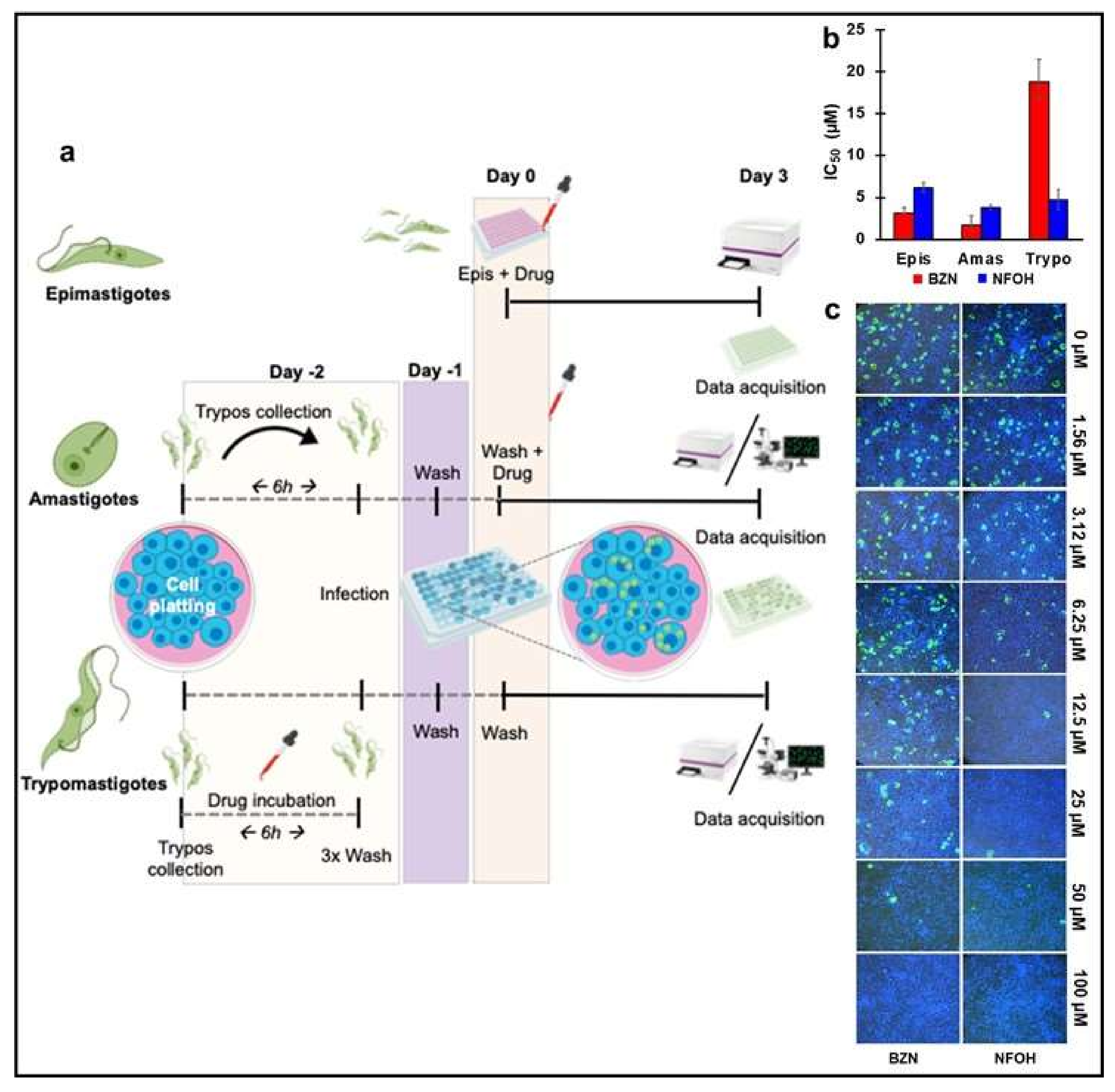
2.1. NFOH Has Greater Anti-Trypomastigote Activity Than BZN In Vitro
2.2. In Vitro Wash Out Assays Reveal the Trypanostatic Effect of NFOH
2.3. NFOH Is More Effective against Chronic Stage T. cruzi Infections
3. Discussion
4. Materials and Methods
4.1. Parasites and Cells Culture
4.2. In Vitro Screening
4.3. Wash Out Assay Using Real Time Microscopy
4.4. Ethics Statement
4.5. In Vivo Infection
4.6. Treatment Schedule
4.7. Bioluminescence Imaging
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malafaia, G.; Rodrigues, A.S.L. Centenário do descobrimento da doença de Chagas: Desafios e perspectivas. Rev. Soc. Bras. Med. Trop. 2010, 43, 483–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Mem. Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef] [Green Version]
- Marin-Neto, J.; Rassi, A.J.; Morillo, C.; Avezum, A.; Connolly, S.; Sosa-Estani, S.; Rosas, F.; Yusuf, S. Rationale and design of a randomized placebo-controlled trial assessing the effects of etiologic treatment in Chagas’ cardiomyopathy: The Benznidazole evaluation for interrupting trypanosomiasis (BENEFIT). Am. Heart J. 2008, 156, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Montgomery, S.P. An estimate of the burden of Chagas disease in the United States. Clin. Infect. Dis. 2009, 49, e52–e54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drugs for Neglected Diseases Initiative (DNDi). Neglected Tropical Diseases. Available online: https://www.dndi.org/diseases-projects/chagas/ (accessed on 27 May 2021).
- World Health Organization (WHO). Chagas disease in Latin America: An epidemiological update based on 2010 estimates. Wkly. Epidemiol. Rec. 2015, 90, 33–44. [Google Scholar]
- CDC, Centers for Diseases Control and Prevention (CDC). Available online: https://www.cdc.gov/parasites/chagas/index.html (accessed on 27 May 2021).
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.L.; Nunes, M.P.; Teixeira, M.M.; Rocha, M.O.C. Diagnosis and management of Chagas disease and cardiomyopathy. Nat. Rev. Cardiol. 2012, 9, 576–589. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Marin-Neto, J.A.; Cunha-Neto, E.; Maciel, B.C.; Simoes, M.V. Pathogenesis of chronic Chagas heart disease. Circulation 2007, 115, 1109–1123. [Google Scholar] [CrossRef]
- Carod-Artal, F.J. Trypanosomiasis, cardiomyopathy and the risk of ischemic stroke. Expert Rev. Cardiovasc. Ther. 2010, 8, 717–728. [Google Scholar] [CrossRef]
- Jabari, S.; de Oliveira, E.C.; Brehmer, A.; da Silveira, A.B.M. Chagasic megacolon: Entericneurons and related structures. Histochem. Cell Biol. 2014, 142, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Scarim, C.B.; Chung, C.M. Nitroheterocyclic derivatives: Privileged scaffold for drug development against Chagas disease. Med. Chem. Res. 2019, 28, 2099–2108. [Google Scholar] [CrossRef]
- Maya, J.D.; Orellana, M.; Ferreira, J.; Kemmerling, U.; López-Muñoz, R.; Morello, A. Chagas disease: Present status of pathogenic mechanisms and chemotherapy. Biol. Res. 2010, 43, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Hasslocher-Moreno, A.M.; do Brasil, P.E.A.A.; de Sousa, A.S.; Xavier, S.S.; Chambela, M.C.; da Silva, G.M.S. Safety of benznidazole use in the treatment of chronic Chagas’ disease. J. Antimicrob. Chemother. 2012, 67, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.D.; Tiecher, F.M.; Balbinot, M.M.; Liarte, D.B.; Scholl, D.; Steindel, M.; Romanha, A. Efficacy of benznidazol treatment for asymptomatic chagasic patients from state of Rio Grande do Sul evaluated during a three years follow-up. Mem. Inst. Oswaldo Cruz 2009, 104, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Fernández, M.L.; Marson, M.E.; Ramirez, J.C.; Mastrantonio, G.; Schijman, A.G.; Altcheh, J.; Riarte, A.R.; Bournissen, F.G. Pharmacokinetic and pharmacodynamics responses in adult patients with Chagas disease treated with a new formulation of benznidazole. Mem. Inst. Oswaldo Cruz 2016, 111, 218–221. [Google Scholar] [CrossRef]
- Andrade, M.C.; Oliveira, M.F.; Nagao-Dias, A.T.; Coêlho, I.C.B.; Cândido, D.S.; Freitas, E.C.; Coelho, H.L.L.; Bezerra, F.S.M. Clinical and serological evolution in chronic Chagas disease patients in a 4-year pharmacotherapy follow-up: A preliminary study. Rev. Soc. Bras. Med. Trop. 2013, 46, 776–778. [Google Scholar] [CrossRef] [Green Version]
- Campos, M.C.; Phelan, J.; Francisco, A.F.; Taylor, M.C.; Lewis, M.D.; Pain, A.; Clark, T.G.; Kelly, J.M. Genome-wide mutagenesis and multi-drug resistance in American trypanosomes induced by the front-line drug benznidazole. Sci. Rep. 2017, 7, 14407. [Google Scholar] [CrossRef] [Green Version]
- Scarim, C.B.; Jornada, D.H.; Chelucci, R.C.; de Almeida, L.; dos Santos, J.L.; Chung, M.C. Current advances in drug discovery for Chagas disease. Eur. J. Med. Chem. 2018, 155, 824–838. [Google Scholar] [CrossRef] [Green Version]
- Scarim, C.B.; Chung, C.M. Current challenges and obstacles to drug development for Chagas disease. Drug Des. Intellect. Prop. Int. J. 2018, 2, 182–184. [Google Scholar] [CrossRef] [Green Version]
- Guido, R.V.C.; Ferreira, E.I.; Nassute, J.C.; Varanda, E.A.; Chung, M.C. Diminuição da atividade mutagênica do pró-fármaco NFOH-121 em relação ao nitrofural (nitrofurazona). Rev. Ciênc Farm. 2001, 22, 319–333. [Google Scholar]
- Chung, M.C.; Bosquesi, P.L.; dos Santos, J.L. A Prodrug Approach to Improve the Physico-Chemical Properties and Decrease the Genotoxicity of Nitro Compounds. Curr. Pharm. Des. 2011, 17, 3515–3526. [Google Scholar] [CrossRef]
- Serafim, E.O.P.; Silva, A.T.A.; Moreno, A.H.; Vizioli, E.O.; Ferreira, E.I.; Peccinini, R.G.; Ribeiro, M.L.; Chung, M.C. Pharmacokinetics of hydroxymethylnitrofurazone, a promising new prodrug for chagas’ disease treatment. Antimicrob. Agents Chemother. 2013, 57, 6106–6109. [Google Scholar] [CrossRef] [Green Version]
- Nogueira Filho, M.A.F.; Padilha, E.C.; De Campos, M.L.; Machado, D.V.P.; Davanço, M.G.; Pestana, K.C.; Chung, M.C.; Peccinini, R.G. Pharmacokinetics of hydroxymethylnitrofurazone and its parent drug nitrofurazone in rabbits. Drug Metab. Lett. 2013, 7, 58–64. [Google Scholar] [CrossRef]
- Davies, C.; Dey, N.; Negrette, O.S.; Parada, L.A.; Basombrio, M.A.; Garg, N.J. Hepatotoxicity in mice of a novel anti-parasite drug candidate hydroxymethylnitrofurazone: A comparison with benznidazole. PLoS Negl. Trop. Dis. 2014, 8, e3231. [Google Scholar] [CrossRef] [Green Version]
- Scarim, C.B.; de Andrade, C.R.; da Rosa, J.A.; dos Santos, J.L.; Chung, M.C. Hydroxymethylnitrofurazone treatment in indeterminate form of chronic Chagas disease: Reduced intensity of tissue parasitism and inflammation—A histopathological study. Int. J. Exp. Pathol. 2018, 99, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.C.; Francisco, A.F.; Jayawardhana, S.; Calderano, S.G.; Lewis, M.D.; Olmo, F.; Beneke, T.; Gluenz, E.; Sunter, J.; Dean, S.; et al. Expanding the toolbox for Trypanosoma cruzi: A parasite line incorporating a bioluminescence-fluorescence dual reporter and streamlined CRISPR/Cas9 functionality for rapid in vivo localisation and phenotyping. PLoS Negl. Trop. Dis. 2018, 12, e0006388. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; De Rycker, M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.D.; Francisco, A.F.; Taylor, M.C.; Burrell-Saward, H.; McLatchie, A.P.; Miles, M.A.; Kelly, J.M. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cell. Microbiol. 2014, 16, 1285–1300. [Google Scholar] [CrossRef] [Green Version]
- Francisco, A.F.; Lewis, M.D.; Jayawardhana, S.; Taylor, M.C.; Chatelain, E.; Kelly, J.M. The limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrob. Agents Chemother. 2015, 59, 4653–4661. [Google Scholar] [CrossRef] [Green Version]
- Francisco, A.F.; Jayawardhana, S.; Lewis, M.D.; White, K.L.; Shackleford, D.M.; Chen, G.; Saunders, J.; Osuna-Cabello, M.; Read, K.D.; Charman, S.A.; et al. Nitroheterocyclic drugs cure experimental Trypanosoma cruzi infections more effectively in the chronic stage than in the acute stage. Sci. Rep. 2016, 6, 35351. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. USA. 2008, 105, 5022–5027. [Google Scholar] [CrossRef] [Green Version]
- Paula, F.R.; Serrano, S.H.P.; Tavares, L.C. Aspects of bioactivity and toxicity of nitrocompounds. Quim. Nov. 2009, 32, 1013–1020. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.N.J.; Docampo, R. Mechanism of toxicity of nitro compounds used in the chemotherapy of Trichomoniasis. Environ. Health Perspect. 1985, 64, 199–208. [Google Scholar] [CrossRef]
- Viodé, C.; Bettache, N.; Cenas, N.; Krauth-Siegel, R.L.; Chauvière, G.; Bakalara, N.; Périé, J. Enzymatic reduction studies of nitroheterocycles. J. Biochem. Pharmacol. 1999, 57, 549–557. [Google Scholar] [CrossRef]
- La-Scalea, M.A.; Trossini, G.H.G.; Menezes, C.M.S.; Chung, M.C.; Ferreira, E.I. Electrochemical reduction using glassy carbon electrode in qqueous medium of a potential anti-Chagas drug: NFOH. J. Electrochem. Soc. 2009, 156, F93–F97. [Google Scholar] [CrossRef]
- La-Scalea, M.A.; Menezes, C.M.S.; Julião, M.S.S.; Chung, M.C.; Serrano, S.H.P.; Ferreira, E.I. Voltammetric behavior of nitrofurazone and its hydroxymethyl prodrug with potential anti-chagas activity. J. Braz. Chem. Soc. 2005, 16, 774–782. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.; Versteeg, L.; Damania, A.; Keegan, B.; Kendricks, A.; Pollet, J.; Cruz-Chan, J.V.; Gusovsky, F.; Hotez, P.J.; Bottazzi, M.E. Vaccine-linked chemotherapy improves benznidazole efficacy for acute Chagas disease. Infect. Immun. 2018, 86, e00876-17. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.C.; Güido, R.V.C.; Martinelli, T.F.; Gonçalves, M.F.; Polli, M.C.; Botelho, K.C.A.; Varanda, E.A.; Colli, W.; Miranda, M.T.M.; Ferreira, E.I. Synthesis and in vitro evaluation of potential antichagasic hydroxymethylnitrofurazone (NFOH-121): A new nitrofurazone prodrug. Bio Org. Med. Chem. 2003, 11, 4779–4783. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarim, C.B.; Olmo, F.; Ferreira, E.I.; Chin, C.M.; Kelly, J.M.; Francisco, A.F. Image-Based In Vitro Screening Reveals the Trypanostatic Activity of Hydroxymethylnitrofurazone against Trypanosoma cruzi. Int. J. Mol. Sci. 2021, 22, 6930. https://doi.org/10.3390/ijms22136930
Scarim CB, Olmo F, Ferreira EI, Chin CM, Kelly JM, Francisco AF. Image-Based In Vitro Screening Reveals the Trypanostatic Activity of Hydroxymethylnitrofurazone against Trypanosoma cruzi. International Journal of Molecular Sciences. 2021; 22(13):6930. https://doi.org/10.3390/ijms22136930
Chicago/Turabian StyleScarim, Cauê Benito, Francisco Olmo, Elizabeth Igne Ferreira, Chung Man Chin, John M. Kelly, and Amanda Fortes Francisco. 2021. "Image-Based In Vitro Screening Reveals the Trypanostatic Activity of Hydroxymethylnitrofurazone against Trypanosoma cruzi" International Journal of Molecular Sciences 22, no. 13: 6930. https://doi.org/10.3390/ijms22136930






