Bioengineered Ferritin Nanocarriers for Cancer Therapy
Abstract
:1. Introduction
2. Targeting Mechanism of Ferritin
3. Tumor Targeting Modification on Ferritin
4. The Role of Ferritin in Cancer Treatment
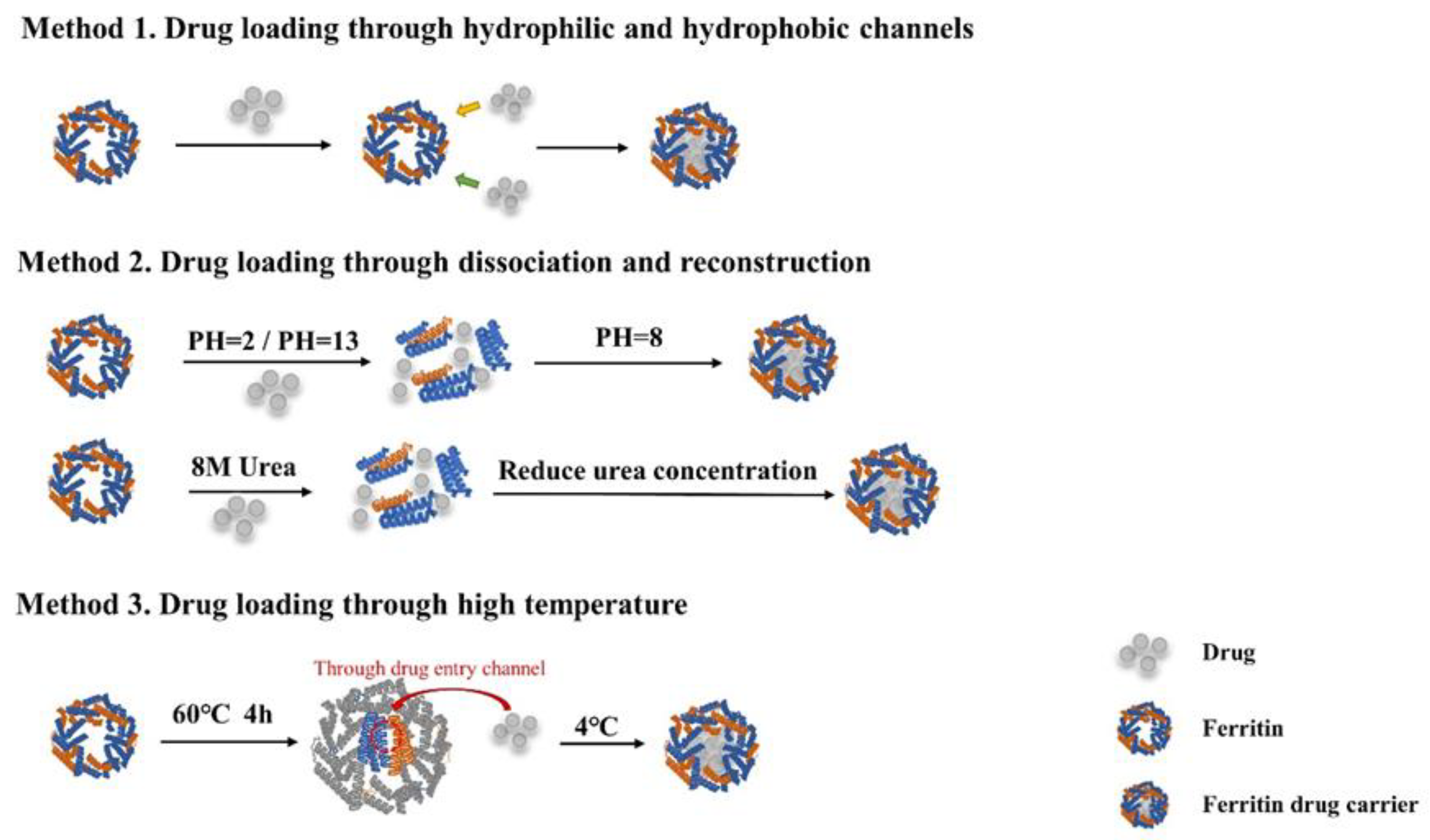
4.1. Strategies for Loading Chemotherapeutics with Ferritin
4.2. Ferritin Nanoparticles Combined with Photothermal Therapy (PTT)
4.3. Ferritin Nanoparticles Combined with Photodynamic Therapy (PDT)
4.4. Application of Ferritin in PTT and PDT
5. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global battle against cancer won’t be won with treatment alone—Effective prevention measures urgently needed to prevent cancer crisis. Cent. Eur. J. Public Health 2014, 22, 23–28.
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart nanoparticles for drug delivery application: Development of versatile nanocarrier platforms in biotechnology and nanomedicine. J. Nanomater. 2019, 2019, 26. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [Green Version]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta 2013, 1836, 245–254. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of nanoparticle-based carriers for targeted drug delivery. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Tosi, G.; Belletti, D.; Pederzoli, F.; Ruozi, B. Apoferritin nanocage as drug reservoir: Is it a reliable drug delivery system? Expert Opin. Drug Deliv. 2016, 13, 1341–1343. [Google Scholar] [CrossRef] [Green Version]
- Petersen, G.H.; Alzghari, S.K.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 2016, 232, 255–264. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 12. [Google Scholar] [CrossRef]
- Bellini, M.; Mazzucchelli, S.; Galbiati, E.; Sommaruga, S.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Colombo, M.; Tortora, P.; Corsi, F.; et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Control. Release 2014, 196, 184–196. [Google Scholar] [CrossRef]
- Thompson, K.; Menzies, S.; Muckenthaler, M.; Torti, F.M.; Wood, T.; Torti, S.V.; Hentze, M.W.; Beard, J.; Connor, J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J. Neurosci. Res. 2003, 71, 46–63. [Google Scholar] [CrossRef]
- Li, W.; Garringer, H.J.; Goodwin, C.B.; Richine, B.; Acton, A.; VanDuyn, N.; Muhoberac, B.B.; Irimia-Dominguez, J.; Chan, R.J.; Peacock, M.; et al. Systemic and cerebral iron homeostasis in ferritin knock-out mice. PLoS ONE 2015, 10, e0117435. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Gao, L.; Yan, X. Human ferritin for tumor detection and therapy. Wiley interdisciplinary reviews. Nanomed. Nanobiotechnol. 2013, 5, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Banyard, S.H.; Stammers, D.K.; Harrison, P.M. Electron density map of apoferritin at 2.8-A resolution. Nature 1978, 271, 282–284. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B.; Kumar, S.U.; Matai, I.; Sachdev, A.; Dubey, P.; Gopinath, P. Ferritin nanocages: A novel platform for biomedical applications. J. Biomed. Nanotechnol. 2014, 10, 2950–2976. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [Green Version]
- Santambrogio, P.; Levi, S.; Arosio, P.; Palagi, L.; Vecchio, G.; Lawson, D.M.; Yewdall, S.J.; Artymiuk, P.J.; Harrison, P.M.; Jappelli, R.; et al. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. J. Biol. Chem. 1992, 267, 14077–14083. [Google Scholar] [CrossRef]
- Kang, S.; Oltrogge, L.M.; Broomell, C.C.; Liepold, L.O.; Prevelige, P.E.; Young, M.; Douglas, T. Controlled assembly of bifunctional chimeric protein cages and composition analysis using noncovalent mass spectrometry. J. Am. Chem. Soc. 2008, 130, 16527–16529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Protein cage nanostructure as drug delivery system: Magnifying glass on apoferritin. Expert Opin. Drug Deliv. 2017, 14, 825–840. [Google Scholar] [CrossRef]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- Li, X.; Qiu, L.; Zhu, P.; Tao, X.; Imanaka, T.; Zhao, J.; Huang, Y.; Tu, Y.; Cao, X. Epidermal growth factor-ferritin H-chain protein nanoparticles for tumor active targeting. Small 2012, 8, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [Green Version]
- Daniels, T.R.; Delgado, T.; Rodriguez, J.A.; Helguera, G.; Penichet, M.L. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006, 121, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Delgado, T.; Helguera, G.; Penichet, M.L. The transferrin receptor part II: Targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 2006, 121, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.L.; He, J.Y.; Fan, K.L.; Yan, X.Y. Ferritin variants: Inspirations for rationally designing protein nanocarriers. Nanoscale 2019, 11, 12449–12459. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.P.; Tang, W.; Chen, H.M.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.Y.; Xie, J. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Chu, C.; Wang, X.; Lin, H.; Wang, J.; Zeng, Y.; Zhu, W.; Wang, Y.J.; Liu, G. Ultra-high loading of sinoporphyrin sodium in ferritin for single-wave motivated photothermal and photodynamic co-therapy. Biomater. Sci. 2017, 5, 1512–1516. [Google Scholar] [CrossRef]
- Kitagawa, T.; Kosuge, H.; Uchida, M.; Iida, Y.; Dalman, R.L.; Douglas, T.; McConnell, M.V. RGD targeting of human ferritin iron oxide nanoparticles enhances in vivo MRI of vascular inflammation and angiogenesis in experimental carotid disease and abdominal aortic aneurysm. J. Magn. Reson. Imaging 2017, 45, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, W.; Zhang, X.; Shao, R.; Li, Z. A pH-induced reversible assembly system with resveratrol-controllable loading and release for enhanced tumor-targeting chemotherapy. Nanoscale Res. Lett. 2019, 14, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falvo, E.; Malagrino, F.; Arcovito, A.; Fazi, F.; Colotti, G.; Tremante, E.; Di Micco, P.; Braca, A.; Opri, R.; Giuffre, A.; et al. The presence of glutamate residues on the PAS sequence of the stimuli-sensitive nano-ferritin improves in vivo biodistribution and mitoxantrone encapsulation homogeneity. J. Control. Release 2018, 275, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Arcovito, A.; Conti, G.; Cipolla, G.; Pitea, M.; Morea, V.; Damiani, V.; Sala, G.; Fracasso, G.; Ceci, P. Engineered human nanoferritin bearing the drug genz-644282 for cancer therapy. Pharmaceutics 2020, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Damiani, V.; Conti, G.; Boschi, F.; Messana, K.; Giacomini, P.; Milella, M.; De Laurenzi, V.; Morea, V.; Sala, G.; et al. High activity and low toxicity of a novel CD71-targeting nanotherapeutic named The-0504 on preclinical models of several human aggressive tumors. J. Exp. Clin. Cancer Res. 2021, 40, 63. [Google Scholar] [CrossRef]
- Cioloboc, D.; Kurtz, D.M. Targeted cancer cell delivery of arsenate as a reductively activated prodrug. JBIC J. Biol. Inorg. Chem. 2020, 25, 441–449. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, Y.; Su, M.; Yu, M.; Zhang, Y.; Cheng, H.; Zheng, H.; Liu, J.; Wang, X.; Lei, Z.; et al. Multifunctional ferritin nanoparticles as theranostics for imaging-guided tumor phototherapy. J. Biomed. Nanotechnol. 2019, 15, 1546–1555. [Google Scholar] [CrossRef]
- Zhai, M.; Wang, Y.; Zhang, L.; Liang, M.; Fu, S.; Cui, L.; Yang, M.; Gong, W.; Li, Z.; Yu, L.; et al. Glioma targeting peptide modified apoferritin nanocage. Drug Deliv. 2018, 25, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhou, X.; Xu, Y.; Fan, S.; Tian, N.; Zhang, W.; Sheng, F.; Lin, J.; Zhong, W. Development of a novel dual-order protein-based nanodelivery carrier that rapidly targets low-grade gliomas with microscopic metastasis in vivo. ACS Omega 2020, 5, 20653–20663. [Google Scholar] [CrossRef]
- Huang, X.; Chisholm, J.; Zhuang, J.; Xiao, Y.; Duncan, G.; Chen, X.; Suk, J.S.; Hanes, J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl. Acad. Sci. USA 2017, 114, E6595–E6602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, Z.; Tang, W.; Zhang, W.; Xie, J. Folic acid conjugated ferritins as photosensitizer carriers for photodynamic therapy. Nanoscale 2015, 7, 10330–10333. [Google Scholar] [CrossRef] [Green Version]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody–drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhu, Y.; Wu, T.; Cheng, J.; Liu, Y. Nanobody-ferritin conjugate for targeted photodynamic therapy. Chemistry 2020, 26, 7442–7450. [Google Scholar] [CrossRef]
- Lee, B.R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered human ferritin nanoparticles for direct delivery of tumor antigens to lymph node and cancer immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef] [Green Version]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; Mannelli, L.D.; Ghelardini, C.; Ferretti, A.M.; et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef]
- Ferraro, G.; Monti, D.M.; Amoresano, A.; Pontillo, N.; Petruk, G.; Pane, F.; Cinellu, M.A.; Merlino, A. Gold-based drug encapsulation within a ferritin nanocage: X-ray structure and biological evaluation as a potential anticancer agent of the Auoxo3-loaded protein. Chem. Commun. 2016, 52, 9518–9521. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.M.; Ferraro, G.; Petruk, G.; Maiore, L.; Pane, F.; Amoresano, A.; Cinellu, M.A.; Merlino, A. Ferritin nanocages loaded with gold ions induce oxidative stress and apoptosis in MCF-7 human breast cancer cells. Dalton Trans. 2017, 46, 15354–15362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuruppu, A.I.; Zhang, L.; Collins, H.; Turyanska, L.; Thomas, N.R.; Bradshaw, T.D. An apoferritin-based drug delivery system for the tyrosine kinase inhibitor gefitinib. Adv. Healthc. Mater. 2015, 4, 2816–2821. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Fan, K.; Wang, L.; Ying, X.; Sanders, A.J.; Guo, T.; Xing, X.; Zhou, M.; Du, H.; Hu, Y.; et al. TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer. Cell Death Dis. 2020, 11, 92. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ma, Y.; Dong, Y.; Zhao, Z.; You, C.; Huang, S.; Li, X.; Wang, F.; Zhang, Y. Novel paclitaxel-loaded nanoparticles based on human H chain ferritin for tumor-targeted delivery. ACS Biomater. Sci. Eng. 2019, 5, 6645–6654. [Google Scholar] [CrossRef]
- Geninatti Crich, S.; Cadenazzi, M.; Lanzardo, S.; Conti, L.; Ruiu, R.; Alberti, D.; Cavallo, F.; Cutrin, J.C.; Aime, S. Targeting ferritin receptors for the selective delivery of imaging and therapeutic agents to breast cancer cells. Nanoscale 2015, 7, 6527–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansourizadeh, F.; Alberti, D.; Bitonto, V.; Tripepi, M.; Sepehri, H.; Khoee, S.; Geninatti Crich, S. Efficient synergistic combination effect of Quercetin with Curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Colloids Surf. B Biointerfaces 2020, 191, 110982. [Google Scholar] [CrossRef]
- Lei, Y.; Hamada, Y.; Li, J.; Cong, L.; Wang, N.; Li, Y.; Zheng, W.; Jiang, X. Targeted tumor delivery and controlled release of neuronal drugs with ferritin nanoparticles to regulate pancreatic cancer progression. J. Control. Release 2016, 232, 131–142. [Google Scholar] [CrossRef]
- Breen, A.F.; Wells, G.; Turyanska, L.; Bradshaw, T.D. Development of novel apoferritin formulations for antitumour benzothiazoles. Cancer Rep. 2019, 2, e1155. [Google Scholar] [CrossRef] [Green Version]
- Tesarova, B.; Dostalova, S.; Smidova, V.; Goliasova, Z.; Skubalova, Z.; Michalkova, H.; Hynek, D.; Michalek, P.; Polanska, H.; Vaculovicova, M.; et al. Surface-PASylation of ferritin to form stealth nanovehicles enhances in vivo therapeutic performance of encapsulated ellipticine. Appl. Mater. Today 2020, 18, 100501. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Jacobson, O.; Wang, Z.; Liu, Y.; Lin, L.; Lin, J.; Lu, N.; Zhang, H.; Tian, R.; et al. Biomineralization-inspired synthesis of copper sulfide–ferritin nanocages as cancer theranostics. ACS Nano 2016, 10, 3453–3460. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Rong, P.; Jin, A.; Yan, X.; Zhang, M.G.; Lin, J.; Hu, H.; Wang, Z.; Yue, X.; Li, W.; et al. Dye-loaded ferritin nanocages for multimodal imaging and photothermal therapy. Adv. Mater. 2014, 26, 6401–6408. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Wang, H.; Cao, H.; Zeng, L.; Wang, Y.; Wang, Z.; Wang, J.; Li, J.; Wang, S.; Zhang, Z.; et al. Deep tumor-penetrated nanocages improve accessibility to cancer stem cells for photothermal-chemotherapy of breast cancer metastasis. Adv. Sci. 2018, 5, 1801012. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Shieh, M.J. Near-infrared fluorescent dye-decorated nanocages to form grenade-like nanoparticles with dual control release for photothermal theranostics and chemotherapy. Bioconjug. Chem. 2018, 29, 1384–1398. [Google Scholar] [CrossRef]
- Li, M.; Wu, D.; Chen, Y.; Shan, G.; Liu, Y. Apoferritin nanocages with Au nanoshell coating as drug carrier for multistimuli-responsive drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 95, 11–18. [Google Scholar] [CrossRef]
- Guo, X.; Mei, J.; Zhang, C. Development of drug dual-carriers delivery system with mitochondria-targeted and pH/heat responsive capacity for synergistic photothermal-chemotherapy of ovarian cancer. Int. J. Nanomed. 2020, 15, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, W.; Ding, L.; Li, X.W.; Wu, Y.; Tang, J.H. Prussian blue-modified ferritin nanoparticles for effective tumor chemo-photothermal combination therapy via enhancing reactive oxygen species production. J. Biomater. Appl. 2019, 33, 1202–1213. [Google Scholar] [CrossRef]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Pang, X.; Wang, X.; Leung, A.W.; Luan, Y.; Zhao, G.; Wang, P.; Xu, C. Preparation of hypocrellin B nanocages in self-assembled apoferritin for enhanced intracellular uptake and photodynamic activity. J. Mater. Chem. B 2017, 5, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jia, S.; Wang, Q.; Ding, X.; Liu, Y.; Yao, H.; Zhou, J. A self-targeting, dual ROS/pH-responsive apoferritin nanocage for spatiotemporally controlled drug delivery to breast cancer. Biomacromolecules 2018, 19, 1026–1036. [Google Scholar] [CrossRef]
- Yao, H.; Zhao, W.; Zhang, S.; Guo, X.; Li, Y.; Du, B. Dual-functional carbon dot-labeled heavy-chain ferritin for self-targeting bio-imaging and chemo-photodynamic therapy. J. Mater. Chem. B 2018, 6, 3107–3115. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Lv, N.; Zhen, Z.; Liu, T.; Gao, S.; Xie, J.; Ma, Q. Photosensitizer-encapsulated ferritins mediate photodynamic therapy against cancer-associated fibroblasts and improve tumor accumulation of nanoparticles. Mol. Pharm. 2018, 15, 3595–3599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhen, Z.; Paschall, A.V.; Xue, L.; Yang, X.; Bebin Blackwell, A.G.; Cao, Z.; Zhang, W.; Wang, M.; Teng, Y.; et al. FAP-targeted photodynamic therapy mediated by ferritin nanoparticles elicits an immune response against cancer cells and cancer associated fibroblasts. Adv. Funct. Mater. 2020, 31, 2007017. [Google Scholar] [CrossRef]
- Kim, M.; Rho, Y.; Jin, K.S.; Ahn, B.; Jung, S.; Kim, H.; Ree, M. pH-dependent structures of ferritin and apoferritin in solution: Disassembly and reassembly. Biomacromolecules 2011, 12, 1629–1640. [Google Scholar] [CrossRef]
- Andrews, S.C. The Ferritin-like superfamily: Evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim. Biophys. Acta-Gen. Subj. 2010, 1800, 691–705. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, X.; Sun, G.; Chen, X.; Yin, Y.; Jin, Y.; Mi, Q.; Ma, L.; Yang, Y.; Yan, X.; et al. A natural drug entry channel in the ferritin nanocage. Nano Today 2020, 35, 100948. [Google Scholar] [CrossRef]
- Inoue, I.; Chiba, M.; Ito, K.; Okamatsu, Y.; Suga, Y.; Kitahara, Y.; Nakahara, Y.; Endo, Y.; Takahashi, K.; Tagami, U.; et al. One-step construction of ferritin encapsulation drugs for cancer chemotherapy. Nanoscale 2021, 13, 1875–1883. [Google Scholar] [CrossRef]
- Brown, J.M.; Giaccia, A.J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 1998, 58, 1408–1416. [Google Scholar] [PubMed]
- Liu, Y.J.; Bhattarai, P.; Dai, Z.F.; Chen, X.Y. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Zha, Z.B.; Deng, Z.J.; Li, Y.Y.; Li, C.H.; Wang, J.R.; Wang, S.M.; Qu, E.Z.; Dai, Z.F. Biocompatible polypyrrole nanoparticles as a novel organic photoacoustic contrast agent for deep tissue imaging. Nanoscale 2013, 5, 4462–4467. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, A.; Manchanda, R.; Lei, T.J.; Carvajal, D.A.; Tang, Y.; Kazmi, S.Z.R.; McGoron, A.J. Comparative study of the optical and heat generation properties of IR820 and indocyanine green. Mol. Imaging 2012, 11, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.R.; Singh, R.N.; Carroll, D.L.; Wood, J.C.S.; D’Agostino, R.B.; Ajayan, P.M.; Torti, F.M.; Torti, S.V. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials 2012, 33, 2961–2970. [Google Scholar] [CrossRef] [Green Version]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
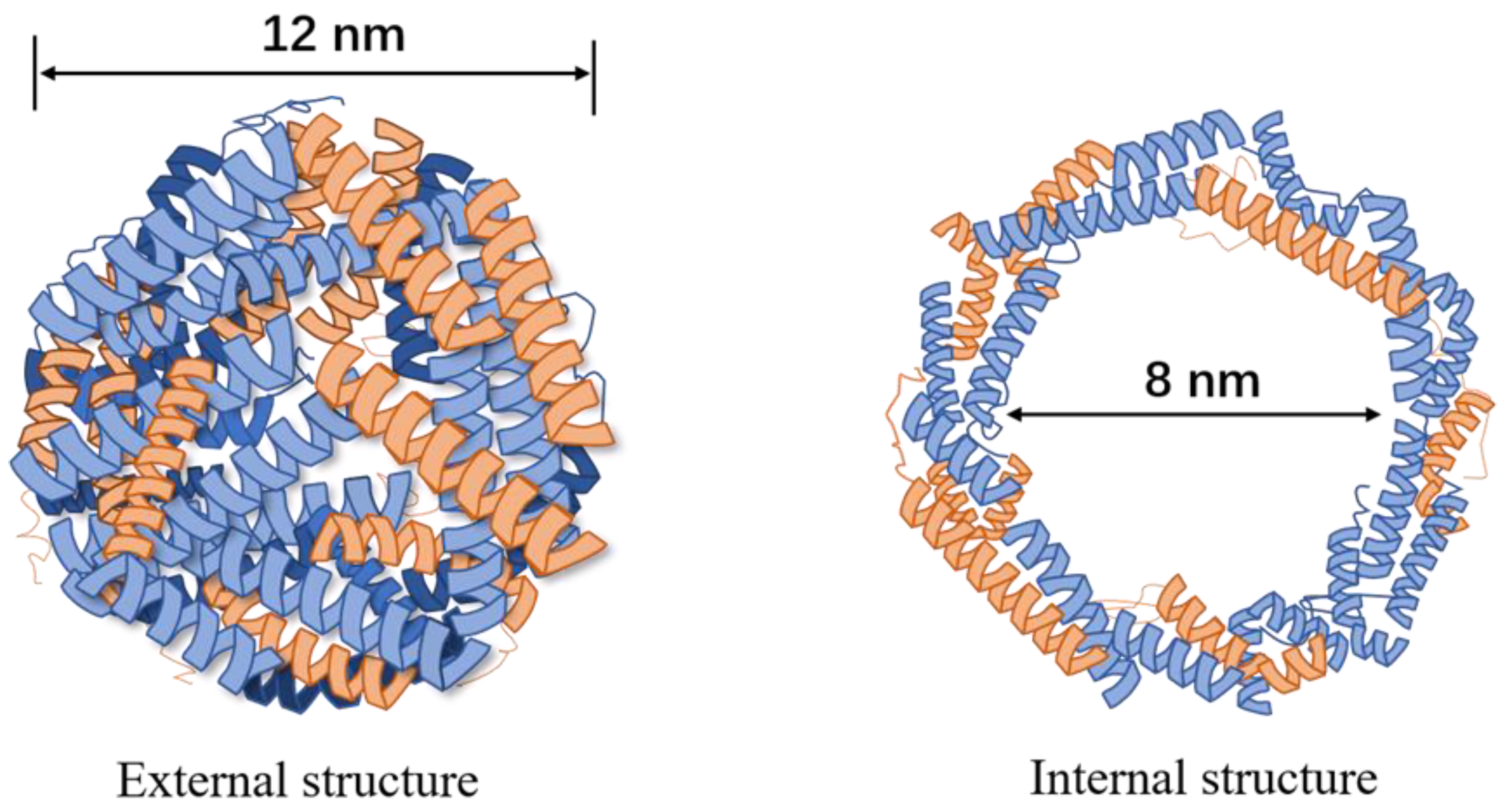
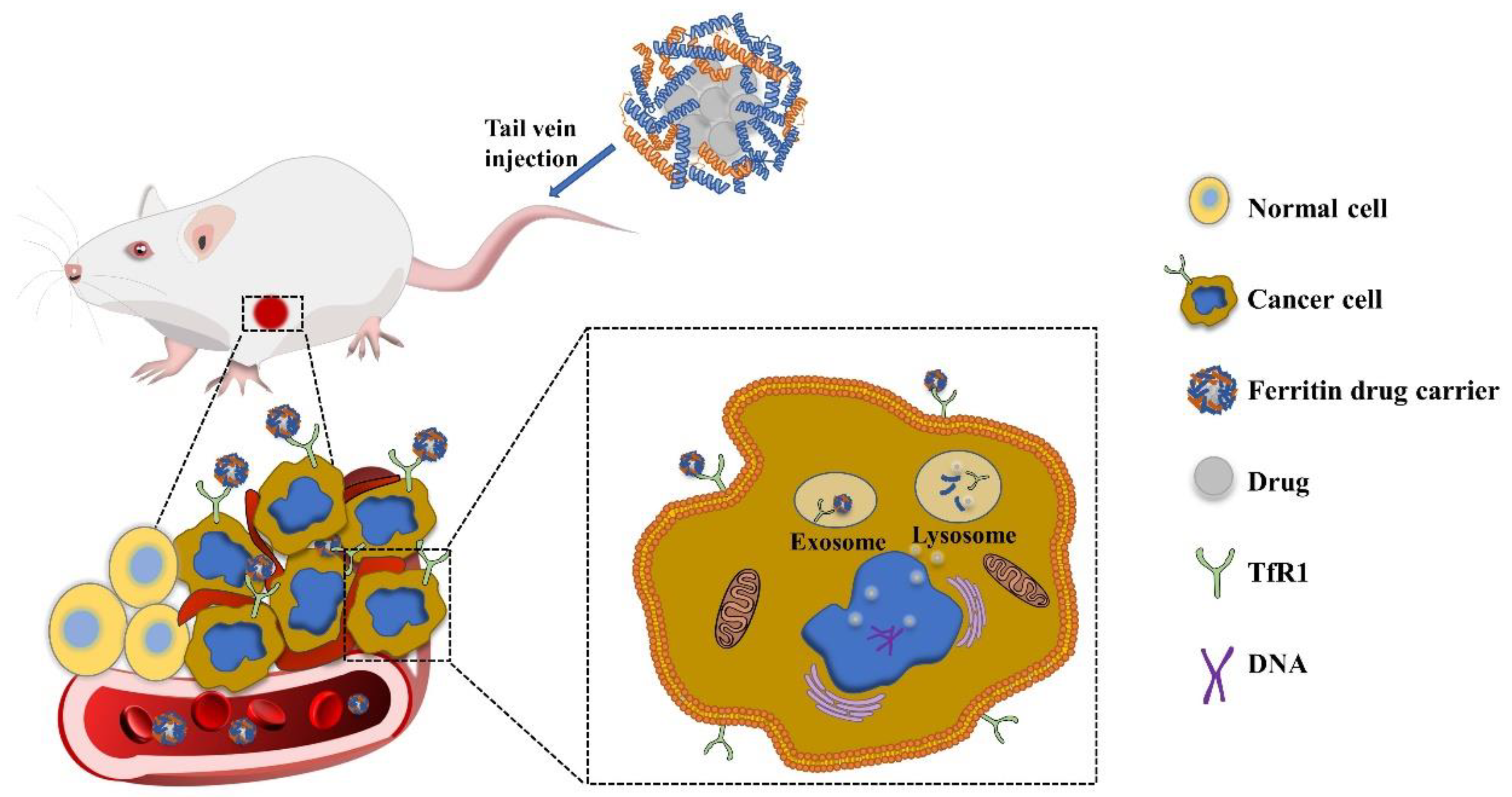
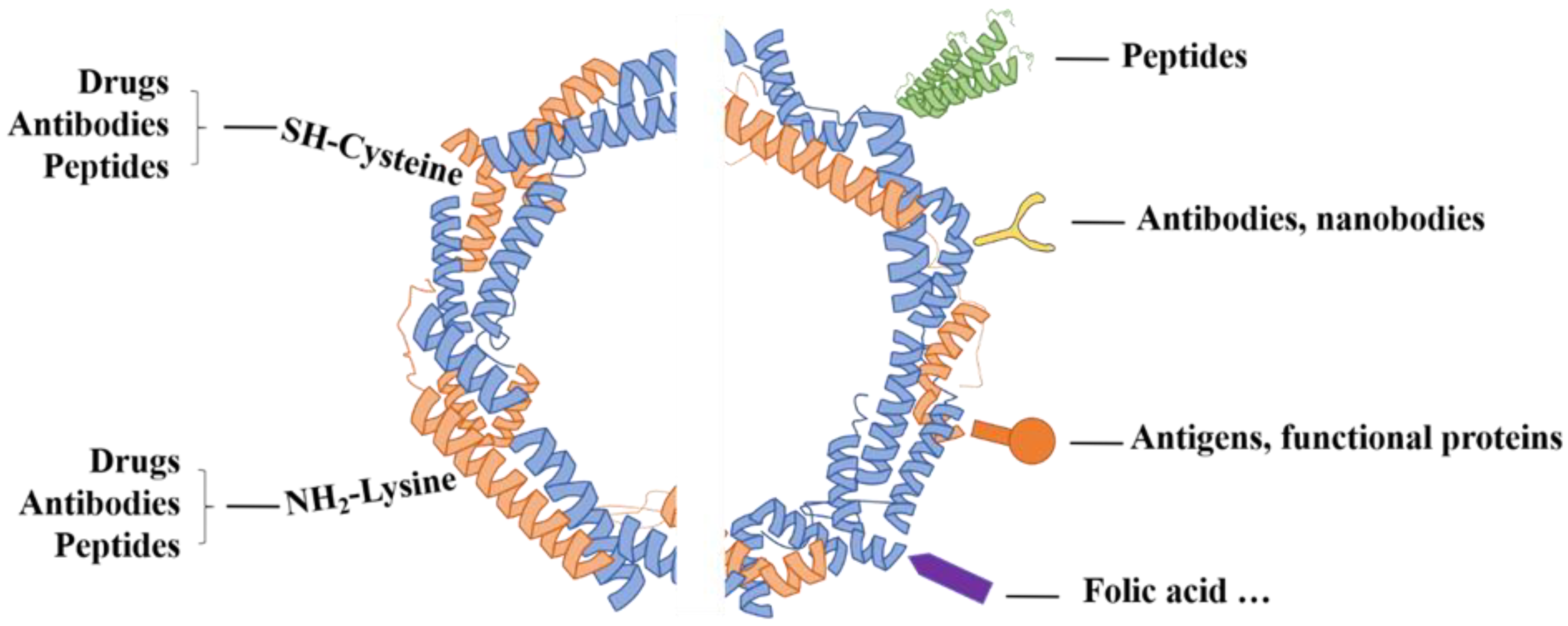

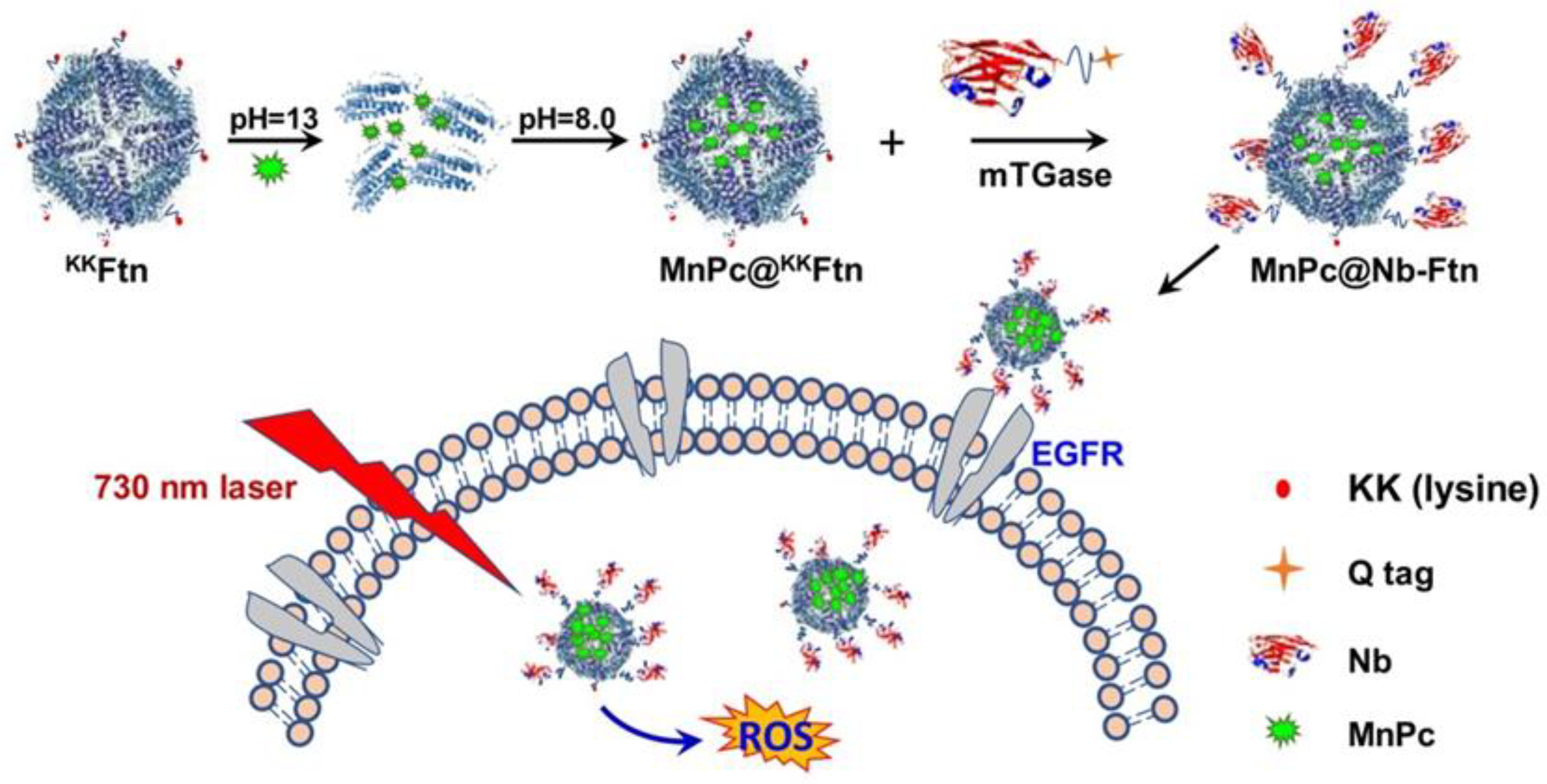

| Ferritin Carrier | Surface Modification | Targeting Receptor | Cancer Type | References |
|---|---|---|---|---|
| Human HFn | RGD peptide | Integrin αvβ3 | All types | [30] |
| human HFn | PASE masking peptide | \ | All types | [35] |
| human HFn | EGF | EGFR | Breast cancer | [23] |
| human HFn | GE11 peptide | EGFR | Breast cancer | [36] |
| Pyrococcus furiosus ferritin | SP94 peptide | GRP78 | Liver cancer | [37] |
| Apoferritin | GKRK peptide | HSPG | Glioma | [39] |
| Human HFn | Angiopep-2 peptide | LRP1 | Glioma | [40] |
| Human HFn | PEG | \ | Airway lung cancer | [41] |
| Human HFn | folic acid | folic acid receptor | \ | [42] |
| Human HFn | Ep1 antibody | CSPG4 | Melanoma | [43] |
| Human HFn | Nb | EGFR | Epidermoid carcinoma | [44] |
| Human HFn | RFP | LNs | Melanoma | [45] |
| Human HFn | MSH | Melanoma cells | Melanoma | [46] |
| Drug Carrier | Encapsulated Drug | Application | Cancer Type | Treatment Effect | References |
|---|---|---|---|---|---|
| Human HFn | Cisplatin | Chemotherapy | Melanoma | Improved the therapeutic index of melanoma | [43] |
| Horse spleen ferritin | Gold-based anticancer drugs | Chemotherapy | All types | The impact on normal cells was significantly reduced | [47,48] |
| Human HFn (MSH and PEG modified | Co (II) | Hyperthermia | Melanoma | Cell viability significantly reduced | [46] |
| Human HFn | Gefitinib | Chemotherapy | Breast cancer | Has enhanced tumor suppression (GI50 = 0.52 × 10−6 M) compared to free Gefitinib (GI50 = 1.66 × 10−6 M) | [49] |
| Human HFn | DOX (Pre-complexation with Cu (II)) | Chemotherapy | Glioblastoma | 89.6% TGI of U87MG subcutaneous tumor models | [29] |
| Human HFn | DOX | Chemotherapy | Gastric cancer | 91.1% TGI of TfR1-positive gastric cancer models | [50] |
| Apoferritin | VCR | Chemotherapy | Glioma | Relative tumor proliferation rate of VCR-loaded apoferritin (36.31 ± 5.52%) was much lower than free VCR (96.34 ± 5.56%) | [39] |
| Human HFn | Paclitaxel | Chemotherapy | Breast cancer | The tumor volume in Taxol group (0.8 cm3) was much lower than PBS group (2.26 cm3) | [51] |
| Apoferritin | Curcumin | Chemotherapy | Breast cancer | The therapeutic dose reached 97 μg/mL | [52] |
| Apoferritin | Quercetin and curcumin | Chemotherapy | Breast cancer | The EC50 for MCF7 reduced to 11 μM | [53] |
| Human HFn | Atropine | Chemotherapy | Pancreatic cancer | The neurogenesis in pancreatic cancer was impaired | [54] |
| Human HFn (PASE masking peptide modified) | Genz-644282 | Chemotherapy | All types | 94.0% TGI of xenograft (subcutaneous) model of pancreatic (HPAF II cells) cancer model; 100% TGI of xenograft PaCa44 pancreatic, triple-negative breast and liver cancer model | [34,35] |
| Apoferritin | GW 610 and amino acid prodrugs | Chemotherapy | Breast and colorectal carcinoma | The Apoferritin-encapsulated Lys modified GW 608 complexes exhibit potent anticancer activity | [55] |
| Horse spleen ferritin (protective PAS peptides or PEG modified) | Ellipticine | Chemotherapy | Breast cancer | All three surface modifications of ferritin displayed beneficial effects on biocompatibility | [56] |
| Human HFn | CuS | PTT | Glioblastoma | 100% tumor elimination was achieved in CuS-Fn group plus laser irradiation | [57] |
| Human HFn | IR820 | PTT | Breast cancer | Eliminated 100% mouse breast cancer cells | [58] |
| Apoferritin | Epirubicin and DBN | Chemotherapy and PTT | Breast cancer | Killed about 80% of CSCs in primary tumor with photodynamic therapy | [59] |
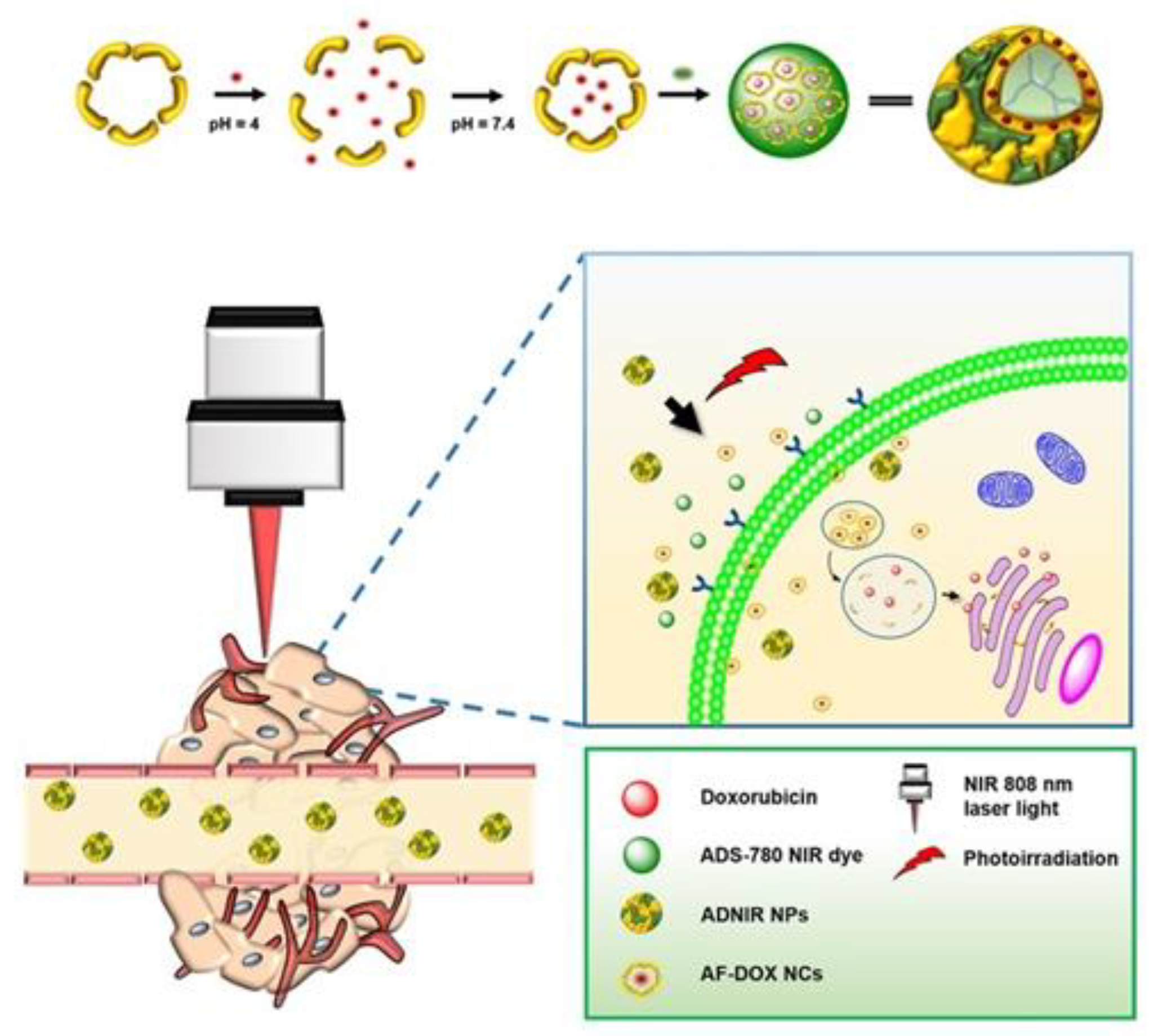
| Apoferritin | DOX and ADNIR | Chemotherapy and PTT | Colon cancer | The tumor size was significantly reduced in a mice HT-29 tumor model | [60] |
| Apoferritin (with Au nanoshell) | DOX | Chemotherapy and PTT | Liver cancer | Hepa1-6 cells have a low viability (4.3%) after chemotherapy and PTT | [61] |
| Apoferritin | RSV and IR780 | PTT | Ovarian cancer | The survival rate was high after 60 days of combined treatment | [62] |
| Human HFn (Prussian blue PB-modified) | gemcitabine GEM | Chemotherapy and PTT | Breast cancer | PB-Ft NPs-assisted photothermo-chemotherapy effectively damaged the 4T1 tumor cells | [63] |
| Apoferritin (RGD modified) | ZnF16Pc | PDT | Breast cancer | The loading rate reached 60% | [64] |
| Apoferritin | HB | PDT | Breast cancer | HB encapsulation efficiency of 85% | [65] |
| Apoferritin | DOX and RB | Chemotherapy and PDT | Breast cancer | The cell inhibition rate was up to ~83% | [66] |
| Human HFn (CDs modified) | DOX | Chemotherapy and PDT | Breast cancer | Simultaneous action of CDs and DOX was the most effective for DNA damage | [67] |
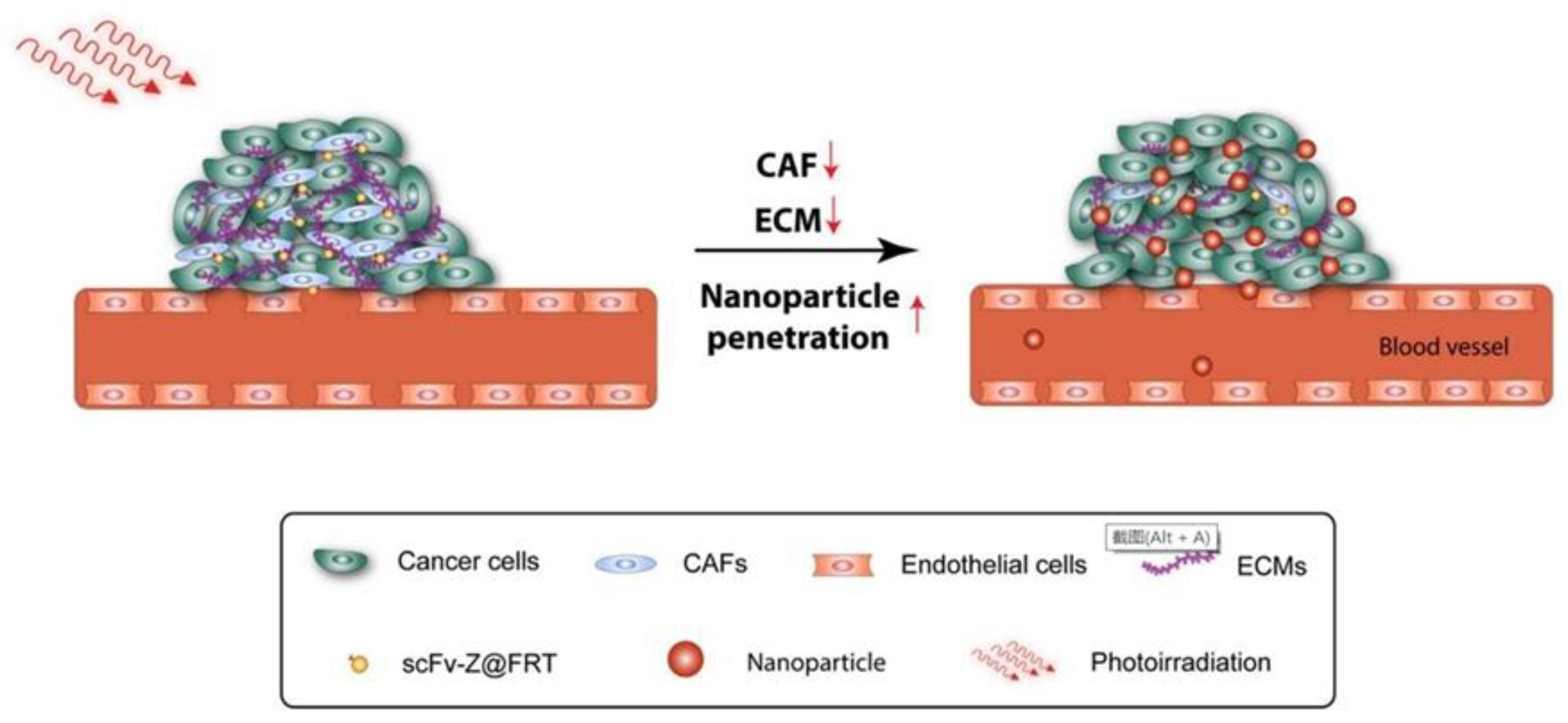
| Human HFn (scFv sequence modified) | ZnF16Pc | PDT | Breast cancer | Selectively killing CAFs under the action of PDT; stimulate immunity against CAFs and induce broad-spectrum anti-cancer immunity | [68,69] |
| Human HFn (RGD peptide modified) | DVDMS | PTT and PDT | Breast cancer | Eliminated 100% mouse breast cancer cells with PTT and PDT | [30] |
| Human HFn (CGKRK peptide modified) | 556-Ph | PTT and PDT | Breast cancer | The tumor in the PTT + PDT group did not recur after 16 days of treatment | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Hong, Y.; Gong, Y.; Zheng, S.; Xie, D. Bioengineered Ferritin Nanocarriers for Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 7023. https://doi.org/10.3390/ijms22137023
Sun X, Hong Y, Gong Y, Zheng S, Xie D. Bioengineered Ferritin Nanocarriers for Cancer Therapy. International Journal of Molecular Sciences. 2021; 22(13):7023. https://doi.org/10.3390/ijms22137023
Chicago/Turabian StyleSun, Xuanrong, Yulu Hong, Yubei Gong, Shanshan Zheng, and Dehui Xie. 2021. "Bioengineered Ferritin Nanocarriers for Cancer Therapy" International Journal of Molecular Sciences 22, no. 13: 7023. https://doi.org/10.3390/ijms22137023
APA StyleSun, X., Hong, Y., Gong, Y., Zheng, S., & Xie, D. (2021). Bioengineered Ferritin Nanocarriers for Cancer Therapy. International Journal of Molecular Sciences, 22(13), 7023. https://doi.org/10.3390/ijms22137023






