CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis
Abstract
:1. Introduction
2. Results
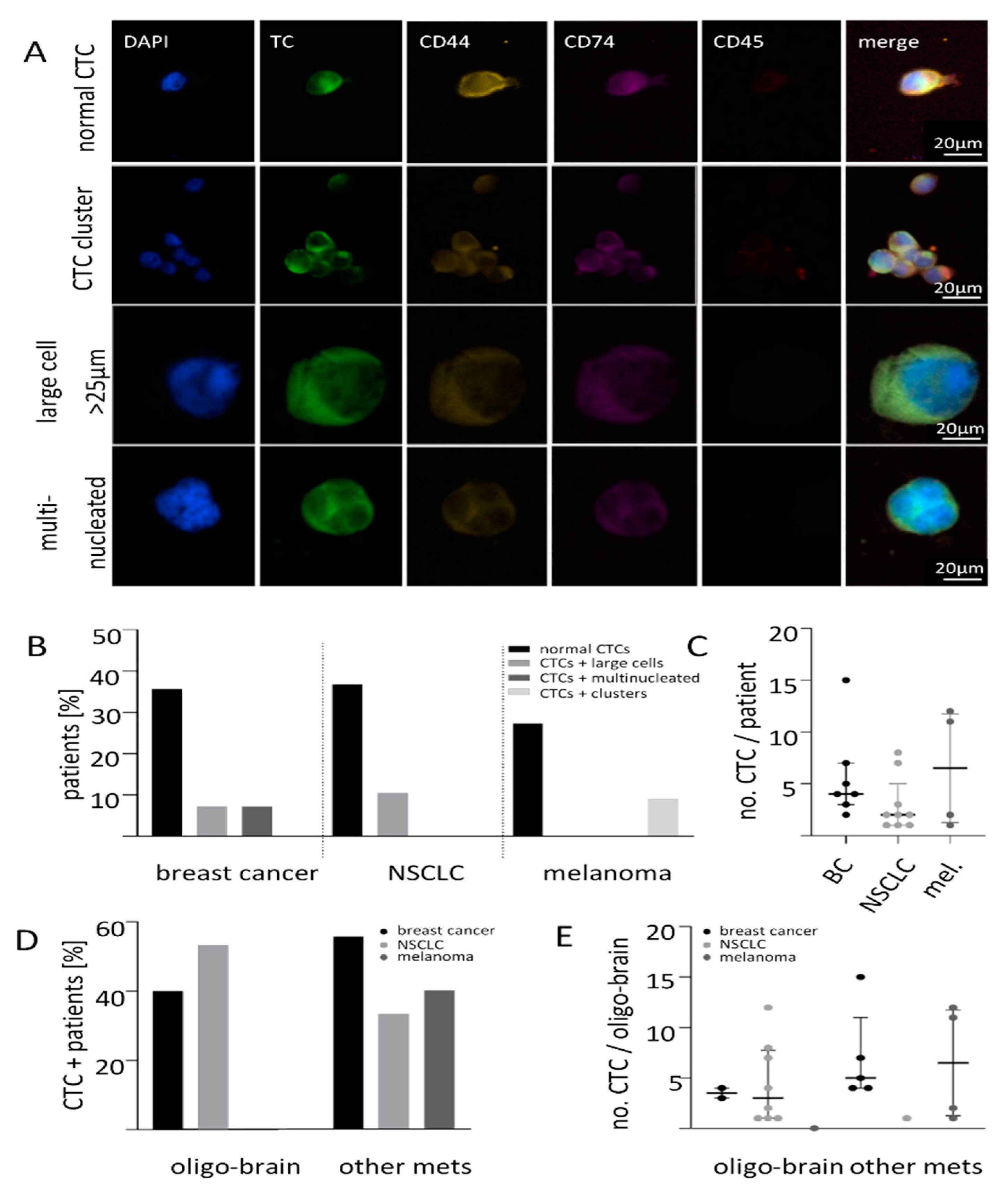
2.1. Size-Based Enrichment of CTCs in Breast Cancer, NSCLC and Melanoma Patients
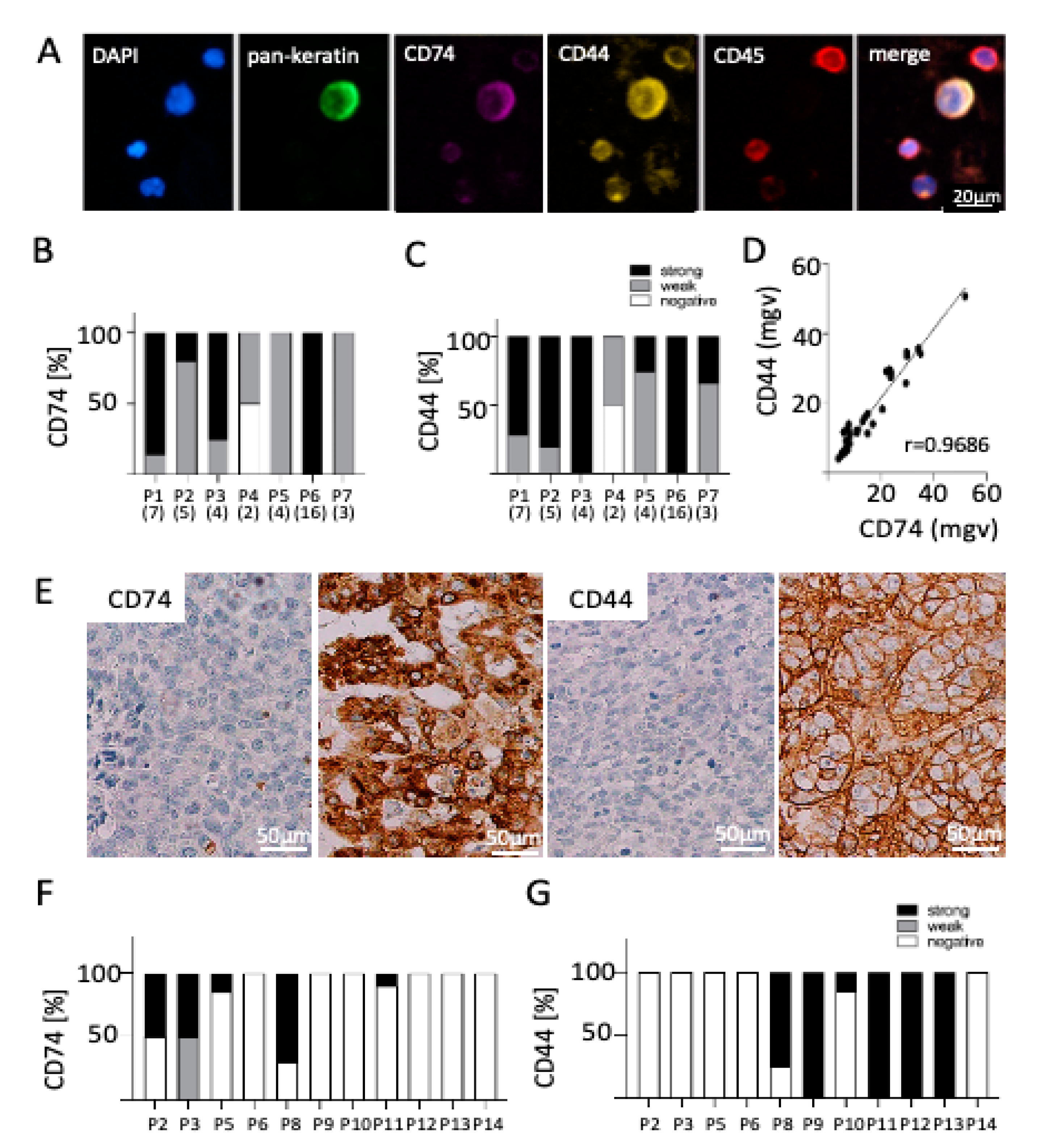
2.2. Expression of CD74 and CD44 on CTCs
2.3. Correlation of CD74 and CD44 Expression on CTCs and Matched BM Tissue
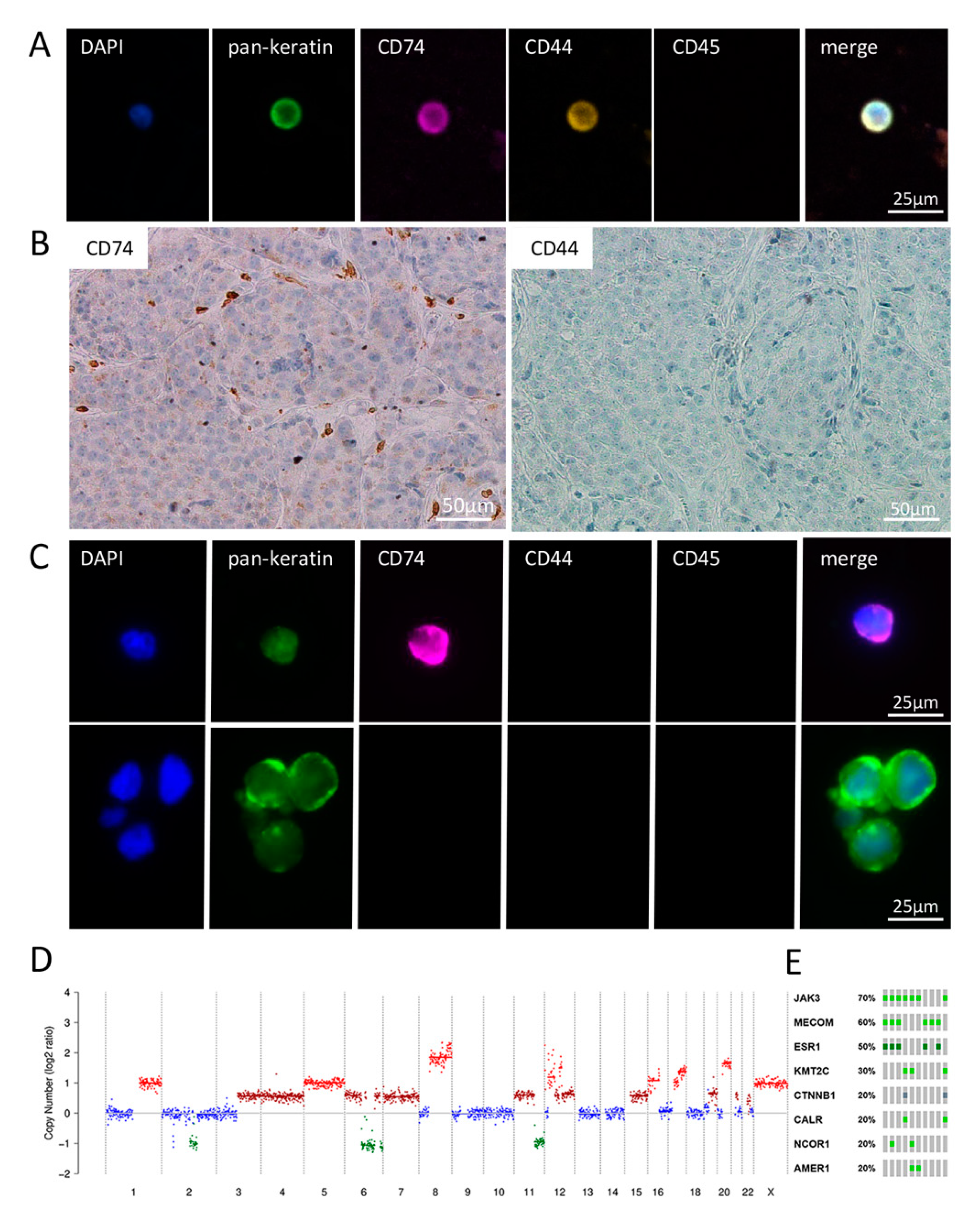
2.4. Detailed Analysis of Blood, CSF and BM Tissue of a HER2-Positive Breast Cancer Patient
3. Discussion
4. Material and Methods
4.1. Patient Materials
4.2. Immunohistochemistry of Brain Metastatic Samples
4.3. Detection of Circulating Tumor Cells and Disseminated Tumor Cells (CTCs and DTCs)
4.4. Whole Genome Amplification (WGA) and Quality Control of CTCs
4.5. Whole Genome Sequencing
4.6. Data Processing
4.7. Mutation Analysis
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Franchino, F.; Ruda, R.; Soffietti, R. Mechanisms and therapy for cancer metastasis to the brain. Front. Oncol. 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Quigley, M.R.; Fukui, O.; Chew, B.; Bhatia, S.; Karlovits, S. The shifting landscape of metastatic breast cancer to the CNS. Neurosurg. Rev. 2013, 36, 377–382. [Google Scholar] [CrossRef]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Muller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, M.C.; Baik, C.S.; Gadi, V.K.; Bhatia, S.; Chow, L.Q. Systemic therapy of brain metastases: Non-small cell lung cancer, breast cancer, and melanoma. Neuro-Oncology 2017, 19, i1–i24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alix-Panabieres, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Ridgway, L.D.; Wetzel, M.D.; Ngo, J.; Yin, W.; Kumar, D.; Goodman, J.C.; Groves, M.D.; Marchetti, D. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 2013, 5, 180ra48. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, A.; Loges, S.; Pantel, K.; Wikman, H. Detection of circulating tumor cells in non-small cell lung cancer. Front. Oncol. 2015, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Riebensahm, C.; Joosse, S.A.; Mohme, M.; Hanssen, A.; Matschke, J.; Goy, Y.; Witzel, I.; Lamszus, K.; Kropidlowski, J.; Petersen, C.; et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res. 2019, 21, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamszus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ssadh, H.A.; Abdulmonem, W.A. Immunophenotyping of the cluster of differentiation 74, migration inhibitory factor, and cluster of differentiation 44 expression on human breast cancer-derived cell lines. Int. J. Health Sci. 2019, 13, 17–24. [Google Scholar]
- Koch, C.; Joosse, S.A.; Schneegans, S.; Wilken, O.J.W.; Janning, M.; Loreth, D.; Muller, V.; Prieske, K.; Banys-Paluchowski, M.; Horst, L.J.; et al. Pre-analytical and analytical variables of label-independent enrichment and automated detection of circulating Tumor cells in cancer patients. Cancers 2020, 12, 442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiner, P.S.; Zinke, J.; Kowalewski, D.J.; Bernatz, S.; Tichy, J.; Ronellenfitsch, M.W.; Thorsen, F.; Berger, A.; Forster, M.T.; Muller, A.; et al. CD74 regulates complexity of tumor cell HLA class II peptidome in brain metastasis and is a positive prognostic marker for patient survival. Acta Neuropathol. Commun. 2018, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Liu, J.; Hofmann, M.; Hamou, M.F.; de Tribolet, N. Differential CD44 expression patterns in primary brain tumours and brain metastases. Br. J. Cancer 1995, 72, 160–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarlane, S.; Coulter, J.A.; Tibbits, P.; O’Grady, A.; McFarlane, C.; Montgomery, N.; Hill, A.; McCarthy, H.O.; Young, L.S.; Kay, E.W.; et al. CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget 2015, 6, 11465–11476. [Google Scholar] [CrossRef] [Green Version]
- Zeiner, P.S.; Preusse, C.; Blank, A.E.; Zachskorn, C.; Baumgarten, P.; Caspary, L.; Braczynski, A.K.; Weissenberger, J.; Bratzke, H.; Reiss, S.; et al. MIF receptor CD74 is restricted to microglia/macrophages, associated with a m1-polarized immune milieu and prolonged patient survival in gliomas. Brain Pathol. 2015, 25, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Gore, Y.; Starlets, D.; Maharshak, N.; Becker-Herman, S.; Kaneyuki, U.; Leng, L.; Bucala, R.; Shachar, I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J. Biol. Chem. 2008, 283, 2784–2792. [Google Scholar] [CrossRef] [Green Version]
- Boral, D.; Vishnoi, M.; Liu, H.N.; Yin, W.; Sprouse, M.L.; Scamardo, A.; Hong, D.S.; Tan, T.Z.; Thiery, J.P.; Chang, J.C.; et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat. Commun. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorges, K.; Wiltfang, L.; Gorges, T.M.; Sartori, A.; Hildebrandt, L.; Keller, L.; Volkmer, B.; Peine, S.; Babayan, A.; Moll, I.; et al. Intra-patient heterogeneity of circulating tumor cells and circulating tumor DNA in blood of melanoma patients. Cancers 2019, 11, 1685. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, A.; Riebensahm, C.; Mohme, M.; Joosse, S.A.; Velthaus, J.L.; Berger, L.A.; Bernreuther, C.; Glatzel, M.; Loges, S.; Lamszus, K.; et al. Frequency of circulating tumor cells (CTC) in patients with brain metastases: Implications as a risk assessment marker in oligo-metastatic disease. Cancers 2018, 10, 527. [Google Scholar] [CrossRef] [Green Version]
- Janni, W.J.; Rack, B.; Terstappen, L.W.; Pierga, J.Y.; Taran, F.A.; Fehm, T.; Hall, C.; de Groot, M.R.; Bidard, F.C.; Friedl, T.W.; et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin. Cancer Res. 2016, 22, 2583–2593. [Google Scholar] [CrossRef] [Green Version]
- Khoja, L.; Lorigan, P.; Zhou, C.; Lancashire, M.; Booth, J.; Cummings, J.; Califano, R.; Clack, G.; Hughes, A.; Dive, C. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 1582–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Mitra, D.; Sullivan, R.J.; Wittner, B.S.; Kimura, A.M.; Pan, S.; Hoang, M.P.; Brannigan, B.W.; Lawrence, D.P.; Flaherty, K.T.; et al. Isolation and molecular characterization of circulating melanoma cells. Cell Rep. 2014, 7, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierga, J.Y.; Bidard, F.C.; Cropet, C.; Tresca, P.; Dalenc, F.; Romieu, G.; Campone, M.; Mahier Ait-Oukhatar, C.; Le Rhun, E.; Goncalves, A.; et al. Circulating tumor cells and brain metastasis outcome in patients with HER2-positive breast cancer: The LANDSCAPE trial. Ann. Oncol. 2013, 24, 2999–3004. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, S.; Xu, W.; Zhang, Z.; Ding, X.; He, J.; Liang, W. Presence of intra-tumoral CD61+ megakaryocytes predicts poor prognosis in non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Schneegans, S.; Luck, L.; Besler, K.; Bluhm, L.; Stadler, J.C.; Staub, J.; Greinert, R.; Volkmer, B.; Kubista, M.; Gebhardt, C.; et al. Pre-analytical factors affecting the establishment of a single tube assay for multiparameter liquid biopsy detection in melanoma patients. Mol. Oncol. 2020, 14, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.; Harding, A. Size does matter: Why polyploid tumor cells are critical drug targets in the war on cancer. Front. Oncol. 2014, 4, 123. [Google Scholar] [CrossRef]
- Ssadh, H.A.; Spencer, P.S.; Alabdulmenaim, W.; Alghamdi, R.; Madar, I.H.; Miranda-Sayago, J.M.; Fernandez, N. Measurements of heterotypic associations between cluster of differentiation CD74 and CD44 in human breast cancer-derived cells. Oncotarget 2017, 8, 92143–92156. [Google Scholar] [CrossRef] [Green Version]
- Baccelli, I.; Schneeweiss, A.; Riethdorf, S.; Stenzinger, A.; Schillert, A.; Vogel, V.; Klein, C.; Saini, M.; Bauerle, T.; Wallwiener, M.; et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 2013, 31, 539–544. [Google Scholar] [CrossRef]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef] [Green Version]
- Palagani, V.; El Khatib, M.; Krech, T.; Manns, M.P.; Malek, N.P.; Plentz, R.R. Decrease of CD44-positive cells correlates with tumor response to chemotherapy in patients with gastrointestinal cancer. Anticancer Res. 2012, 32, 1747–1755. [Google Scholar] [PubMed]
- Bucala, R.; Shachar, I. The integral role of CD74 in antigen presentation, MIF signal transduction, and B cell survival and homeostasis. Mini-Rev. Med. Chem. 2015, 14, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, H. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adalsteinsson, V.A.; Ha, G.; Freeman, S.S.; Choudhury, A.D.; Stover, D.G.; Parsons, H.A.; Gydush, G.; Reed, S.C.; Rotem, D.; Rhoades, J.; et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Scheffler, K.; Halpern, A.L.; Bekritsky, M.A.; Noh, E.; Kallberg, M.; Chen, X.; Kim, Y.; Beyter, D.; Krusche, P.; et al. Strelka2: Fast and accurate calling of germline and somatic variants. Nat. Methods 2018, 15, 591–594. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The ensembl variant effect predictor. Genome Biol. 2016, 17, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosse, S.A. In-Silico Online–Statistical Tools. Available online: http://in-silico.online (accessed on June 2020).
| Breast Cancer | CTC | Lung Cancer | CTC | Melanoma | CTC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | ||||||||||||
| Total n | 14 | 7 | 7 | Total n | 18 | 9 | 9 | Total n | 11 | 7 | 4 | ||||||
| % | % | p | % | % | p | % | % | p | |||||||||
| Survival | 0.155 | Survival | 0.501 | Survival | 0.049 | ||||||||||||
| Alive | 5 | 20.0 | 80.0 | Alive | 4 | 50.0 | 50.0 | Alive | 6 | 100.0 | 0.0 | ||||||
| Dead | 9 | 66.7 | 33.3 | Dead | 14 | 57.1 | 42.9 | Dead | 5 | 20.0 | 80.0 | ||||||
| ER | 8 | 37.5 | 62.5 | 0.486 | BRAF | 0 | - | - | - | BRAF | 8 | 50.0 | 50.0 | - | |||
| PR | 6 | 33.3 | 66.7 | 0.486 | EGFR | 1 | 0.0 | 100.0 | - | PD-L1 | 3 | 66.7 | 33.3 | - | |||
| HER2 | 10 | 40.0 | 60.0 | 0.409 | NRAS | 3 | 66.7 | 33.3 | - | ||||||||
| BRCA1 | 2 | 100.0 | 0.0 | - | |||||||||||||
| Metastases | Metastases | Metastases | |||||||||||||||
| Bone | 10 | 0.999 | Bone | 18 | 0.999 | Bone | 6 | 0.206 | |||||||||
| No | 5 | 60.0 | 40.0 | No | 17 | 55.6 | 44.4 | No | 2 | 0.0 | 100.0 | ||||||
| Yes | 5 | 40.0 | 60.0 | Yes | 1 | 0.0 | 100.0 | Yes | 4 | 75.0 | 25.0 | ||||||
| Liver | 9 | 0.999 | Liver | 18 | 0.999 | Liver | 8 | 0.003 | |||||||||
| No | 7 | 42.9 | 57.1 | No | 17 | 55.6 | 44.4 | No | 3 | 0.0 | 100.0 | ||||||
| Yes | 2 | 50.0 | 50.0 | Yes | 1 | 100.0 | 0.0 | Yes | 5 | 100.0 | 0.0 | ||||||
| Lung | 10 | 0.999 | Lung | 18 | 0.999 | Lung | 8 | - | |||||||||
| No | 5 | 40.0 | 60.0 | No | 17 | 55.6 | 44.4 | No | 0 | - | - | ||||||
| Yes | 5 | 40.0 | 60.0 | Yes | 1 | 100.0 | 0.0 | Yes | 8 | 50.0 | 50.0 | ||||||
| Lymph node | 11 | 0.437 | Lymph node | 18 | 0.999 | Lymph node | 8 | - | |||||||||
| No | 7 | 42.9 | 57.1 | No | 10 | 54.5 | 45.5 | No | 0 | ||||||||
| Yes | 4 | 75.0 | 25.0 | Yes | 8 | 62.5 | 37.5 | Yes | 8 | 62.5 | 37.5 | ||||||
| Other organ | 9 | 0.487 | Other organ | 18 | 0.999 | Other organ | 9 | 0.999 | |||||||||
| No | 5 | 40.0 | 60.0 | No | 15 | 50.0 | 50.0 | No | 1 | 100.0 | 0.0 | ||||||
| Yes | 4 | 75.0 | 25.0 | Yes | 3 | 100.0 | 0.0 | Yes | 8 | 50.0 | 50.0 | ||||||
| Oligo brain | 9 | 0.999 | Oligo brain | 18 | 0.394 | Oligo brain | 11 | 0.999 | |||||||||
| No | 7 | 42.9 | 57.1 | No | 3 | 54.5 | 45.5 | No | 10 | 60.0 | 40.0 | ||||||
| Yes | 2 | 60.0 | 40.0 | Yes | 15 | 47.0 | 53.0 | Yes | 1 | 100.0 | 0.0 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loreth, D.; Schuette, M.; Zinke, J.; Mohme, M.; Piffko, A.; Schneegans, S.; Stadler, J.; Janning, M.; Loges, S.; Joosse, S.A.; et al. CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. Int. J. Mol. Sci. 2021, 22, 6993. https://doi.org/10.3390/ijms22136993
Loreth D, Schuette M, Zinke J, Mohme M, Piffko A, Schneegans S, Stadler J, Janning M, Loges S, Joosse SA, et al. CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. International Journal of Molecular Sciences. 2021; 22(13):6993. https://doi.org/10.3390/ijms22136993
Chicago/Turabian StyleLoreth, Desiree, Moritz Schuette, Jenny Zinke, Malte Mohme, Andras Piffko, Svenja Schneegans, Julia Stadler, Melanie Janning, Sonja Loges, Simon A. Joosse, and et al. 2021. "CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis" International Journal of Molecular Sciences 22, no. 13: 6993. https://doi.org/10.3390/ijms22136993
APA StyleLoreth, D., Schuette, M., Zinke, J., Mohme, M., Piffko, A., Schneegans, S., Stadler, J., Janning, M., Loges, S., Joosse, S. A., Lamszus, K., Westphal, M., Müller, V., Glatzel, M., Matschke, J., Gebhardt, C., Schneider, S. W., Belczacka, I., Volkmer, B., ... Wikman, H. (2021). CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. International Journal of Molecular Sciences, 22(13), 6993. https://doi.org/10.3390/ijms22136993







