Dietary Sphingolipids Contribute to Health via Intestinal Maintenance
Abstract
:1. Introduction
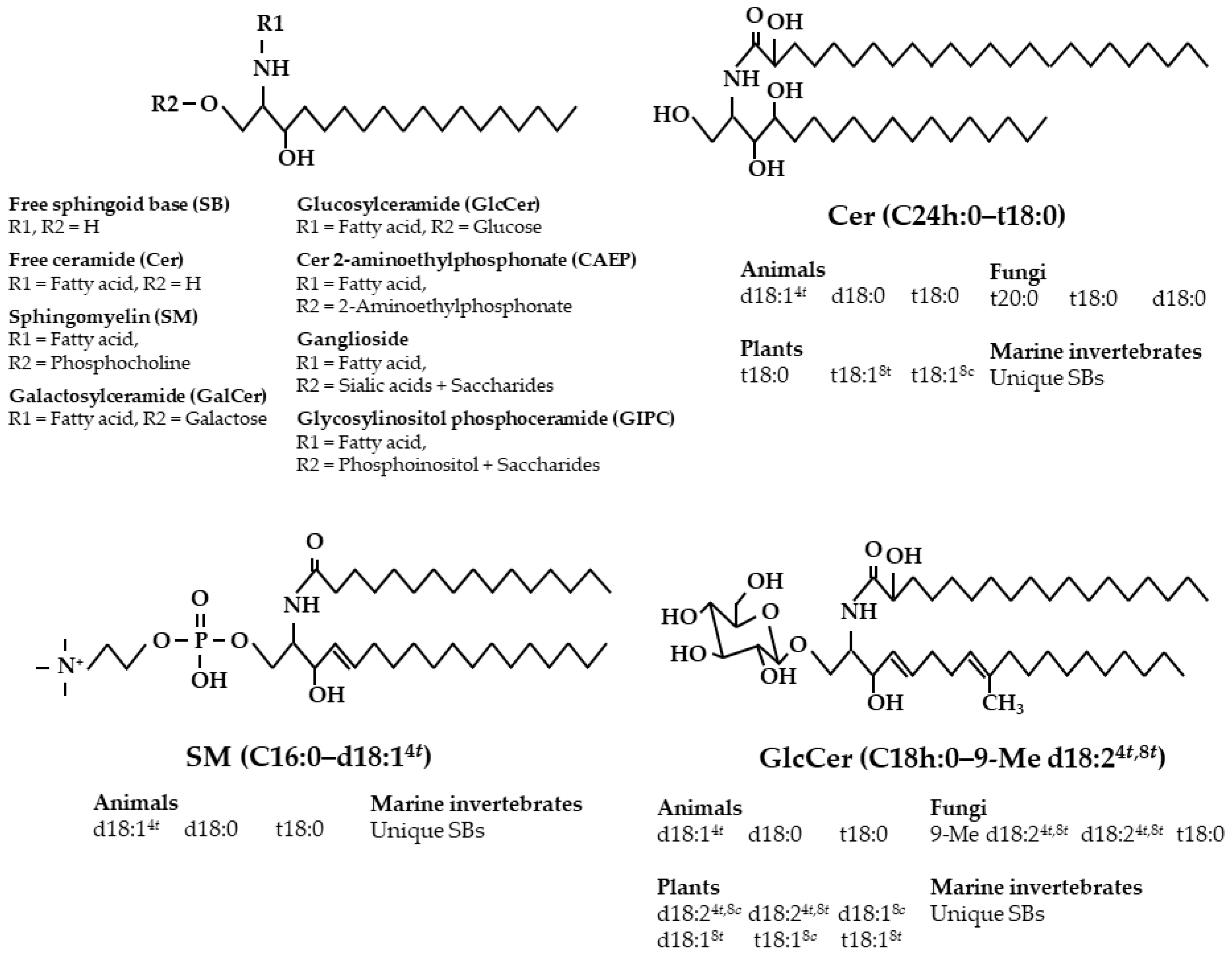
2. Diversity of Sphingolipid Classes and Base Composition in Foods
3. Intake of Sphingolipids from Daily Diets
4. Digestion and Absorption of Various Sphingolipids
5. Suppression of Intestinal Cancer by Dietary Sphingolipids
5.1. Sphingomyelin
5.2. Glycosphingolipids
5.3. Cer and SB
5.4. Foods Containing Sphingolipids
5.5. Apoptotic and Anti-Proliferative Effects of Sphingolipids on Colon Cancer Cells
6. Suppression of Intestinal Inflammation by Dietary Sphingolipids
6.1. Sphingomyelin
6.2. Glycosphingolipids
6.3. Extracts Containing Sphingolipids from Foods
6.4. Effects of Sphingolipids on Ex Vivo and In Vitro Inflammation
7. Effects of Sphingolipids on Lipid Absorption and Energy Metabolism
8. Other Beneficial Effects of Dietary Sphingolipids
9. Conclusions
Funding
Conflicts of Interest
References
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737. [Google Scholar]
- Hofmanová, J.; Straková, N.; Vaculová, A.H.; Tylichová, Z.; Šafa, B.; Skender, B.; Kozubík, A. Interaction of dietary fatty acids with tumour necrosis factor family cytokines during colon inflammation and cancer. Mediat. Inflamm. 2014, 2014, 848632. [Google Scholar] [CrossRef] [Green Version]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Yamashita, S.; Kikuchi, N.; Kinoshita, M.; Miyazawa, T. Chemical properties and nutritional value of plant-origin glucosylceramide. J. Nutr. Sci. Vitaminol. 2019, 65, S153–S157. [Google Scholar] [CrossRef]
- Vesper, H.; Schmelz, E.M.; Nikolova-Karakashian, M.N.; Dillehay, D.L.; Lynch, D.V.; Merrill, A.H., Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T. Dietary Control of Ganglioside Expression in Mammalian Tissues. Int. J. Mol. Sci. 2019, 21, 177. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Fong, B.Y.; MacGibbon, A.K.H.; Norris, G. Qualitative and quantitative study of glycosphingolipids in human milk and bovine milk using high performance liquid chromatography–data-dependent acquisition–mass spectrometry. Molecules 2020, 25, 4024. [Google Scholar] [CrossRef]
- Hellgren, L.J. Occurrence of bioactive sphingolipids in meat and fish products. Eur. J. Lipid Sci. Technol. 2001, 103, 661–667. [Google Scholar] [CrossRef]
- Duan, J.; Sugawara, T.; Hirata, T. Rapid quantitative analysis of sphingolipids in seafood using HPLC with evaporative light-scattering detection: Its application in tissue distribution of sphingolipids in fish. J. Oleo Sci. 2010, 59, 509–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, T.; Sugita, M. Sphingolipids in lower animals. Prog. Lipid Res. 1993, 32, 25–45. [Google Scholar] [CrossRef]
- Fujino, Y.; Ohnishi, M. Species of sphingolipids in rice grain. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1982, 58, 36–39. [Google Scholar] [CrossRef] [Green Version]
- Markham, J.E.; Li, J.; Cahoon, E.B.; Jaworski, J.G. Separation and identification of major plant sphingolipid classes from leaves. J. Biol. Chem. 2006, 281, 22684–22694. [Google Scholar] [CrossRef] [Green Version]
- Buré, C.; Cacas, J.L.; Mongrand, S.; Schmitter, J.M. Characterization of glycosyl inositol phosphoryl ceramides from plants and fungi by mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 995–1010. [Google Scholar] [CrossRef]
- Kimura, K.; Kinoshita, M.; Takakuwa, N.; Tamura, M.; Oda, Y.; Ohnishi, M. Content and constituent properties of sphingolipid classes in Saccharomyces kluyveri. J. Oleo Sci. 2006, 55, 623–627. [Google Scholar] [CrossRef]
- Ogawa, S.; Tachimoto, H.; Kaga, T. Elevation of ceramide in Acetobacter malorum S24 by low pH stress and high temperature stress. J. Biosci. Bioeng. 2010, 109, 32–36. [Google Scholar] [CrossRef]
- Okino, N.; Li, M.; Qu, Q.; Nakagawa, T.; Hayashi, Y.; Matsumoto, M.; Ishibashi, Y.; Ito, M. Two bacterial glycosphingolipid synthases responsible for the synthesis of glucuronosylceramide and α-galactosylceramide. J. Biol. Chem. 2020, 295, 10709–10725. [Google Scholar] [CrossRef]
- Han, C.; Xia, K.; Yang, J.; Zhang, H.; DeLisa, M.P.; Liang, X. Investigation of lipid profile in Acetobacter pasteurianus Ab3 against acetic acid stress during vinegar production. Extremophiles 2020, 24, 909–922. [Google Scholar] [CrossRef]
- Yamashita, S.; Higaki, C.; Kanai, A.; Kikuchi, N.; Suzuki, D.; Kinoshita, M.; Miyazawa, T. Sphingolipid properties in sake rice cultivars and changes during polishing and brewing. J. Oleo Sci. 2021, 70, 263–273. [Google Scholar] [CrossRef]
- Yamashita, S.; Higaki, C.; Kikuchi, N.; Suzuki, D.; Kinoshita, M.; Miyazawa, T. Sake (rice wine) brewing hydrolyzes highly polar sphingolipids to ceramides and increases free sphingoid bases. J. Oleo Sci. 2021. Preprint. [Google Scholar] [CrossRef]
- Omae, F.; Miyazaki, M.; Enomoto, A.; Suzuki, M.; Suzuki, Y.; Suzuki, A. DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem. J. 2004, 379, 687–695. [Google Scholar] [CrossRef]
- Miyazawa, T.; Ito, S.; Fujino, Y. Isolation of cerebroside from pea seeds. Agric. Biol. Chem. 1974, 38, 1387–1391. [Google Scholar] [CrossRef]
- Aida, K.; Takakuwa, N.; Kinoshita, M.; Sugawara, T.; Imai, H.; Ono, J.; Ohnishi, M. Properties and physiological effects of plant cerebroside species as functional lipids. In Advanced Research on Plant Lipids; Murata, N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2015; pp. 233–236. [Google Scholar]
- Fujino, Y.; Ohnishi, M. Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 1976, 486, 161–171. [Google Scholar]
- Takakuwa, N.; Tanji, M.; Oda, Y.; Ohnishi, M. Distribution of 9-methyl sphingoid base in mushrooms and its effects on the fluidity of phospholipid liposomes. J. Oleo Sci. 2002, 51, 741–747. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Zaima, N.; Yamamoto, A.; Sakai, S.; Noguchi, R.; Hirata, T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci. Biotechnol. Biochem. 2006, 70, 2906–2912. [Google Scholar] [CrossRef]
- Shah, A.K.M.A.; Kinoshita, M.; Kurihara, H.; Ohnishi, M.; Takahashi, K. Glycosylceramides obtain from the starfish Asterias amurensis Lütken. J. Oleo Sci. 2008, 57, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Itonori, S.; Kajiwara, C.; Hada, N.; Takeda, T.; Sugita, M. Structural elucidation of the neutral glycosphingolipids, mono-, di-, tri-and tetraglycosylceramides from the marine crab Erimacrus isenbeckii. J. Oleo Sci. 2014, 63, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Tomonaga, N.; Manabe, Y.; Sugawara, T. Digestion of ceramide 2-aminoethylphosphonate, a sphingolipid from the jumbo flying squid Dosidicus gigas, in mice. Lipids 2017, 52, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Mikami, D.; Sakai, S.; Nishimukai, M.; Yuyama, K.; Mukai, K.; Igarashi, Y. Structure-dependent absorption of atypical sphingoid long-chain bases from digestive tract into lymph. Lipids Health Dis. 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, B.; Leppimäki, P.; Axberg, M.; Slotte, J.P. Analysis of natural and synthetic sphingomyelins using high-performance thin-layer chromatography. Eur. J. Biochem. 1999, 266, 997–1002. [Google Scholar] [CrossRef]
- Yunoki, K.; Renaguli, M.; Kinoshita, M.; Matsuyama, H.; Mawatari, S.; Fujino, T.; Kodama, Y.; Sugiyama, M.; Ohnishi, M. Dietary sphingolipids ameliorate disorders of lipid metabolism in Zucker fatty rats. J. Agric. Food Chem. 2010, 58, 7030–7035. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, N.; Saito, K.; Ohnishi, M.; Oda, Y. Determination of glucosylceramide contents in crop tissues and by-products from their processing. Bioresour. Technol. 2005, 96, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Ogawa, T.; Ono, J.; Miyashita, R.; Aida, K.; Oda, Y.; Ohnishi, M. Analysis of sphingolipid classes and their contents in meals. Biosci. Biotechnol. Biochem. 2008, 72, 222–225. [Google Scholar] [CrossRef]
- Sugawara, T.; Miyazawa, T. Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection. Lipids 1999, 34, 1231–1237. [Google Scholar] [CrossRef]
- Nilsson, Å. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim. Biophys. Acta 1968, 164, 575–584. [Google Scholar] [CrossRef]
- Nilsson, Å. Metabolism of cerebroside in the intestinal tract of the rat. Biochim. Biophys. Acta 1969, 176, 339–347. [Google Scholar] [CrossRef]
- Hasi, R.Y.; Miyagi, M.; Kida, T.; Fukuta, T.; Kogure, K.; Hayashi, J.; Kawakami, R.; Kanemaru, K.; Tanaka, T. Quantitative analysis of glycosylinositol phosphoceramide and phytoceramide 1-phosphate in vegetables. J. Nutr. Sci. Vitaminol. 2019, 65, S175–S179. [Google Scholar] [CrossRef] [Green Version]
- Tomonaga, N.; Tsuduki, T.; Manabe, Y.; Sugawara, T. Sphingoid bases of dietary ceramide 2-aminoethylphosphonate, a marine sphingolipid, absorb into lymph in rats. J. Lipid Res. 2019, 60, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Ohta, K.; Hiraki, S.; Miyanabe, M.; Ueki, T.; Aida, K.; Manabe, Y.; Sugawara, T. Appearance of intact molecules of dietary ceramides prepared from soy sauce lees and rice glucosylceramides in mouse plasma. J. Agric. Food Chem. 2021. Preprint. [Google Scholar] [CrossRef]
- Dickson, J.J.; Messer, M. Intestinal neuraminidase activity of suckling rats and other mammals. Relationship to the sialic acid content of milk. Biochem. J. 1978, 170, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, Å. Role of sphingolipids in infant gut health and immunity. J. Pediatr. 2016, 173, S53–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelz, E.M.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H., Jr. Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glycosphingolipids in 1, 2-dimethylhydrazine-treated CF1 mice. J. Nutr. 2000, 130, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Leese, H.J.; Semenza, G. On the identity between the small intestinal enzymes phlorizin hydrolase and glycosylceramidase. J. Biol. Chem. 1973, 248, 8170–8173. [Google Scholar] [CrossRef]
- Sugawara, T.; Kinoshita, M.; Ohnishi, M.; Nagata, J.; Saito, M. Digestion of maize sphingolipids in rats and uptake of sphingadienine by Caco-2 cells. J. Nutr. 2003, 133, 2777–2782. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Kinoshita, M.; Ohnishi, M.; Tsuzuki, T.; Miyazawa, T.; Nagata, J.; Hirata, T.; Saito, M. Efflux of sphingoid bases by P-glycoprotein in human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2004, 68, 2541–2546. [Google Scholar] [CrossRef] [Green Version]
- Fujii, A.; Manabe, Y.; Aida, K.; Tsuduki, T.; Hirata, T.; Sugawara, T. Selective absorption of dietary sphingoid bases from the intestine via efflux by p-glycoprotein in rats. J. Nutr. Sci. Vitaminol. 2017, 63, 44–50. [Google Scholar] [CrossRef] [Green Version]
- García-Barros, M.; Coant, N.; Truman, J.P.; Snider, A.J.; Hannun, Y.A. Sphingolipids in colon cancer. Biochim. Biophys. Acta 2014, 1841, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, B.; Gao, S.; Duan, R.D. Dietary sphingomyelin inhibits colonic tumorigenesis with an up-regulation of alkaline sphingomyelinase expression in ICR mice. Anticancer Res. 2008, 28, 3631–3635. [Google Scholar]
- Schmelz, E.M.; Dillehay, D.L.; Webb, S.K.; Reiter, A.; Adams, J.; Merrill, A.H., Jr. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: Implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996, 56, 4936–4941. [Google Scholar]
- Schmelz, E.M.; Bushnev, A.S.; Dillehay, D.L.; Liotta, D.C.; Merrill, A.H., Jr. Suppression of aberrant colonic crypt foci by synthetic sphingomyelins with saturated or unsaturated sphingoid base backbones. Nutr. Cancer 1997, 28, 81–85. [Google Scholar] [CrossRef]
- Lemonnier, L.A.; Dillehay, D.L.; Vespremi, M.J.; Abrams, J.; Brody, E.; Schmelz, E.M. Sphingomyelin in the suppression of colon tumors: Prevention versus intervention. Arch. Biochem. Biophys. 2003, 419, 129–138. [Google Scholar] [CrossRef]
- Exon, J.H.; South, E.H. Effects of sphingomyelin on aberrant colonic crypt foci development, colon crypt cell proliferation and immune function in an aging rat tumor model. Food Chem. Toxicol. 2003, 41, 471–476. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Belobrajdic, D.; Young, G.P. The potential of sphingomyelin as a chemopreventive agent in AOM-induced colon cancer model: Wild-type and p53+/− mice. Mol. Nutr. Food Res. 2008, 52, 558–566. [Google Scholar] [CrossRef]
- Aida, K.; Kinoshita, M.; Tanji, M.; Sugawara, T.; Tamura, M.; Ono, J.; Ueno, N.; Ohnishi, M. Prevention of aberrant crypt foci formation by dietary maize and yeast cerebrosides in 1,2-dimethylhydrazine-treated mice. J. Oleo Sci. 2005, 54, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, M.; Aida, K.; Tokuji, Y.; Sugawara, T.; Ohnishi, M. Effects of dietary plant cerebroside on gene expression in the large intestine of 1,2-dimethylhydrazine (DMH)-treated mice determined by DNA microarray analysis. J. Food Lipids 2009, 16, 200–208. [Google Scholar] [CrossRef]
- Yamashita, S.; Sakurai, R.; Hishiki, K.; Aida, K.; Kinoshita, M. Effects of dietary plant-origin glucosylceramide on colon cytokine contents in DMH-treated mice. J. Oleo Sci. 2017, 66, 157–160. [Google Scholar] [CrossRef]
- Inamine, M.; Suzui, M.; Morioka, T.; Kinjo, T.; Kaneshiro, T.; Sugishita, T.; Okada, T.; Yoshimi, N. Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats. Cancer Sci. 2005, 96, 876–881. [Google Scholar] [CrossRef]
- Symolon, H.; Schmelz, E.M.; Dillehay, D.L.; Merrill, A.H., Jr. Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J. Nutr. 2004, 134, 1157–1161. [Google Scholar] [CrossRef] [Green Version]
- Schmelz, E.M.; Roberts, P.C.; Kustin, E.M.; Lemonnier, L.A.; Sullards, M.C.; Dillehay, D.L.; Merrill, A.H., Jr. Modulation of intracellular β-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001, 61, 6723–6729. [Google Scholar]
- Symolon, H.; Bushnev, A.; Peng, Q.; Ramaraju, H.; Mays, S.G.; Allegood, J.C.; Pruett, S.T.; Sullards, M.C.; Dillehay, D.L.; Liotta, D.C.; et al. Enigmol: A novel sphingolipid analogue with anticancer activity against cancer cell lines and in vivo models for intestinal and prostate cancer. Mol. Cancer Ther. 2011, 10, 648–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariyawasam, K.M.G.R.M.; Yamashita, S.; Fukuda, K.; Ohwada, T.; Kinoshita, M. Preventing the formation of aberrant crypt foci in 1,2-dimethylhydrazine-treated mice by dietary milk and maitake mushroom (Grifola frondosa). Milk Sci. 2019, 68, 85–93. [Google Scholar]
- Kariyawasam, K.M.G.R.M.; Yamashita, S.; Fukuma, N.; Kinoshita, M. Dietary milk and maitake mushroom (Grifola frondosa) modulate inflammation-related cytokines and apoptosis related-proteins in colon of 1,2-dimethylhydrazine-treated mice. Milk Sci. 2020, 69, 21–28. [Google Scholar]
- Arai, K.; Yamazaki, T.; Tokuji, Y.; Kawahara, M.; Ohba, K.; Hironaka, K.; Kinoshita, M.; Ohnishi, M. Effects of Chinese yam storage protein on formation of aberrant crypt foci in 1,2-dimethylhydrazine-treated mice. J. Food Nutr. Res. 2013, 52, 139–145. [Google Scholar]
- Yamashita, S.; Yamamoto, M.; Hirakawa, K.; Kikuchi, N.; Kinoshita, M.; Miyazawa, T. Extraction of lipophilic fraction from polished rice improves its ameliorative effect on intestinal impairment. J. Oleo Sci. 2019, 68, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Hata, M.; Kikuchi, N.; Kinoshita, M.; Miyazawa, T. Effects of dietary ethanol extracts from sake rice and sake lees on intestinal impairment in mice. J. Oleo Sci. 2020, 69, 929–939. [Google Scholar] [CrossRef]
- Schmelz, E.M.; Bushnev, A.S.; Dillehay, D.L.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H., Jr. Ceramide-β-d-glucuronide: Synthesis, digestion, and suppression of early markers of colon carcinogenesis. Cancer Res. 1999, 59, 5768–5772. [Google Scholar]
- Ahn, E.H.; Schroeder, J.J. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp. Biol. Med. 2002, 227, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Schroeder, J.J. Induction of apoptosis by sphingosine, sphinganine, and C2-ceramide in human colon cancer cells, but not by C2-dihydroceramide. Anticancer Res. 2010, 30, 2881–2884. [Google Scholar]
- Sugawara, T.; Kinoshita, M.; Ohnishi, M.; Miyazawa, T. Apoptosis induction by wheat-flour sphingoid bases in DLD-1 human colon cancer cells. Biosci. Biotechnol. Biochem. 2002, 66, 2228–2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aida, K.; Kinoshita, M.; Sugawara, T.; Ono, J.; Miyazawa, T.; Ohnishi, M. Apoptosis inducement by plant and fungus sphingoid bases in human colon cancer cells. J. Oleo Sci. 2004, 53, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Morad, S.A.; Madigan, J.P.; Levin, J.C.; Abdelmageed, N.; Karimi, R.; Rosenberg, D.W.; Kester, M.; Shanmugavelandy, S.S.; Cabot, M.C. Tamoxifen magnifies therapeutic impact of ceramide in human colorectal cancer cells independent of p53. Biochem. Pharmacol. 2013, 85, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Rahmaniyan, M.; Curley, R.W., Jr.; Obeid, L.M.; Hannun, Y.A.; Kraveka, J.M. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J. Biol. Chem. 2011, 286, 24754–24764. [Google Scholar] [CrossRef] [Green Version]
- Snider, A.J.; Kawamori, T.; Bradshaw, S.G.; Orr, K.A.; Gilkeson, G.S.; Hannun, Y.A.; Obeid, L.M. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2009, 23, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Pulkoski-Gross, M.J.; Uys, J.D.; Orr-Gandy, K.A.; Coant, N.; Bialkowska, A.B.; Szulc, Z.M.; Bai, A.; Bielawska, A.; Townsend, D.M.; Hannun, Y.A.; et al. Novel sphingosine kinase-1 inhibitor, LCL351, reduces immune responses in murine DSS induced colitis. Prostaglandins Other Lipid Mediat. 2017, 130, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, T.; Bell, R.M.; Hannun, Y.A. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J. Biol. Chem. 1989, 264, 19076–19080. [Google Scholar] [CrossRef]
- Ohnishi, T.; Hashizume, C.; Taniguchi, M.; Furumoto, H.; Han, J.; Gao, R.; Kinami, S.; Kosaka, T.; Okazaki, T. Sphingomyelin synthase 2 deficiency inhibits the induction of murine colitis-associated colon cancer. FASEB J. 2017, 31, 3816–3830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffel, M.J.; Helke, K.; Lu, P.; Bowers, J.S.; Ogretmen, B.; Garrett-Mayer, E.; Paulos, C.M.; Voelkel-Johnson, C. Adoptive transfer of ceramide synthase 6 deficient splenocytes reduces the development of colitis. Sci. Rep. 2017, 7, 15552. [Google Scholar] [CrossRef] [PubMed]
- Furuya, H.; Ohkawara, S.; Nagashima, K.; Asanuma, N.; Hino, T. Dietary sphingomyelin alleviates experimental inflammatory bowel disease in mice. Int. J. Vitam. Nutr. Res. 2008, 78, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Fischbeck, A.; Leucht, K.; Frey-Wagner, I.; Bentz, S.; Pesch, T.; Kellermeier, S.; Krebs, M.; Fried, M.; Rogler, G.; Hausmann, M.; et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut 2011, 60, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor γ expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar] [CrossRef] [Green Version]
- Sakata, A.; Ochiai, T.; Shimeno, H.; Hikishima, S.; Yokomatsu, T.; Shibuya, S.; Toda, A.; Eyanagi, R.; Soeda, S. Acid sphingomyelinase inhibition suppresses lipopolysaccharide-mediated release of inflammatory cytokines from macrophages and protects against disease pathology in dextran sulphate sodium-induced colitis in mice. Immunology 2007, 122, 54–64. [Google Scholar] [CrossRef]
- Fekry, B.; Jeffries, K.A.; Esmaeilniakooshkghazi, A.; Szulc, Z.M.; Knagge, K.J.; Kirchner, D.R.; Horita, D.A.; Krupenko, S.A.; Krupenko, N.I. C16-ceramide is a natural regulatory ligand of p53 in cellular stress response. Nat. Commun. 2018, 9, 4149. [Google Scholar] [CrossRef] [PubMed]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Müller, D.; Schneider, M.; et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Mizobuchi, Y.; Tokuji, Y.; Aida, K.; Yamashita, S.; Ohnishi, M.; Kinoshita, M. Effects of dietary plant-origin glucosylceramide on bowel inflammation in DSS-treated mice. J. Oleo Sci. 2015, 64, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Suh, M.; Thomson, B.; Ma, D.W.; Ramanujam, K.; Thomson, A.B.; Clandinin, M.T. Dietary ganglioside inhibits acute inflammatory signals in intestinal mucosa and blood induced by systemic inflammation of Escherichia coli lipopolysaccharide. Shock 2007, 28, 112–117. [Google Scholar] [CrossRef]
- Park, E.J.; Thomson, A.B.; Clandinin, M.T. Protection of intestinal occludin tight junction protein by dietary gangliosides in lipopolysaccharide-induced acute inflammation. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 321–328. [Google Scholar] [CrossRef]
- Norris, G.H.; Blesso, C.N. Dietary and endogenous sphingolipid metabolism in chronic inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Akada, K.; Matsumoto, S.; Kinoshita, M. Effects of dietary ethanol extract from fruiting bodies of golden oyster mushroom (Pleurotus citrinopileatus) on chronic colon inflammation in mice treated with dextran sulfate sodium salt. Mushroom Sci. Biotechnol. 2020, 28, 7–14. [Google Scholar]
- Yamashita, S.; Seino, T.; Inobe, M.; Jutanom, M.; Matsumoto, S.; Kinoshita, M. Polar lipid fraction from golden oyster mushrooms (Pleurotus citrinopileatus) suppresses colon injuries from inflammatory stresses in vivo and in vitro. J. Oleo Sci. 2020, 69, 751–757. [Google Scholar] [CrossRef]
- Schnabl, K.L.; Larsen, B.; van Aerde, J.E.; Lees, G.; Evans, M.; Belosevic, M.; Field, C.; Thomson, A.B.; Clandinin, M.T. Gangliosides protect bowel in an infant model of necrotizing enterocolitis by suppressing proinflammatory signals. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 382–392. [Google Scholar] [CrossRef]
- Yamashita, S.; Seino, T.; Aida, K.; Kinoshita, M. Effects of plant sphingolipids on inflammatory stress in differentiated Caco-2 cells. J. Oleo Sci. 2017, 66, 1337–1342. [Google Scholar] [CrossRef] [Green Version]
- Jutanom, M.; Higaki, C.; Yamashita, S.; Nakagawa, K.; Matsumoto, S.; Kinoshita, M. Effects of sphingolipid fractions from golden oyster mushroom (Pleurotus citrinopileatus) on apoptosis induced by inflammatory stress in an intestinal tract in vitro model. J. Oleo Sci. 2020, 69, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Noriris, G.H.; Porter, C.M.; Jiang, J.; Blesso, C.N. Dietary milk sphingomyelin reduces systemic inflammation in diet-induced obese mice and inhibits LPS activity in macrophages. Beverages 2017, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, H.; Yoshida, T.; Sanaki, T.; Shigaki, S.; Morita, H.; Oyama, M.; Mitsui, M.; Tanaka, Y.; Nakano, T.; Mitsutake, S.; et al. Possible roles of long-chain sphingomyelines and sphingomyelin synthase 2 in mouse macrophage inflammatory response. Biochem. Biophys. Res. Commun. 2017, 482, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Noriris, G.H.; Jiang, J.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef]
- Chen, H.; Born, E.; Mathur, S.N.; Johlin, F.C., Jr.; Field, F.J. Sphingomyelin content of intestinal cell membranes regulates cholesterol absorption. Evidence for pancreatic and intestinal cell sphingomyelinase activity. Biochem. J. 1992, 286, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Kirby, R.J.; Zheng, S.; Tso, P.; Howles, P.N.; Hui, D.Y. Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine: A process mediated via ceramide hydrolysis. J. Biol. Chem. 2001, 277, 4104–4109. [Google Scholar] [CrossRef] [Green Version]
- Garmy, N.; Taieb, N.; Yahi, N.; Fantini, J. Interaction of cholesterol with sphingosine: Physicochemical characterization and impact on intestinal absorption. J. Lipid Res. 2005, 46, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.Y.; Zhang, T.T.; Dong, Z.; Shi, H.H.; Xu, J.; Mao, X.Z.; Wang, Y.M.; Xue, C.H. Dietary supplementation with exogenous sea-cucumber-derived ceramides and glucosylceramides alleviates insulin resistance in high-fructose-diet-fed rats by upregulating the IRS/PI3K/Akt signaling pathway. J. Agric. Food Chem. 2021. Preprint. [Google Scholar] [CrossRef]
- Simon, K.W.; Tait, L.; Miller, F.; Cao, C.; Davy, K.P.; LeRoith, T.; Schmelz, E.M. Suppression of breast xenograft growth and progression in nude mice: Implications for the use of orally administered sphingolipids as chemopreventive agents against breast cancer. Food Funct. 2010, 1, 90–98. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yazama, H.; Donishi, R.; Koyama, S.; Fukuhara, T.; Takeuchi, H. Inhibitory effects of glucosylceramide on tumorigenesis induced by a carcinogen in mice. Laryngoscope 2020, 130, E593–E597. [Google Scholar] [CrossRef] [Green Version]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese traditional dietary fungus koji Aspergillus oryzae functions as a prebiotic for Blautia coccoides through glycosylceramide: Japanese dietary fungus koji is a new prebiotic. Springerplus 2016, 5, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.T.; Le, H.H.; Johnson, E.L. Dietary sphinganine is selectively assimilated by members of the mammalian gut microbiome. J. Lipid Res. 2021, 62, 100034. [Google Scholar] [CrossRef]
- Ono, J.; Kinoshita, M.; Aida, K.; Tamura, M.; Ohnishi, M. Effects of dietary glucosylceramide on dermatitis in atopic dermatitis model mice. Eur. J. Lipid Sci. Technol. 2010, 112, 708–711. [Google Scholar] [CrossRef]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Sugawara, T.; Hirose, M.; Aida, K.; Sakai, S.; Fujii, A.; Hirata, T. Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp. Dermatol. 2012, 21, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Sato, A.; Hattori, Y.; Matsumoto, T.; Yokoyama, K.; Kanai, A. Dietary rice bran extract improves TEWL of whole body. Jpn. Pharmacol. Ther. 2013, 41, 1051–1059. [Google Scholar]
- Oba, C.; Morifuji, M.; Ichikawa, S.; Ito, K.; Kawahata, K.; Yamaji, T.; Asami, Y.; Itou, H.; Sugawara, T. Dietary milk sphingomyelin prevents disruption of skin barrier function in hairless mice after UV-B irradiation. PLoS ONE 2015, 10, e0136377. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, Y.; Ban, M.; Kishi, M.; Ono, T.; Masaki, H. Safety and efficacy of oral intake of ceramide-containing acetic acid bacteria for improving the stratum corneum hydration: A randomized, double-blind, placebo-controlled study over 12 weeks. J. Oleo Sci. 2020, 69, 1497–1508. [Google Scholar] [CrossRef]
- Ueda, O.; Hasegawa, M.; Kitamura, S. Distribution in skin of ceramide after oral administration to rats. Drug Metab. Pharmacokinet. 2009, 24, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Ueda, O.; Uchiyama, T.; Nakashima, M. Distribution and metabolism of sphingosine in skin after oral administration to mice. Drug Metab. Pharmacokinet. 2010, 25, 456–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirakura, Y.; Kikuchi, K.; Matsumura, K.; Mukai, K.; Mitsutake, S.; Igarashi, Y. 4,8-Sphingadienine and 4-hydroxy-8-sphingenine activate ceramide production in the skin. Lipids Health Dis. 2012, 11, 108. [Google Scholar] [CrossRef] [Green Version]
- Rohrhofer, J.; Zwirzitz, B.; Selberherr, E.; Untersmayr, E. The impact of dietary sphingolipids on intestinal microbiota and gastrointestinal immune homeostasis. Front. Immunol. 2021, 12, 5704. [Google Scholar] [CrossRef]
| SM | ||||
|---|---|---|---|---|
| Fatty Chain | Bovine Milk [34] | Bovine Brain [34] | Egg Yolk [34] | Chicken Skin [35] |
| Acyl group | nonhydroxy | |||
| C16:0 | 14 | 3 | 66 | 44 |
| C18:0 | 3 | 42 | 10 | 20 |
| C20:0 | 1 | 6 | 4 | 4 |
| C22:0 | 22 | 7 | 6 | 7 |
| C23:0 | 32 | 3 | 2 | 2 |
| C24:0 | 19 | 6 | 5 | 6 |
| C24:1 | 5 | 27 | 3 | 8 |
| Others | 4 | 6 | 4 | 9 |
| Sphingoid base | ||||
| d16:0 | 9 | |||
| d17:0 | 15 | |||
| d17:1 | 8 | |||
| Me-d17:1 | 11 | |||
| d18:0 | 10 | 19 | 7 | 2 |
| d18:14t | 44 | 82 | 93 | 98 |
| d19:0 | 4 | |||
| GlcCer | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fatty Chain | Rice [26] | Wheat [26] | Rye [26] | Maize [26] | Soybean [26] | Konjac [26] | Apple [36] | Yeast [26] | Mushroom [28] |
| Acyl group | α-hydroxy | ||||||||
| C16h:0 | 1 | 39 | 26 | 6 | 82 | 19 | 64 | 1 | 80 |
| C18h:0 | 6 | 7 | 5 | 16 | <1 | 32 | <1 | 99 | 12 |
| C20h:0 | 50 | 38 | 36 | 39 | <1 | 14 | <1 | ||
| C22h:0 | 14 | 4 | 8 | 12 | 7 | 17 | 12 | <1 | |
| C23h:0 | 7 | 2 | |||||||
| C24h:0 | 21 | 4 | 7 | 21 | 8 | 6 | 15 | 3 | |
| C24h:1 | 1 | 8 | |||||||
| C26h:0 | 3 | 2 | 2 | 3 | 1 | 1 | <1 | ||
| Others | 5 | 5 | 8 | 3 | 2 | 11 | 1 | 3 | |
| Sphingoid base | |||||||||
| d18:0 | 1 | 5 | 4 | 1 | <1 | <1 | 1 | 1 | |
| d18:14t | 4 | 1 | <1 | 3 | <1 | 1 | <1 | 1 | |
| d18:18c | <1 | 50 | 50 | <1 | 5 | 3 | <1 | ||
| d18:18t | 1 | 21 | 21 | <1 | <1 | 1 | <1 | ||
| d18:24t,8c | 45 | 9 | 5 | 54 | 24 | 51 | 34 | ||
| d18:24t,8t | 13 | 4 | 3 | 17 | 49 | 11 | 11 | 20 | <1 |
| 9Me-d18:24t,8t | 78 | 97 | |||||||
| t18:0 | 7 | 1 | 2 | 2 | <1 | 1 | 1 | 2 | 1 |
| t18:18c | 26 | 8 | 12 | 21 | 12 | 31 | 36 | ||
| t18:18t | 3 | 1 | 2 | 2 | 9 | 1 | 17 | ||
| Diet * | Animal | Diet Duration | DMH Treatment | Effects | Refs. |
|---|---|---|---|---|---|
| 0.1% buttermilk- and powdered milk-derived SM | Female CF1 mice6 wks of age | For 4 wks from 1 wk | Once i.p. per wk for 6 wks | ACF formation⬇ | [53] |
| after final DMH i.p. | 40 mg/kg bw | ||||
| 0.025%, 0.05%, and 0.1% buttermilk-derived SM | For 34 wks from 1 wk | Once i.p. per wk for 6 wks | Colonic tumor incidence⬌; Adenoma progression to adenocarcinoma⬇ | ||
| after final DMH i.p. | 20 mg/kg bw | ||||
| 0.1% milk-derived SM, synthetic SM (C16-d18:14t), | Female CF1 mice | For 4wks from 1 wk | Once i.p. per wk for 6 wks | ACF formation⬇ | [54] |
| and dihydoSM (C16-d18:0) | 6 wks of age | after final DMH i.p. | 40 mg/kg bw | ||
| 0.025% and 0.1% syntetic glucuronylceramide | Female CF1 mice | For 4 wks from 1 wk | Once i.p. per wk for 6 wks | ACF formation⬇ | [70] |
| (C16-d18:14t) | 6 wks of age | after final DMH i.p. | 30 mg/kg bw | ||
| 0.025% and 0.1% milk-derived GlcCer, LacCer, | Female CF1 mice | For 4 wks from 1 wk | Once i.p. per wk for 6 wks | ACF formation⬇; Cell proliferation in crypt⬇ (these data also contained | [46] |
| and ganglioside (GD3) | 6 wks of age | after final DMH i.p. | 30 mg/kg bw | 0.1% milk-derived and synthetic SM diets) | |
| 0.05% milk-derived SM | Female CF1 mice | For 7 wks from 1 wk | Once i.p. per wk for 6 wks | Colonic tumor incidence⬇; Adenoma and carcinoma⬆ | [55] |
| 5 wks of age | prior to first DMH i.p. | 30 mg/kg bw | |||
| Female CF1 mice | For 44 wks from 1 wk | Once i.p. per wk for 6 wks | Cell proliferation in crypt⬇; Apoptotic inhibition in crypts⬇ | ||
| 6 wks of age | after final DMH i.p. | 30 mg/kg bw | |||
| 0.05% milk-derived SM based on AIN-93 | Female ICR mice | For 22 wks after final | Once i.p. per wk for 6 wks | Colonic tumor formation⬇; Colonic expression and production of alk-SMase⬆; | [52] |
| 5 wks of age | DMH i.p. | 30 mg/kg bw | Alk-SMase activity in colon mucosa⬆; Alk-SMase activity in colon content⬌ | ||
| 0.1% and 0.5% maize-derived GlcCer | Male BALB/c mice | For 80 ds from 10 ds | Once i.p. per wk for 10 wks | ACF formation⬇ | [58] |
| and 0.1% yeast-derived GlcCer | 5 wks of age | before first DMH i.p. | 15 mg/kg bw | ||
| 0.1% maize-derived GlcCer | Male BALB/c mice | For 80 ds from 10 ds | Once i.p. per wk for 10 wks | Colonic mRNA expression: Wnt signaling pathway suppression (Soggy-1 and others)⬆, | [59] |
| 5 wks of age | prior to first DMH i.p. | 15 mg/kg bw | MAP-kinase pathway activation (Ras-associated protein and other)⬇, Caspase family⬌ | ||
| 0.1% maize-derived GlcCer | Male BALB/c mice | For 59 ds from 10 ds | Once i.p. per wk for 7 wks | ACF formation⬇; Colonic production of inflammation-related cytokines (IP-10, MIG, | [60] |
| 5 wks of age | prior to first DMH i.p. | 15 mg/kg bw | RANTES, I-TAC, IL-23, TNF-α, and M-CSF)⬇ | ||
| 0.02% and 0.1% rice-derived GlcCer based on CE-2 | Male F344 rats | For 5 wks from 1 wk | Once i.p. per 2 wks for 4 wks | ACF and BCAC formation⬇; | [61] |
| 42 wks of age | prior to first DMH i.p. | 40 mg/kg bw | Cell proliferation in ACF and BCAC⬇; Cell apoptosis in ACF and BCAC⬌ | ||
| 15% polished rice and 0.05% rice extract | Male BALB/c mice 5 wks of age | For 9 wks from 2 wks prior to first DMH i.p. | Once i.p. per wk for 7 wks 15 mg/kg bw | ACF formation⬇ only by rice extract diet (having the same GlcCer level as rice diet); Colonic production of inflammation-related cytokines (IP-10, MIG, I-TAC, TNF-α, M-CSF, and others)⬇ by rice extract diet | [68] |
| 0.09% sake rice extract and 4.21% sake lees extract | Male BALB/c mice | For 9 wks from 2 wks | Once i.p. per wk for 7 wks | ACF formation⬇; Colonic inflammation and oxidation (TNF-α and malondialdehyde)⬇; | [69] |
| 5 wks of age | prior to first DMH i.p. | 15 mg/kg bw | Colonic production of apoptosis-related proteins (Bcl-2, cleaved caspase-3, and others)⬇ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Kinoshita, M.; Miyazawa, T. Dietary Sphingolipids Contribute to Health via Intestinal Maintenance. Int. J. Mol. Sci. 2021, 22, 7052. https://doi.org/10.3390/ijms22137052
Yamashita S, Kinoshita M, Miyazawa T. Dietary Sphingolipids Contribute to Health via Intestinal Maintenance. International Journal of Molecular Sciences. 2021; 22(13):7052. https://doi.org/10.3390/ijms22137052
Chicago/Turabian StyleYamashita, Shinji, Mikio Kinoshita, and Teruo Miyazawa. 2021. "Dietary Sphingolipids Contribute to Health via Intestinal Maintenance" International Journal of Molecular Sciences 22, no. 13: 7052. https://doi.org/10.3390/ijms22137052
APA StyleYamashita, S., Kinoshita, M., & Miyazawa, T. (2021). Dietary Sphingolipids Contribute to Health via Intestinal Maintenance. International Journal of Molecular Sciences, 22(13), 7052. https://doi.org/10.3390/ijms22137052






