Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-β Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke
Abstract
:1. Introduction
2. Results
2.1. ELF-PEMFs Increased Viability and Alleviated the Negative Effects of CSE in SCP-1 Cells
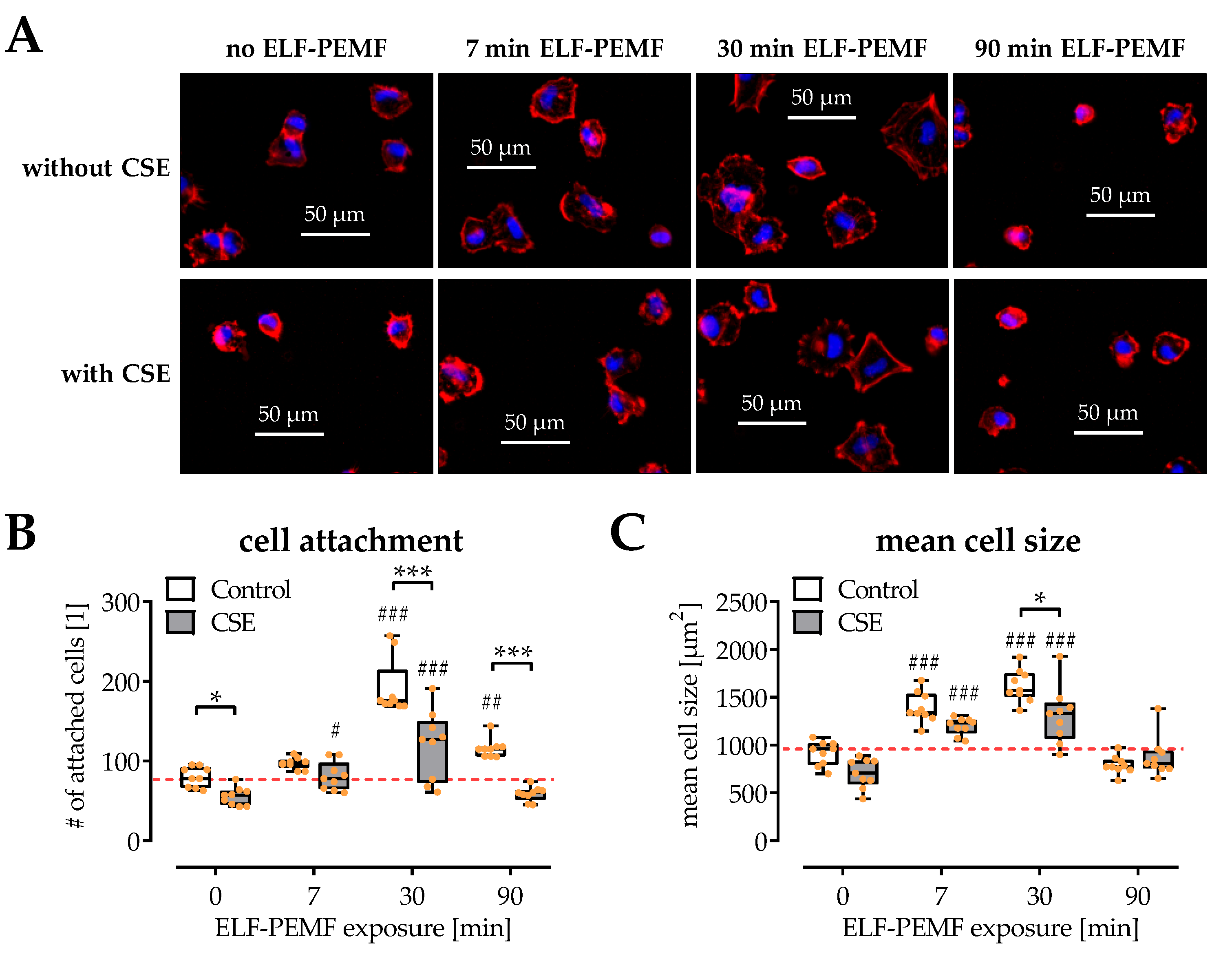
2.2. SCP-1 Cell Adhesion and Spreading Are Enhanced by ELF-PEMF Exposure
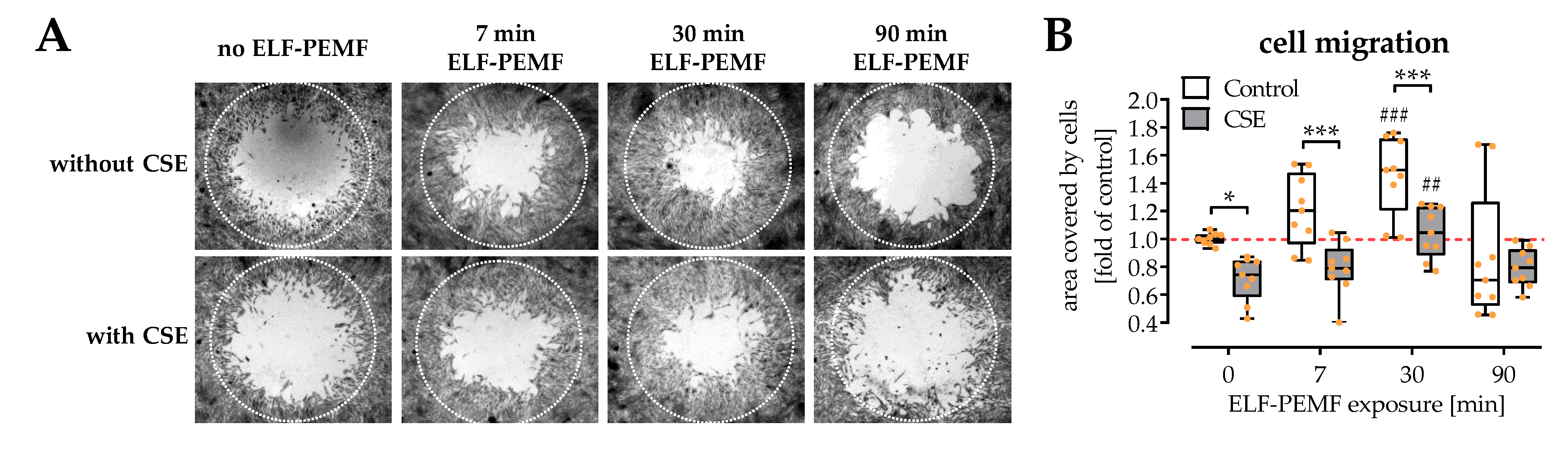
2.3. Exposure to ELF-PEMFs Enhances Migration of SCP-1 Cells
2.4. ELF-PEMF Exposure Intensified TGF-β Signaling in SCP-1 Cells
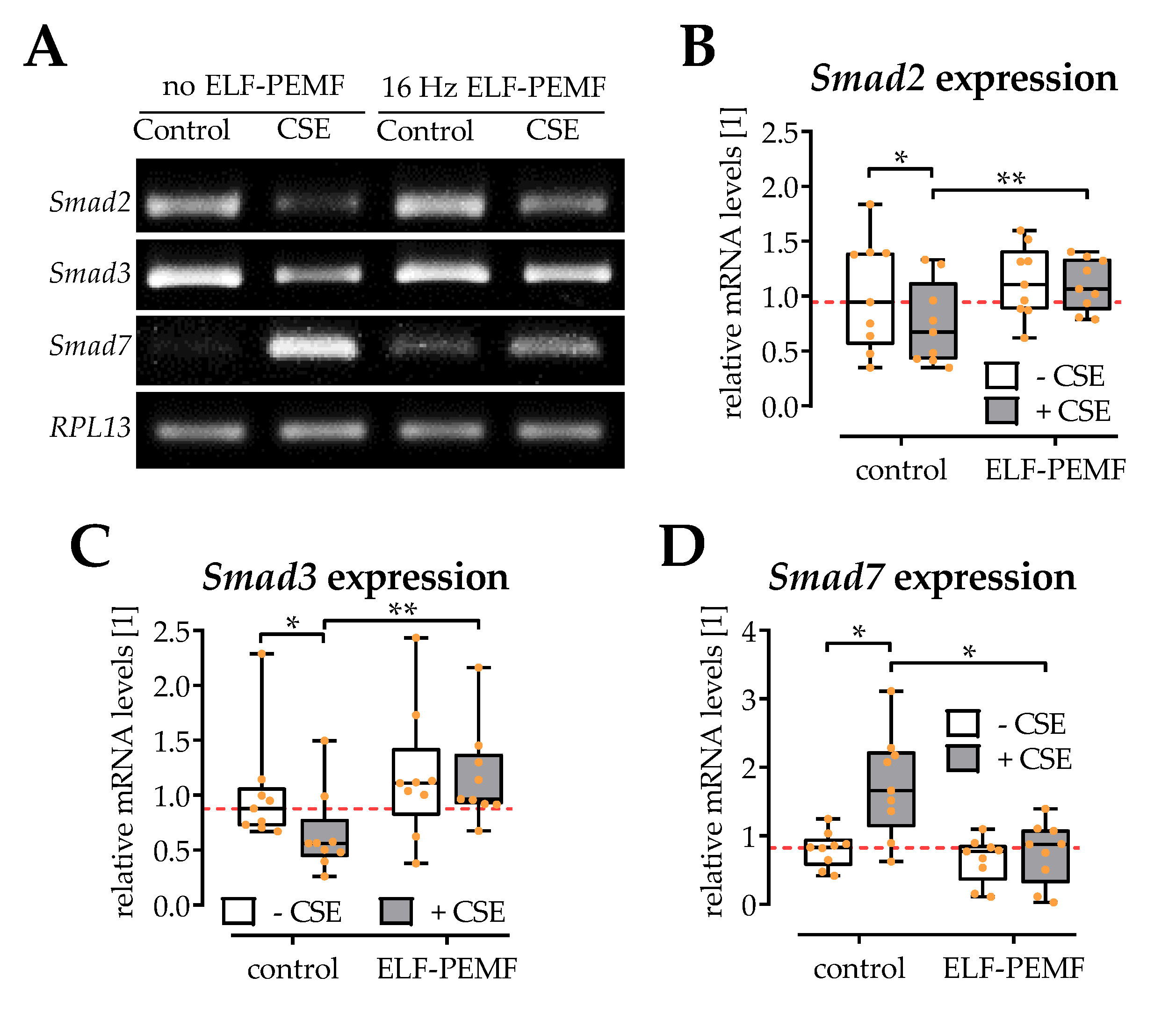
2.5. ELF-PEMF Exposure Reversed CSE-Mediated Changes in Smad2, -3, and -7 Expression
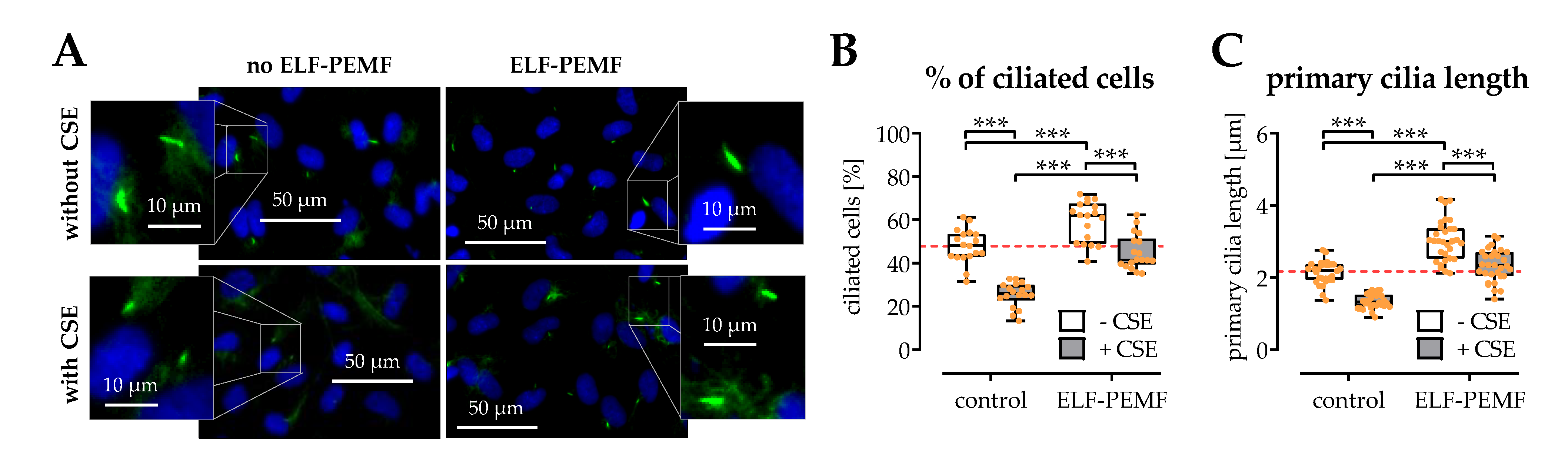
2.6. The Structural Integrity of Primary Cilia Is Related to the Protective Effects of 16 Hz ELF-PEMFs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. ELF-PEMF Device and Exposure
4.3. Cigarette Smoke Extract (CSE) Preparation
4.4. Cell Viability
4.5. Live Staining and Cytoskeleton Staining
4.6. Cell Migration Assay
4.7. Immunofluorescent (IF) Staining for Primary Cilia
4.8. Transient Cells Infection and Reporter Assay
4.9. Conventional RT-PCR
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | cigarette smoking |
| CSE | cigarette smoke extract |
| ELF-PEMF | extremely low frequency pulsed electromagnetic field |
| EtOH | ethanol |
| ERK1/2 | extracellular signal-regulated kinases 1 and 2 |
| MSCs | mesenchymal stem cells |
| PI3K | phosphoinositide 3-kinase |
| ROS | reactive oxygen species |
| SRB | Sulforhodamine B |
| TRITC | tetramethylrhodamine |
References
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Frohlich, T.; Nussler, A.K. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Cao, H.; Cu, F.; Xu, D.; Lei, Y.; Tan, Y.; Magdalou, J.; Wang, H.; Chen, L. Nicotine-induced retardation of chondrogenesis through down-regulation of IGF-1 signaling pathway to inhibit matrix synthesis of growth plate chondrocytes in fetal rats. Toxicol. Appl. Pharmacol. 2013, 269, 25–33. [Google Scholar] [CrossRef]
- Cheng, Y.; Gu, W.; Zhang, G.; Li, X.; Guo, X. Activation of Notch1 signaling alleviates dysfunction of bone marrow-derived mesenchymal stem cells induced by cigarette smoke extract. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 3133–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Q.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellstrom, D.; Andersson, E.M. Smoking-Induced Risk of Osteoporosis Is Partly Mediated by Cadmium From Tobacco Smoke: TheMrOSSweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef] [Green Version]
- Tamaki, J.; Iki, M.; Fujita, Y.; Kouda, K.; Yura, A.; Kadowaki, E.; Sato, Y.; Moon, J.S.; Tomioka, K.; Okamoto, N.; et al. Impact of smoking on bone mineral density and bone metabolism in elderly men: The Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Osteoporos. Int. 2011, 22, 133–141. [Google Scholar] [CrossRef]
- Scolaro, J.A.; Schenker, M.L.; Yannascoli, S.; Baldwin, K.; Mehta, S.; Ahn, J. Cigarette smoking increases complications following fracture: A systematic review. J. Bone Joint Surg. Am. 2014, 96, 674–681. [Google Scholar] [CrossRef]
- Kaku, M.; Komatsu, Y. Functional Diversity of Ciliary Proteins in Bone Development and Disease. Curr. Osteoporos. Rep. 2017, 15, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Schroter, S.; Aspera-Werz, R.H.; Eisler, W.; Falldorf, K.; Ronniger, M.; Nussler, A.K. Translational Insights into Extremely Low Frequency Pulsed Electromagnetic Fields (ELF-PEMFs) for Bone Regeneration after Trauma and Orthopedic Surgery. J. Clin. Med. 2019, 8, 2028. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, I. Classic Fundamental Aspects of Fracture Treatment. Clin. Orthop. Relat. Res. 1977, 124, 5–8. [Google Scholar]
- Bhandari, M.; Fong, K.; Sprague, S.; Williams, D.; Petrisor, B. Variability in the definition and perceived causes of delayed unions and nonunions: A cross-sectional, multinational survey of orthopaedic surgeons. J. Bone Joint Surg. Am. 2012, 94, e1091–e1096. [Google Scholar] [CrossRef]
- Ehnert, S.; Falldorf, K.; Fentz, A.K.; Ziegler, P.; Schroter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekumar, V.; Aspera-Werz, R.; Ehnert, S.; Strobel, J.; Tendulkar, G.; Heid, D.; Schreiner, A.; Arnscheidt, C.; Nussler, A.K. Resveratrol protects primary cilia integrity of human mesenchymal stem cells from cigarette smoke to improve osteogenic differentiation in vitro. Arch. Toxicol. 2018, 92, 1525–1538. [Google Scholar] [CrossRef]
- Jacobs, C.R.; Temiyasathit, S.; Castillo, A.B. Osteocyte mechanobiology and pericellular mechanics. Annu. Rev. Biomed. Eng. 2010, 12, 369–400. [Google Scholar] [CrossRef]
- Seeger-Nukpezah, T.; Golemis, E.A. The extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012, 24, 652–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, G.; Moghaddam, A.; Reumann, M.; Wangler, B.; Breier, L.; Wentzensen, A.; Henle, P.; Weiss, S. TGF-beta1 as a pathophysiological factor in fracture healing. Unfallchirurg 2007, 110, 130–136. [Google Scholar] [CrossRef]
- Ehnert, S.; Sreekumar, V.; Aspera-Werz, R.H.; Sajadian, S.O.; Wintermeyer, E.; Sandmann, G.H.; Bahrs, C.; Hengstler, J.G.; Godoy, P.; Nussler, A.K. TGF-beta(1) impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia. J. Mol. Med. 2017, 95, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.A.; Ajbro, K.D.; Koefoed, K.; Vestergaard, M.L.; Veland, I.R.; Henriques de Jesus, M.P.; Pedersen, L.B.; Benmerah, A.; Andersen, C.Y.; Larsen, L.A.; et al. TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013, 3, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Satta, M.; Lago-Docampo, M.; Bea-Mascato, B.; Solarat, C.; Castro-Sanchez, S.; Christensen, S.T.; Valverde, D. ALMS1 Regulates TGF-beta Signaling and Morphology of Primary Cilia. Front. Cell Dev. Biol. 2021, 9, 623829. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-beta signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Bykova, D.; Biermann, S.; Fecht, L.; De Zwart, P.M.; Nussler, A.K.; Stuby, F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-beta Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int. J. Mol. Sci. 2018, 19, 2239. [Google Scholar] [CrossRef] [Green Version]
- Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schroter, S.; Relja, B.; Nussler, A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghaddam, A.; Weiss, S.; Wolfl, C.G.; Schmeckenbecher, K.; Wentzensen, A.; Grutzner, P.A.; Zimmermann, G. Cigarette smoking decreases TGF-b1 serum concentrations after long bone fracture. Injury 2010, 41, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Fentz, A.-K.; Schreiner, A.; Birk, J.; Wilbrand, B.; Ziegler, P.; Reumann, M.K.; Wang, H.; Falldorf, K.; Nussler, A.K. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of *O2(-) and H2O2. Sci. Rep. 2017, 7, 14544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, N.; Xiao, Z.; Cao, L.; Buechel, M.M.; David, V.; Roan, E.; Quarles, L.D. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J. Cell Sci. 2012, 125, 1945–1957. [Google Scholar]
- Zhou, J.; Gao, Y.H.; Zhu, B.Y.; Shao, J.L.; Ma, H.P.; Xian, C.J.; Chen, K.M. Sinusoidal Electromagnetic Fields Increase Peak Bone Mass in Rats by Activating Wnt10b/beta-Catenin in Primary Cilia of Osteoblasts. J. Bone Miner. Res. 2019, 34, 1336–1351. [Google Scholar] [CrossRef]
- Daish, C.; Blanchard, R.; Fox, K.; Pivonka, P.; Pirogova, E. The Application of Pulsed Electromagnetic Fields (PEMFs) for Bone Fracture Repair: Past and Perspective Findings. Ann. Biomed. Eng. 2018, 46, 525–542. [Google Scholar] [CrossRef]
- Ziegler, P.; Nussler, A.K.; Wilbrand, B.; Falldorf, K.; Springer, F.; Fentz, A.K.; Eschenburg, G.; Ziegler, A.; Stockle, U.; Maurer, E.; et al. Pulsed Electromagnetic Field Therapy Improves Osseous Consolidation after High Tibial Osteotomy in Elderly Patients-A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Med. 2019, 8, 2008. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Alharbi, M.; Graves, D.; Yang, S. IFT80 Is Required for Fracture Healing Through Controlling the Regulation of TGF-beta Signaling in Chondrocyte Differentiation and Function. J. Bone Miner. Res. 2020, 35, 571–582. [Google Scholar] [CrossRef]
- Xie, Y.-F.; Shi, W.G.; Zhou, J.; Gao, Y.-H.; Li, S.-F.; Fang, Q.-Q.; Wang, M.-G.; Ma, H.-P.; Wang, J.-F.; Xian, C.J.; et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone 2016, 93, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-L.; Zhou, J.; Ma, H.-P.; Ma, X.-N.; Gao, Y.-H.; Shi, W.-G.; Fang, Q.-Q.; Ren, Q.; Xian, C.J.; Chen, K.-M. Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol. Cell Endocrinol. 2015, 404, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Alomari, N.; Nauli, S.M. Primary Cilium-Dependent Signaling Mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Ferland, R.J. Primary cilia proteins: Ciliary and extraciliary sites and functions. Cell Mol. Life Sci. 2018, 75, 1521–1540. [Google Scholar] [CrossRef]
- Spasic, M.; Jacobs, C.R. Lengthening primary cilia enhances cellular mechanosensitivity. Eur. Cell Mater. 2017, 33, 158–168. [Google Scholar] [CrossRef]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix control of transforming growth factor-beta function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.G. Endocytic regulation of TGF-beta signaling. Cell Res. 2009, 19, 58–70. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Mogensen, J.B.; Christensen, S.T. Endocytic Control of Cellular Signaling at the Primary Cilium. Trends Biochem. Sci. 2016, 41, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xin, F.; Jiang, W. Underlying Signaling Pathways and Therapeutic Applications of Pulsed Electromagnetic Fields in Bone Repair. Cell Physiol. Biochem. 2018, 46, 1581–1594. [Google Scholar] [CrossRef]
- Selvamurugan, N.; He, Z.; Rifkin, D.; Dabovic, B.; Partridge, N.C. Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-beta Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation. Stem Cells Int. 2017, 2017, 2450327. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H.; Ning, B.; Ma, X.E.; Gong, W.M.; Jia, T.H. Regulatory roles of microRNA-21 in fibrosis through interaction with diverse pathways (Review). Mol. Med. Rep. 2016, 13, 2359–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Han, W.; Giraldo, C.; De Li, Y.; Block, E.R. Effect of cigarette smoke extract on nitric oxide synthase in pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]



| Target Gene | Gene Bank Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Amplicon Size [bp] | Ta [°C] | Number of Cycles |
|---|---|---|---|---|---|---|
| Smad2 | NM_001003652.3 | CAAACCAGGTCTCTTGATGG | GAGGCGGAAGTTCTGTTAGG | 259 | 60 | 35 |
| Smad3 | NM_005902.2 | GGAGAAATGGTGCGAGAAGG | GAAGGCGAACTCACACAGC | 259 | 60 | 35 |
| Smad7 | NM_005904.1 | TTCGGACAACAAGAGTCAGC | AAGCCTTGATGGAGAAACC | 200 | 60 | 35 |
| RPL13 | NM_012423.3 | AAGTACCAGGCAGTGACAG | CCTGTTTCCGTAGCCTCATG | 100 | 56 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Aspera-Werz, R.H.; Menger, M.M.; Falldorf, K.; Ronniger, M.; Stacke, C.; Histing, T.; Nussler, A.K.; Ehnert, S. Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-β Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke. Int. J. Mol. Sci. 2021, 22, 7036. https://doi.org/10.3390/ijms22137036
Chen Y, Aspera-Werz RH, Menger MM, Falldorf K, Ronniger M, Stacke C, Histing T, Nussler AK, Ehnert S. Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-β Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke. International Journal of Molecular Sciences. 2021; 22(13):7036. https://doi.org/10.3390/ijms22137036
Chicago/Turabian StyleChen, Yangmengfan, Romina H. Aspera-Werz, Maximilian M. Menger, Karsten Falldorf, Michael Ronniger, Christina Stacke, Tina Histing, Andreas K. Nussler, and Sabrina Ehnert. 2021. "Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-β Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke" International Journal of Molecular Sciences 22, no. 13: 7036. https://doi.org/10.3390/ijms22137036
APA StyleChen, Y., Aspera-Werz, R. H., Menger, M. M., Falldorf, K., Ronniger, M., Stacke, C., Histing, T., Nussler, A. K., & Ehnert, S. (2021). Exposure to 16 Hz Pulsed Electromagnetic Fields Protect the Structural Integrity of Primary Cilia and Associated TGF-β Signaling in Osteoprogenitor Cells Harmed by Cigarette Smoke. International Journal of Molecular Sciences, 22(13), 7036. https://doi.org/10.3390/ijms22137036







