The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin–Angiotensin Pathways
Abstract
:1. Introduction
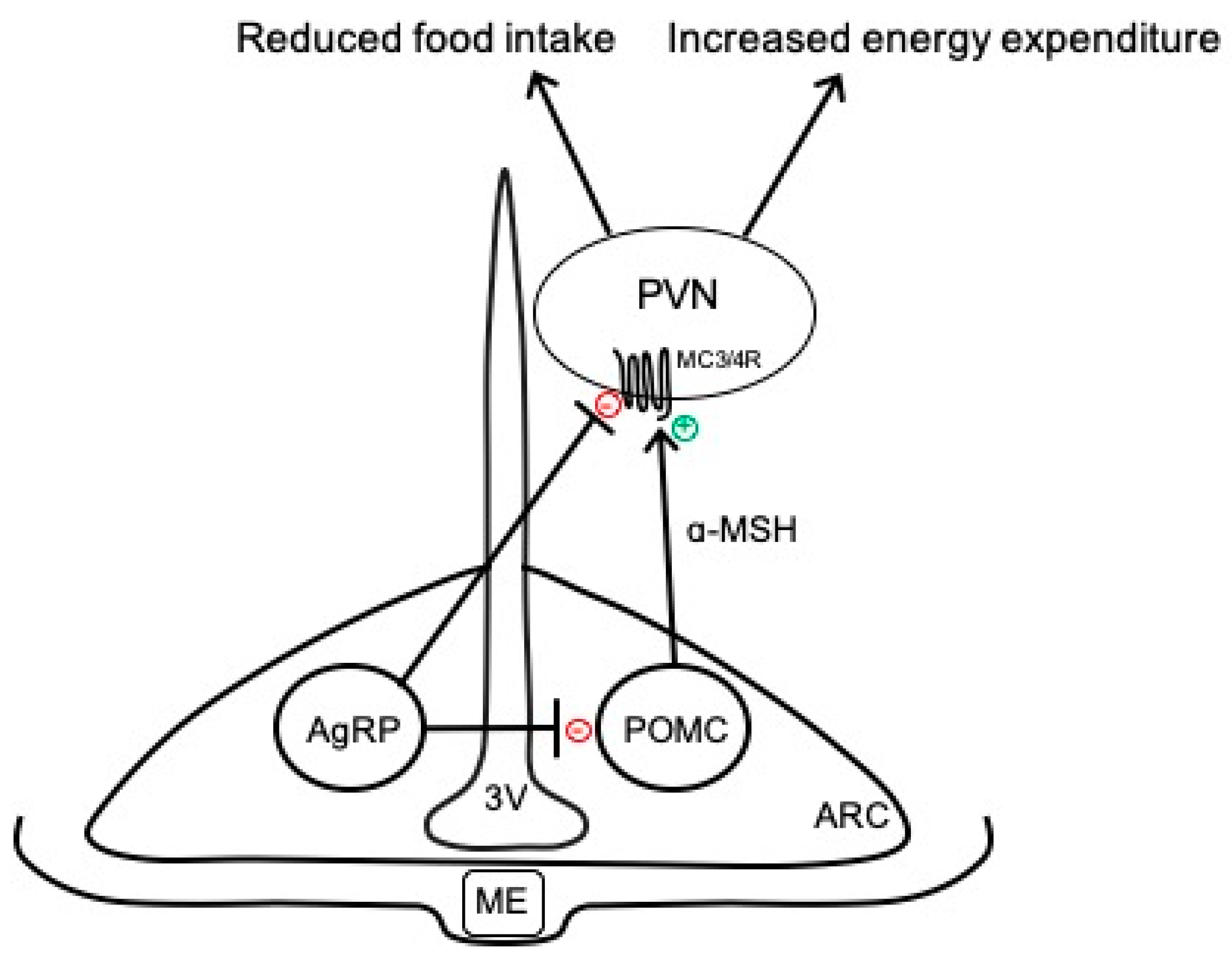
2. Overview of Arcuate Neurocircuits Controlling Energy Balance
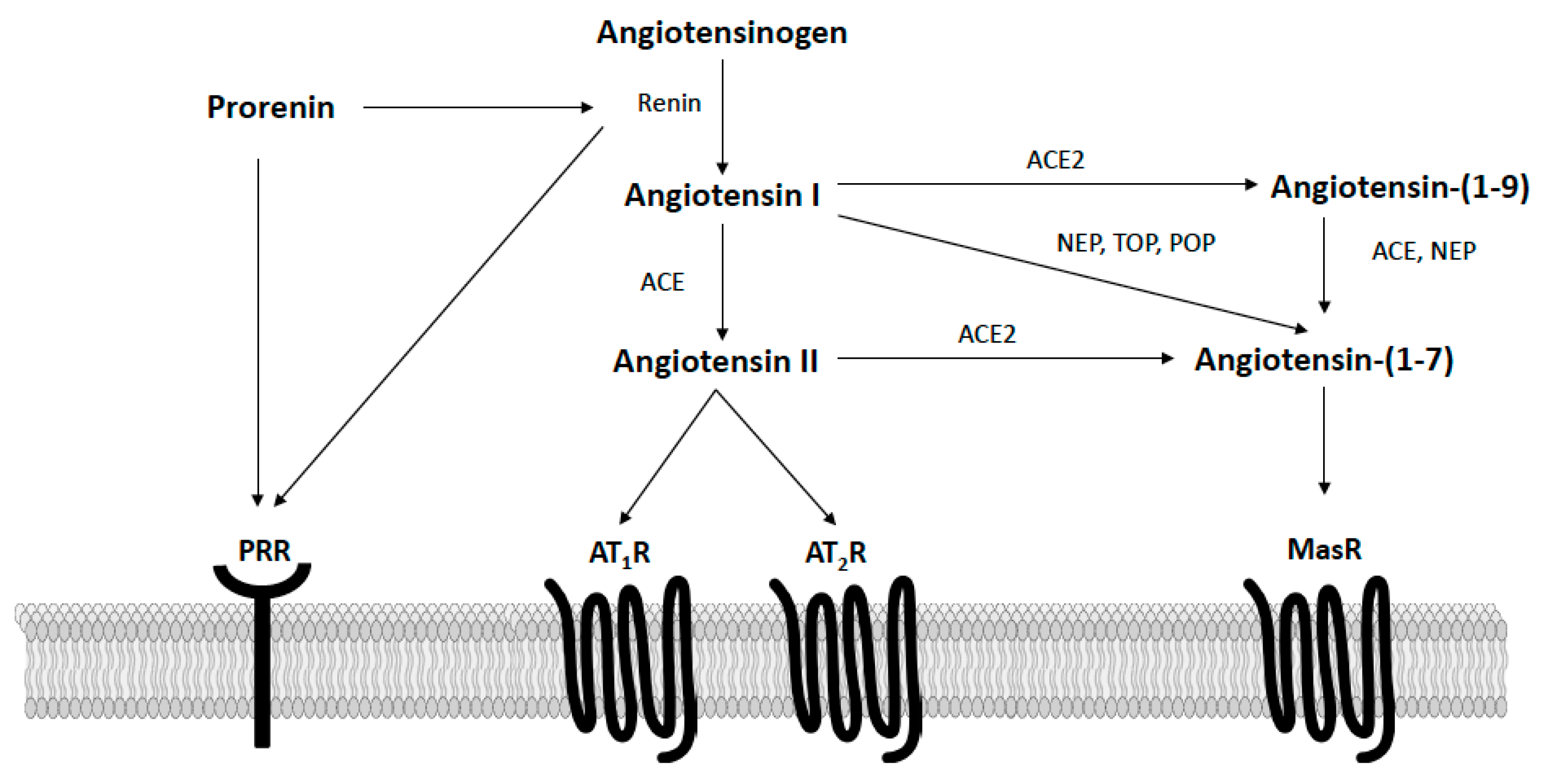
3. Circulating Renin–Angiotensin System in Obesity
4. Renin–Angiotensin Interactions with Arcuate Neurocircuits for Metabolic Regulation
4.1. Angiotensin II Pathways
4.2. Prorenin Pathways
4.3. Angiotensin-(1–7) Pathways
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hales, C.M. Prevalence of Obesity among Adults and Youth: United States, 2015–2016; NCHS Data Brief no. 346; National Center for Health Statistics: Hyattsville, MD, USA, 2017; pp. 1–8.
- Sullivan, P.W.; Morrato, E.H.; Ghushchyan, V.; Wyatt, H.R.; Hill, J.O. Obesity, Inactivity, and the Prevalence of Diabetes and Diabetes-Related Cardiovascular Comorbidities in the U.S., 2000–2002. Diabetes Care 2005, 28, 1599–1603. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association’s Strategic Impact Goal through 2020 and Beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-Term Weight Loss Maintenance for Obesity: A Multidisciplinary Approach. Diabetes Metab. Syndr. Obes. 2016, 9, 37–46. [Google Scholar]
- Rodgers, R.J.; Tschöp, M.H.; Wilding, J.P.H. Anti-Obesity Drugs: Past, Present and Future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mark, A.L. Cardiovascular Side Effects of Anti-Obesity Drugs: A Yellow Flag in the Race to Effective, Safe Pharmacotherapy for Obesity. Circulation 2009, 120, 719–721. [Google Scholar] [CrossRef]
- Grill, H.J. Distributed Neural Control of Energy Balance: Contributions from Hindbrain and Hypothalamus. Obesity 2006, 14, 216S–221S. [Google Scholar] [CrossRef]
- Münzberg, H.; Qualls-Creekmore, E.; Berthoud, H.-R.; Morrison, C.D.; Yu, S. Neural Control of Energy Expenditure. Handb. Exp. Pharmacol. 2016, 233, 173–194. [Google Scholar] [PubMed] [Green Version]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Rahmouni, K.; López, M. Traveling from the Hypothalamus to the Adipose Tissue: The Thermogenic Pathway. Redox Biol. 2017, 12, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K. Cardiovascular Regulation by the Arcuate Nucleus of the Hypothalamus: Neurocircuitry and Signaling Systems. Hypertension 2016, 67, 1064–1071. [Google Scholar] [CrossRef]
- Mercer, A.J.; Hentges, S.T.; Meshul, C.K.; Low, M.J. Unraveling the Central Proopiomelanocortin Neural Circuits. Front. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Jégou, S.; Cone, R.D.; Eberlé, A.N.; Vaudry, H. Melanocortins. In Handbook of Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2013; pp. 838–844. ISBN 978-0-12-385095-9. [Google Scholar]
- Cai, M.; Hruby, V.J. The Melanocortin Receptor System: A Target for Multiple Degenerative Diseases. Curr. Protein Pept. Sci. 2016, 17, 488–496. [Google Scholar] [CrossRef]
- Vargas-Castillo, A.; Fuentes-Romero, R.; Rodriguez-Lopez, L.A.; Torres, N.; Tovar, A.R. Understanding the Biology of Thermogenic Fat: Is Browning A New Approach to the Treatment of Obesity? Arch. Med. Res. 2017, 48, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Leyva, S.; Diano, S. Hormonal Regulation of the Hypothalamic Melanocortin System. Front. Physiol. 2014, 5, 480. [Google Scholar] [CrossRef] [PubMed]
- Toda, C.; Santoro, A.; Kim, J.D.; Diano, S. POMC Neurons: From Birth to Death. Annu. Rev. Physiol. 2017, 79, 209–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, C.; Claret, M.; Zeltser, L.M.; Williams, K.W.; Yeo, G.S.H.; Tschöp, M.H.; Diano, S.; Brüning, J.C.; Cota, D. POMC Neuronal Heterogeneity in Energy Balance and beyond: An Integrated View. Nat. Metab. 2021, 3, 299–308. [Google Scholar] [CrossRef]
- Williams, K.W.; Margatho, L.O.; Lee, C.E.; Choi, M.; Lee, S.; Scott, M.M.; Elias, C.F.; Elmquist, J.K. Segregation of Acute Leptin and Insulin Effects in Distinct Populations of Arcuate Proopiomelanocortin Neurons. J. Neurosci. 2010, 30, 2472–2479. [Google Scholar] [CrossRef] [Green Version]
- Hentges, S.T.; Nishiyama, M.; Overstreet, L.S.; Stenzel-Poore, M.; Williams, J.T.; Low, M.J. GABA Release from Proopiomelanocortin Neurons. J. Neurosci. 2004, 24, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Wittmann, G.; Hrabovszky, E.; Lechan, R.M. Distinct Glutamatergic and GABAergic Subsets of Hypothalamic Pro-Opiomelanocortin Neurons Revealed by in Situ Hybridization in Male Rats and Mice. J. Comp. Neurol. 2013, 521, 3287–3302. [Google Scholar] [CrossRef] [Green Version]
- Suyama, S.; Yada, T. New Insight into GABAergic Neurons in the Hypothalamic Feeding Regulation. J. Physiol. Sci. 2018, 68, 717–722. [Google Scholar] [CrossRef]
- Tupone, D.; Madden, C.J.; Morrison, S.F. Autonomic Regulation of Brown Adipose Tissue Thermogenesis in Health and Disease: Potential Clinical Applications for Altering BAT Thermogenesis. Front. Neurosci. 2014, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Song, N.-J.; Chang, S.-H.; Li, D.Y.; Villanueva, C.J.; Park, K.W. Induction of Thermogenic Adipocytes: Molecular Targets and Thermogenic Small Molecules. Exp. Mol. Med. 2017, 49, e353. [Google Scholar] [CrossRef] [Green Version]
- Bartness, T.J.; Ryu, V. Neural Control of White, Beige and Brown Adipocytes. Int. J. Obes. Suppl. 2015, 5, S35–S39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef]
- Finlin, B.S.; Memetimin, H.; Confides, A.L.; Kasza, I.; Zhu, B.; Vekaria, H.J.; Harfmann, B.; Jones, K.A.; Johnson, Z.R.; Westgate, P.M.; et al. Human Adipose Beiging in Response to Cold and Mirabegron. JCI Insight 2018, 3, e121510. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, X.F. Altered Hypothalamic C-Fos-like Immunoreactivity in Diet-Induced Obese Mice. Brain Res. Bull. 1999, 49, 215–219. [Google Scholar] [CrossRef]
- Xin, X.; Storlien, L.H.; Huang, X.F. Hypothalamic C-Fos-like Immunoreactivity in High-Fat Diet-Induced Obese and Resistant Mice. Brain Res. Bull. 2000, 52, 235–242. [Google Scholar] [CrossRef]
- Newton, A.J.; Hess, S.; Paeger, L.; Vogt, M.C.; Fleming Lascano, J.; Nillni, E.A.; Brüning, J.C.; Kloppenburg, P.; Xu, A.W. AgRP Innervation onto POMC Neurons Increases with Age and Is Accelerated with Chronic High-Fat Feeding in Male Mice. Endocrinology 2013, 154, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Morselli, L.L.; Wagner, V.A.; Balapattabi, K.; Sapouckey, S.A.; Knudtson, K.L.; Rahmouni, K.; Cui, H.; Sigmund, C.D.; Kwitek, A.E.; et al. Single-Nucleus RNA Sequencing of the Hypothalamic Arcuate Nucleus of C57BL/6J Mice After Prolonged Diet-Induced Obesity. Hypertension 2020, 76, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Diano, S.; Liu, Z.-W.; Jeong, J.K.; Dietrich, M.O.; Ruan, H.-B.; Kim, E.; Suyama, S.; Kelly, K.; Gyengesi, E.; Arbiser, J.L.; et al. Peroxisome Proliferation-Associated Control of Reactive Oxygen Species Sets Melanocortin Tone and Feeding in Diet-Induced Obesity. Nat. Med. 2011, 17, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betz, M.J.; Enerbäck, S. Targeting Thermogenesis in Brown Fat and Muscle to Treat Obesity and Metabolic Disease. Nat. Rev. Endocrinol. 2018, 14, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, K.; Morgan, D.A.; Morgan, G.M.; Mark, A.L.; Haynes, W.G. Role of Selective Leptin Resistance in Diet-Induced Obesity Hypertension. Diabetes 2005, 54, 2012–2018. [Google Scholar] [CrossRef] [Green Version]
- Mark, A.L. Selective Leptin Resistance Revisited. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R566–R581. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Brown, N.J. The Renin-Angiotensin-Aldosterone System and Glucose Homeostasis. Trends Pharmacol. Sci. 2011, 32, 734–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, T. The Role of the Renin-Angiotensin System in the Development of Cardiovascular Disease. Am. J. Cardiol. 2002, 89, 3A–9A, discussion 10A. [Google Scholar] [CrossRef]
- Miller, A.J.; Arnold, A.C. The Renin-Angiotensin System in Cardiovascular Autonomic Control: Recent Developments and Clinical Implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Lemarié, C.A.; Schiffrin, E.L. The Angiotensin II Type 2 Receptor in Cardiovascular Disease. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 19–31. [Google Scholar] [CrossRef]
- Jayasooriya, A.P.; Mathai, M.L.; Walker, L.L.; Begg, D.P.; Denton, D.A.; Cameron-Smith, D.; Egan, G.F.; McKinley, M.J.; Rodger, P.D.; Sinclair, A.J.; et al. Mice Lacking Angiotensin-Converting Enzyme Have Increased Energy Expenditure, with Reduced Fat Mass and Improved Glucose Clearance. Proc. Natl. Acad. Sci. USA 2008, 105, 6531–6536. [Google Scholar] [CrossRef] [Green Version]
- Massiera, F.; Seydoux, J.; Geloen, A.; Quignard-Boulange, A.; Turban, S.; Saint-Marc, P.; Fukamizu, A.; Negrel, R.; Ailhaud, G.; Teboul, M. Angiotensinogen-Deficient Mice Exhibit Impairment of Diet-Induced Weight Gain with Alteration in Adipose Tissue Development and Increased Locomotor Activity. Endocrinology 2001, 142, 5220–5225. [Google Scholar] [CrossRef]
- Kouyama, R.; Suganami, T.; Nishida, J.; Tanaka, M.; Toyoda, T.; Kiso, M.; Chiwata, T.; Miyamoto, Y.; Yoshimasa, Y.; Fukamizu, A.; et al. Attenuation of Diet-Induced Weight Gain and Adiposity through Increased Energy Expenditure in Mice Lacking Angiotensin II Type 1a Receptor. Endocrinology 2005, 146, 3481–3489. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, N.; Li, F.; Hua, K.; Deng, J.; Wang, C.-H.; Bowers, R.R.; Bartness, T.J.; Kim, H.-S.; Harp, J.B. Increased Energy Expenditure, Dietary Fat Wasting, and Resistance to Diet-Induced Obesity in Mice Lacking Renin. Cell Metab. 2007, 6, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Weisinger, H.S.; Begg, D.P.; Egan, G.F.; Jayasooriya, A.P.; Lie, F.; Mathai, M.L.; Sinclair, A.J.; Wark, J.D.; Weisinger, R.S. Angiotensin Converting Enzyme Inhibition from Birth Reduces Body Weight and Body Fat in Sprague-Dawley Rats. Physiol. Behav. 2008, 93, 820–825. [Google Scholar] [CrossRef]
- Carter, C.S.; Cesari, M.; Ambrosius, W.T.; Hu, N.; Diz, D.; Oden, S.; Sonntag, W.E.; Pahor, M. Angiotensin-Converting Enzyme Inhibition, Body Composition, and Physical Performance in Aged Rats. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Zorad, S.; Dou, J.; Benicky, J.; Hutanu, D.; Tybitanclova, K.; Zhou, J.; Saavedra, J.M. Long-Term Angiotensin II AT1 Receptor Inhibition Produces Adipose Tissue Hypotrophy Accompanied by Increased Expression of Adiponectin and PPARgamma. Eur. J. Pharmacol. 2006, 552, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuissa, H.; Jones, P.G.; Marso, S.P.; O’Keefe, J.H. Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers for Prevention of Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. J. Am. Coll. Cardiol. 2005, 46, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, C.; Bramlage, P.; Kirch, W.; Ferrario, C.M. Role of the Vasodilator Peptide Angiotensin-(1–7) in Cardiovascular Drug Therapy. Vasc. Health Risk Manag. 2007, 3, 125–137. [Google Scholar]
- Loloi, J.; Miller, A.J.; Bingaman, S.S.; Silberman, Y.; Arnold, A.C. Angiotensin-(1–7) Contributes to Insulin-Sensitizing Effects of Angiotensin-Converting Enzyme Inhibition in Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1204–E1211. [Google Scholar] [CrossRef] [Green Version]
- Schuchard, J.; Winkler, M.; Stölting, I.; Schuster, F.; Vogt, F.M.; Barkhausen, J.; Thorns, C.; Santos, R.A.; Bader, M.; Raasch, W. Lack of Weight Gain after Angiotensin AT1 Receptor Blockade in Diet-Induced Obesity Is Partly Mediated by an Angiotensin-(1–7)/Mas-Dependent Pathway. Br. J. Pharmacol. 2015, 172, 3764–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benter, I.F.; Yousif, M.H.M.; Anim, J.T.; Cojocel, C.; Diz, D.I. Angiotensin-(1–7) Prevents Development of Severe Hypertension and End-Organ Damage in Spontaneously Hypertensive Rats Treated with L-NAME. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H684–H691. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.A. Angiotensin-(1–7). Hypertension 2014, 63, 1138–1147. [Google Scholar] [CrossRef]
- White, M.C.; Fleeman, R.; Arnold, A.C. Sex Differences in the Metabolic Effects of the Renin-Angiotensin System. Biol. Sex. Differ. 2019, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Medina, D.; Arnold, A.C. Angiotensin-(1–7): Translational Avenues in Cardiovascular Control. Am. J. Hypertens. 2019, 32, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.; Alenina, N.; Young, D.; Santos, R.A.S.; Touyz, R.M. The Meaning of Mas. Hypertension 2018, 72, 1072–1075. [Google Scholar] [CrossRef] [Green Version]
- Rukavina Mikusic, N.L.; Silva, M.G.; Mazzitelli, L.R.; Santos, R.A.S.; Gómez, K.A.; Grecco, H.E.; Gironacci, M.M. Interaction Between the Angiotensin-(1–7) Mas Receptor and the Dopamine D2 Receptor: Implications in Inflammation. Hypertension 2021, 77, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Nixon, P.A.; Chappell, M.C.; Diz, D.I.; Russell, G.B.; Shaltout, H.A.; O’Shea, T.M.; Washburn, L.K. Obesity Is Associated with Higher Blood Pressure and Higher Levels of Angiotensin II but Lower Angiotensin-(1–7) in Adolescents Born Preterm. J. Pediatr. 2019, 205, 55–60.e1. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.M.; Otero, Y.F.; Bracy, D.P.; Wasserman, D.H.; Biaggioni, I.; Arnold, A.C. Chronic Angiotensin-(1–7) Improves Insulin Sensitivity in High-Fat Fed Mice Independent of Blood Pressure. Hypertension 2016, 67, 983–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Castillo, A.; Tobon-Cornejo, S.; Del Valle-Mondragon, L.; Torre-Villalvazo, I.; Schcolnik-Cabrera, A.; Guevara-Cruz, M.; Pichardo-Ontiveros, E.; Fuentes-Romero, R.; Bader, M.; Alenina, N.; et al. Angiotensin-(1–7) Induces Beige Fat Thermogenesis through the Mas Receptor. Metabolism 2020, 103, 154048. [Google Scholar] [CrossRef]
- Morimoto, H.; Mori, J.; Nakajima, H.; Kawabe, Y.; Tsuma, Y.; Fukuhara, S.; Kodo, K.; Ikoma, K.; Matoba, S.; Oudit, G.Y.; et al. Angiotensin 1–7 Stimulates Brown Adipose Tissue and Reduces Diet-Induced Obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E131–E138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-B.; Liu, C.; Niu, W.-Y.; Xin, Z.; Yu, M.; Feng, J.-P.; Yang, J.-K. Contributions of Renin-Angiotensin System-Related Gene Interactions to Obesity in a Chinese Population. PLoS ONE 2012, 7, e42881. [Google Scholar] [CrossRef] [Green Version]
- Lazartigues, E. Inflammation and Neurogenic Hypertension: A New Role for the Circumventricular Organs? Circ. Res. 2010, 107, 166–167. [Google Scholar] [CrossRef] [Green Version]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Biancardi, V.C.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating Angiotensin II Gains Access to the Hypothalamus and Brain Stem during Hypertension via Breakdown of the Blood-Brain Barrier. Hypertension 2014, 63, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Salameh, T.S.; Logsdon, A.F.; Hanson, A.J.; Erickson, M.A.; Banks, W.A. Blood-Brain Barriers in Obesity. AAPS J. 2017, 19, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.L.; Sigmund, C.D. Minireview: Overview of the Renin-Angiotensin System—An Endocrine and Paracrine System. Endocrinology 2003, 144, 2179–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Riet, L.; van Esch, J.H.M.; Roks, A.J.M.; van den Meiracker, A.H.; Danser, A.H.J. Hypertension: Renin-Angiotensin-Aldosterone System Alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Claflin, K.E.; Sandgren, J.A.; Lambertz, A.M.; Weidemann, B.J.; Littlejohn, N.K.; Burnett, C.M.L.; Pearson, N.A.; Morgan, D.A.; Gibson-Corley, K.N.; Rahmouni, K.; et al. Angiotensin AT1A Receptors on Leptin Receptor-Expressing Cells Control Resting Metabolism. J. Clin. Investig. 2017, 127, 1414–1424. [Google Scholar] [CrossRef] [Green Version]
- Sapouckey, S.A.; Deng, G.; Sigmund, C.D.; Grobe, J.L. Potential Mechanisms of Hypothalamic Renin-Angiotensin System Activation by Leptin and DOCA-Salt for the Control of Resting Metabolism. Physiol. Genomics 2017, 49, 722–732. [Google Scholar] [CrossRef]
- Aldred, G.P.; Chai, S.Y.; Song, K.; Zhuo, J.; MacGregor, D.P.; Mendelsohn, F.A. Distribution of Angiotensin II Receptor Subtypes in the Rabbit Brain. Regul. Pept. 1993, 44, 119–130. [Google Scholar] [CrossRef]
- Jöhren, O.; Sanvitto, G.L.; Egidy, G.; Saavedra, J.M. Angiotensin II AT1A Receptor MRNA Expression Is Induced by Estrogen-Progesterone in Dopaminergic Neurons of the Female Rat Arcuate Nucleus. J. Neurosci. 1997, 17, 8283–8292. [Google Scholar] [CrossRef] [Green Version]
- McKinley, M.J.; Allen, A.M.; Clevers, J.; Paxinos, G.; Mendelsohn, F.A. Angiotensin Receptor Binding in Human Hypothalamus: Autoradiographic Localization. Brain Res. 1987, 420, 375–379. [Google Scholar] [CrossRef]
- Deng, G.; Grobe, J.L. The Renin-Angiotensin System in the Arcuate Nucleus Controls Resting Metabolic Rate. Curr. Opin. Nephrol. Hypertens. 2019, 28, 120–127. [Google Scholar] [CrossRef]
- Sapouckey, S.A.; Morselli, L.L.; Deng, G.; Patil, C.N.; Balapattabi, K.; Oliveira, V.; Claflin, K.E.; Gomez, J.; Pearson, N.A.; Potthoff, M.J.; et al. Exploration of Cardiometabolic and Developmental Significance of Angiotensinogen Expression by Cells Expressing the Leptin Receptor or Agouti-Related Peptide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R855–R869. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Arellano, M.P.; Solano-Flores, L.P.; Ciriello, J. Arcuate Nucleus Inputs onto Subfornical Organ Neurons That Respond to Plasma Hypernatremia and Angiotensin II. Brain Res. 1996, 707, 308–313. [Google Scholar] [CrossRef]
- Donadio, M.V.F.; Gomes, C.M.; Sagae, S.C.; Franci, C.R.; Anselmo-Franci, J.A.; Lucion, A.B.; Sanvitto, G.L. Estradiol and Progesterone Modulation of Angiotensin II Receptors in the Arcuate Nucleus of Ovariectomized and Lactating Rats. Brain Res. 2006, 1083, 103–109. [Google Scholar] [CrossRef]
- Donadio, M.V.F.; Sagae, S.C.; Franci, C.R.; Anselmo-Franci, J.A.; Lucion, A.B.; Sanvitto, G.L. Angiotensin II Receptors in the Arcuate Nucleus Mediate Stress-Induced Reduction of Prolactin Secretion in Steroid-Primed Ovariectomized and Lactating Rats. Brain Res. 2004, 1006, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, A.; Tsutsumi, K.; Shigematsu, K.; Saavedra, J.M. Reproductive Hormones Modulate Angiotensin II AT1 Receptors in the Dorsomedial Arcuate Nucleus of the Female Rat. Endocrinology 1993, 133, 939–941. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, A.D.; Pioquinto, D.J.; Nguyen, D.; Wang, L.; Smith, J.A.; Hiller, H.; Sumners, C. Obesity Induces Neuroinflammation Mediated by Altered Expression of the Renin-Angiotensin System in Mouse Forebrain Nuclei. Physiol. Behav. 2014, 136, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Worker, C.J.; Li, W.; Feng, C.; Souza, L.A.C.; Gayban, A.J.B.; Cooper, S.G.; Afrin, S.; Romanick, S.; Ferguson, B.S.; Feng Earley, Y. The Neuronal (pro)Renin Receptor and Astrocyte Inflammation in the Central Regulation of Blood Pressure and Blood Glucose in Mice Fed a High-Fat Diet. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E765–E778. [Google Scholar] [CrossRef]
- Campbell, D.J. Critical Review of Prorenin and (pro)Renin Receptor Research. Hypertension 2008, 51, 1259–1264. [Google Scholar] [CrossRef] [Green Version]
- Hennrikus, M.; Gonzalez, A.A.; Prieto, M.C. The Prorenin Receptor in the Cardiovascular System and Beyond. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H139–H145. [Google Scholar] [CrossRef]
- Mohsin, M.; Souza, L.A.C.; Aliabadi, S.; Worker, C.J.; Cooper, S.G.; Afrin, S.; Murata, Y.; Xiong, Z.; Feng Earley, Y. Increased (Pro)Renin Receptor Expression in the Hypertensive Human Brain. Front. Physiol. 2020, 11, 606811. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.B.; Harlan, S.M.; Morgan, D.A.; Guo, D.-F.; Cui, H.; Rahmouni, K. Differential Contribution of POMC and AgRP Neurons to the Regulation of Regional Autonomic Nerve Activity by Leptin. Mol. Metab. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Oliveira, V.; Kwitek, A.E.; Sigmund, C.D.; Morselli, L.L.; Grobe, J.L. Recent Advances in Hypertension: Intersection of Metabolic and Blood Pressure Regulatory Circuits in the Central Nervous System. Hypertension 2021, 77, 1061–1068. [Google Scholar] [CrossRef]
- Wang, C.; He, Y.; Xu, P.; Yang, Y.; Saito, K.; Xia, Y.; Yan, X.; Hinton, A., Jr.; Yan, C.; Ding, H.; et al. TAp63 Contributes to Sexual Dimorphism in POMC Neuron Functions and Energy Homeostasis. Nat. Commun. 2018, 9, 1544. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Lane, M.V.; Numan, S.; Dellovade, T.L. Distribution of Estrogen Receptor α and β in the Mouse Central Nervous System: In Vivo Autoradiographic and Immunocytochemical Analyses. J. Comp. Neurol. 2004, 473, 270–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Jung, Y.-S.; Kwon, H.-J.; Seong, J.K.; Granneman, J.G.; Lee, Y.-H. Sex Differences in Sympathetic Innervation and Browning of White Adipose Tissue of Mice. Biol. Sex. Differ. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupte, M.; Thatcher, S.E.; Boustany-Kari, C.M.; Shoemaker, R.; Yiannikouris, F.; Zhang, X.; Karounos, M.; Cassis, L.A. Angiotensin Converting Enzyme 2 Contributes to Sex Differences in the Development of Obesity Hypertension in C57BL/6 Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1392–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shoemaker, R.; Powell, D.; Su, W.; Thatcher, S.; Cassis, L. Differential Effects of Mas Receptor Deficiency on Cardiac Function and Blood Pressure in Obese Male and Female Mice. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H459–H468. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.; Tannock, L.R.; Su, W.; Gong, M.; Gurley, S.B.; Thatcher, S.E.; Yiannikouris, F.; Ensor, C.M.; Cassis, L.A. Adipocyte Deficiency of ACE2 Increases Systolic Blood Pressures of Obese Female C57BL/6 Mice. Biol. Sex. Differ. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M.C.; Miller, A.J.; Loloi, J.; Bingaman, S.S.; Shen, B.; Wang, M.; Silberman, Y.; Lindsey, S.H.; Arnold, A.C. Sex Differences in Metabolic Effects of Angiotensin-(1–7) Treatment in Obese Mice. Biol. Sex. Differ. 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| RAS Receptor | Neuronal Subpopulations * |
|---|---|
| AT1aR | AgRP (Sst3) |
| AT1bR | No expression |
| AT2R | POMC (vGlut2 11) |
| PRR | POMC (GABA 7), POMC (vGlut2 11), AgRP (Sst3), AgRP (GABA 14) |
| MasR | POMC (GABA 7), POMC (vGlut2 11), AgRP (GABA 14) |
| RAS Receptor | Functions | (Ref.) Species, Sex |
|---|---|---|
| AT1aR | Increase resting metabolic rate; no effect on blood pressure | [67,73] Mice, Male and Female |
| AT1bR | Unknown | |
| AT2R | Unknown | |
| PRR | Increase blood pressure and impair glycemic control; no effect on body weight or food intake | [79] Mice, Male |
| MasR | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehay, D.; Silberman, Y.; Arnold, A.C. The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin–Angiotensin Pathways. Int. J. Mol. Sci. 2021, 22, 7050. https://doi.org/10.3390/ijms22137050
Mehay D, Silberman Y, Arnold AC. The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin–Angiotensin Pathways. International Journal of Molecular Sciences. 2021; 22(13):7050. https://doi.org/10.3390/ijms22137050
Chicago/Turabian StyleMehay, Darren, Yuval Silberman, and Amy C. Arnold. 2021. "The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin–Angiotensin Pathways" International Journal of Molecular Sciences 22, no. 13: 7050. https://doi.org/10.3390/ijms22137050
APA StyleMehay, D., Silberman, Y., & Arnold, A. C. (2021). The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin–Angiotensin Pathways. International Journal of Molecular Sciences, 22(13), 7050. https://doi.org/10.3390/ijms22137050







