Vitamin D Supplementation: Oxidative Stress Modulation in a Mouse Model of Ovalbumin-Induced Acute Asthmatic Airway Inflammation
Abstract
:1. Introduction
2. Results
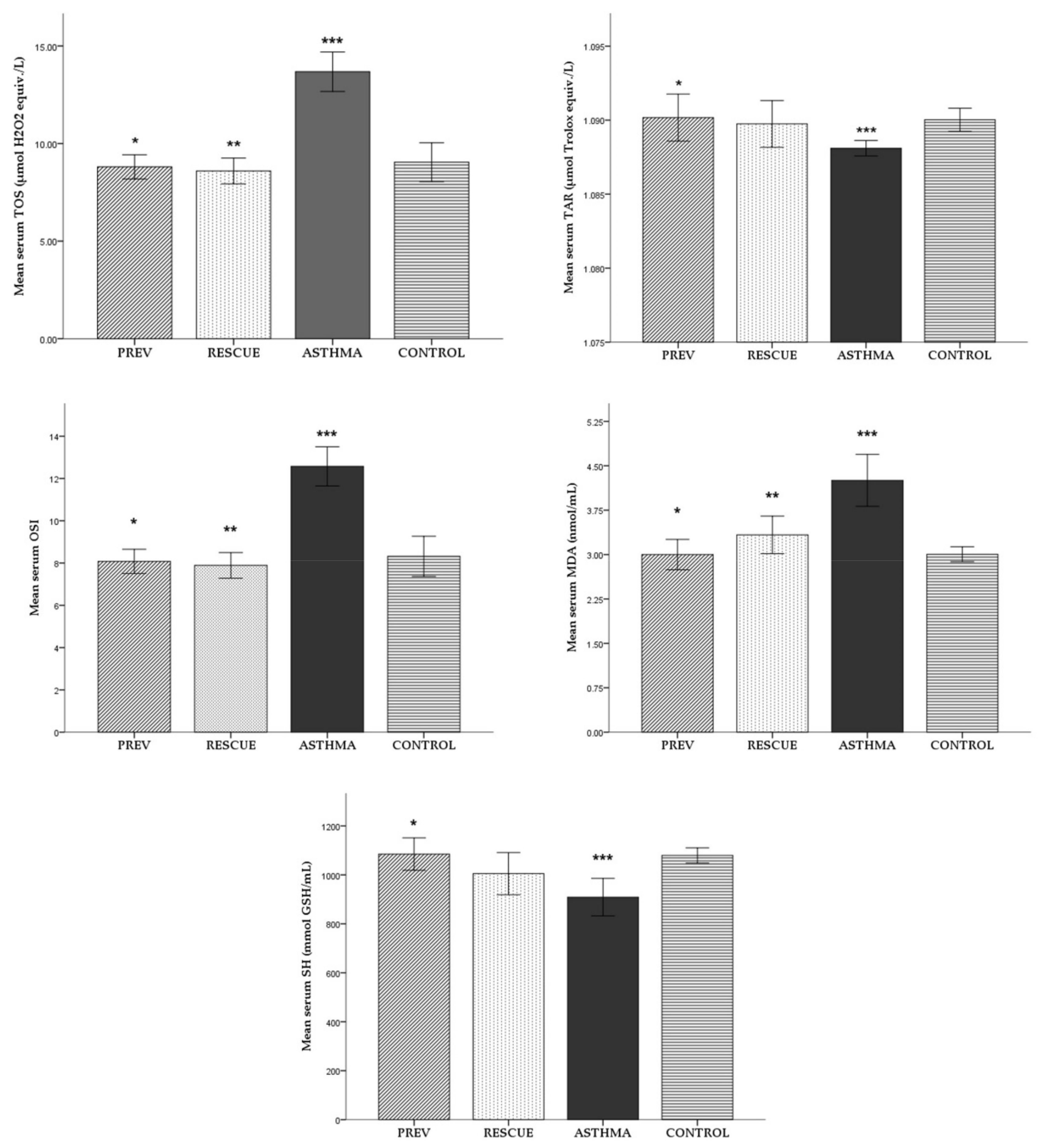
2.1. Oxidative Stress
2.1.1. Serum
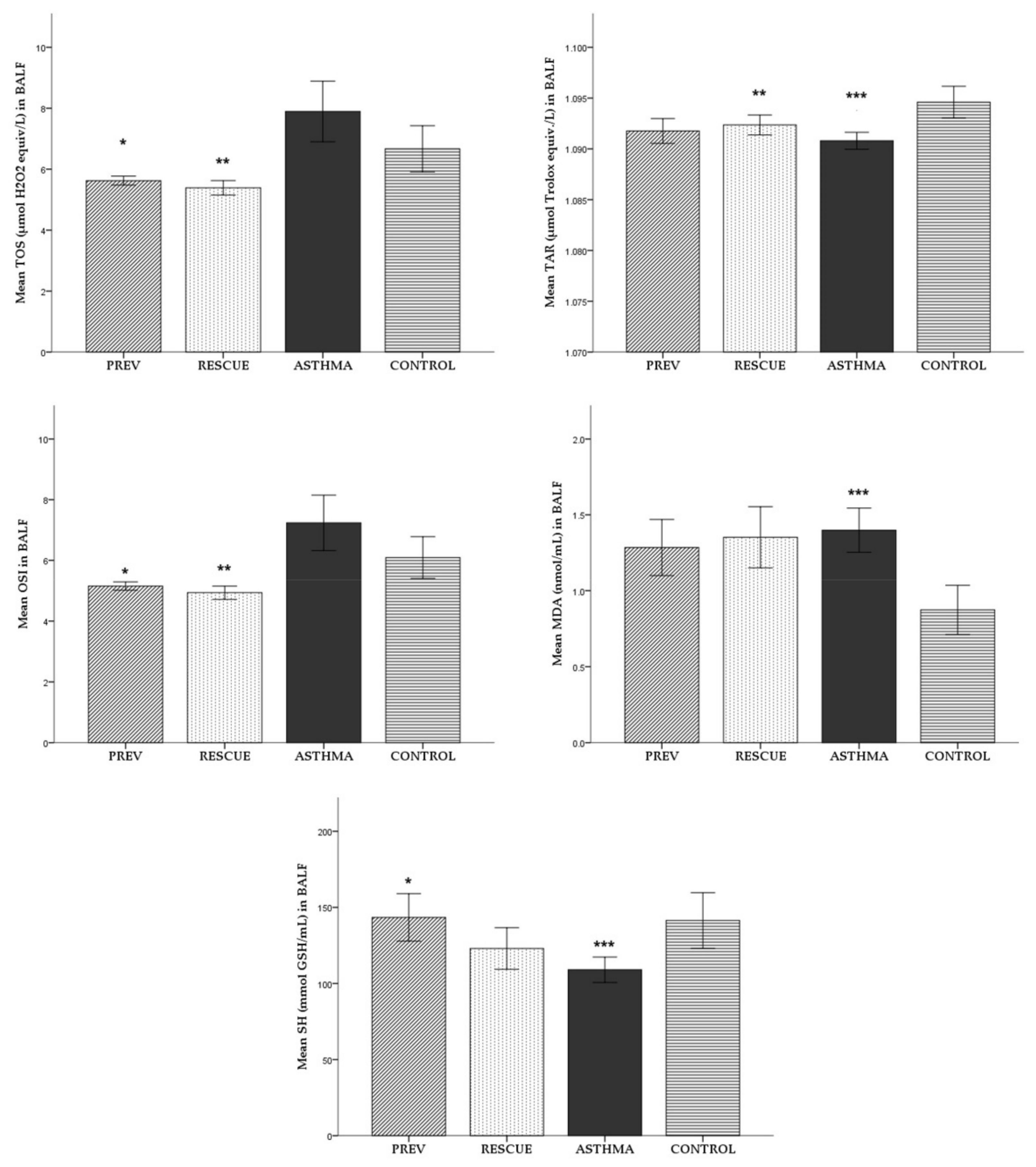
2.1.2. Broncho-Alveolar Lavage Fluid (BALF)
2.1.3. Lung Tissue Homogenate (LTH)
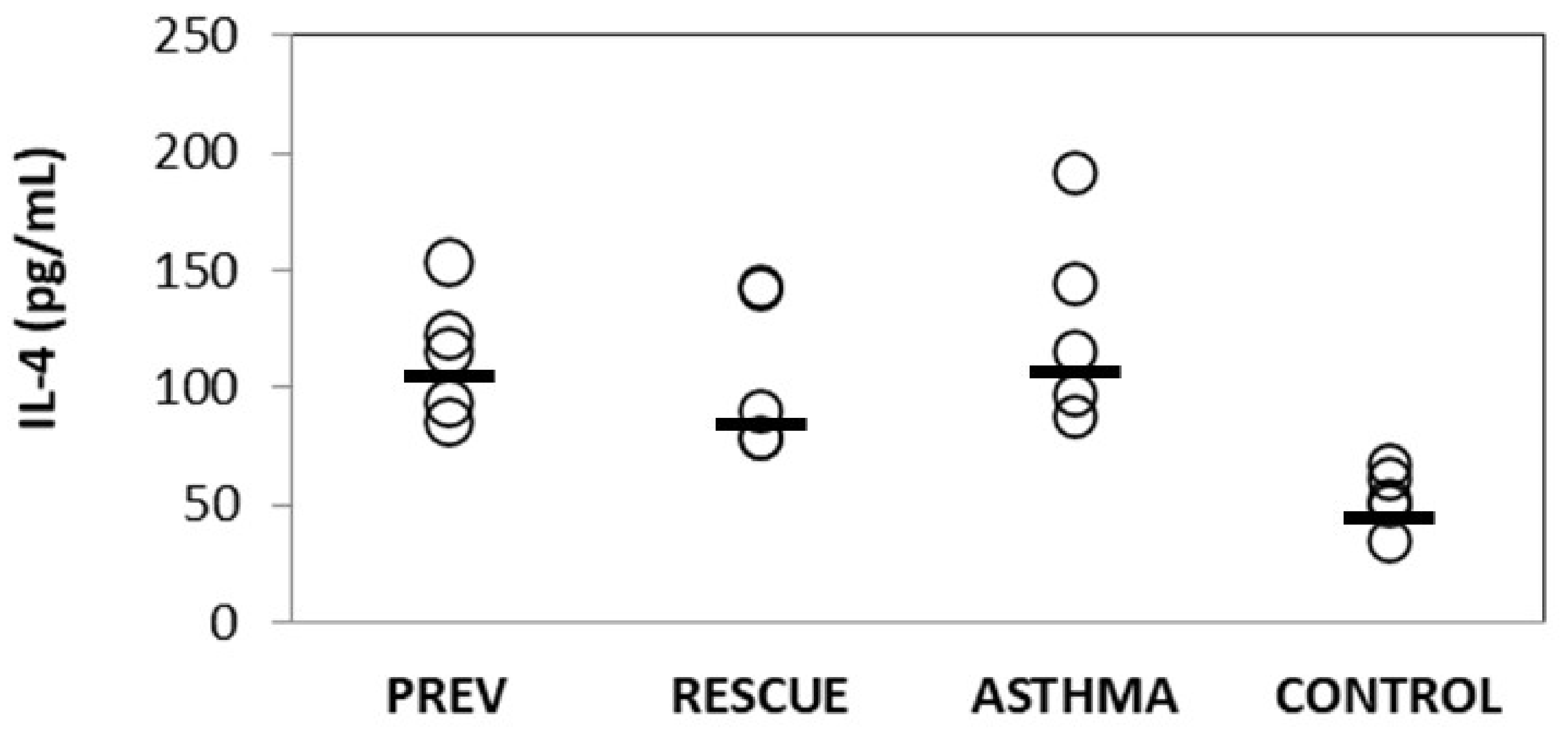
2.2. Inflammation
2.2.1. Inflammatory Cytokines
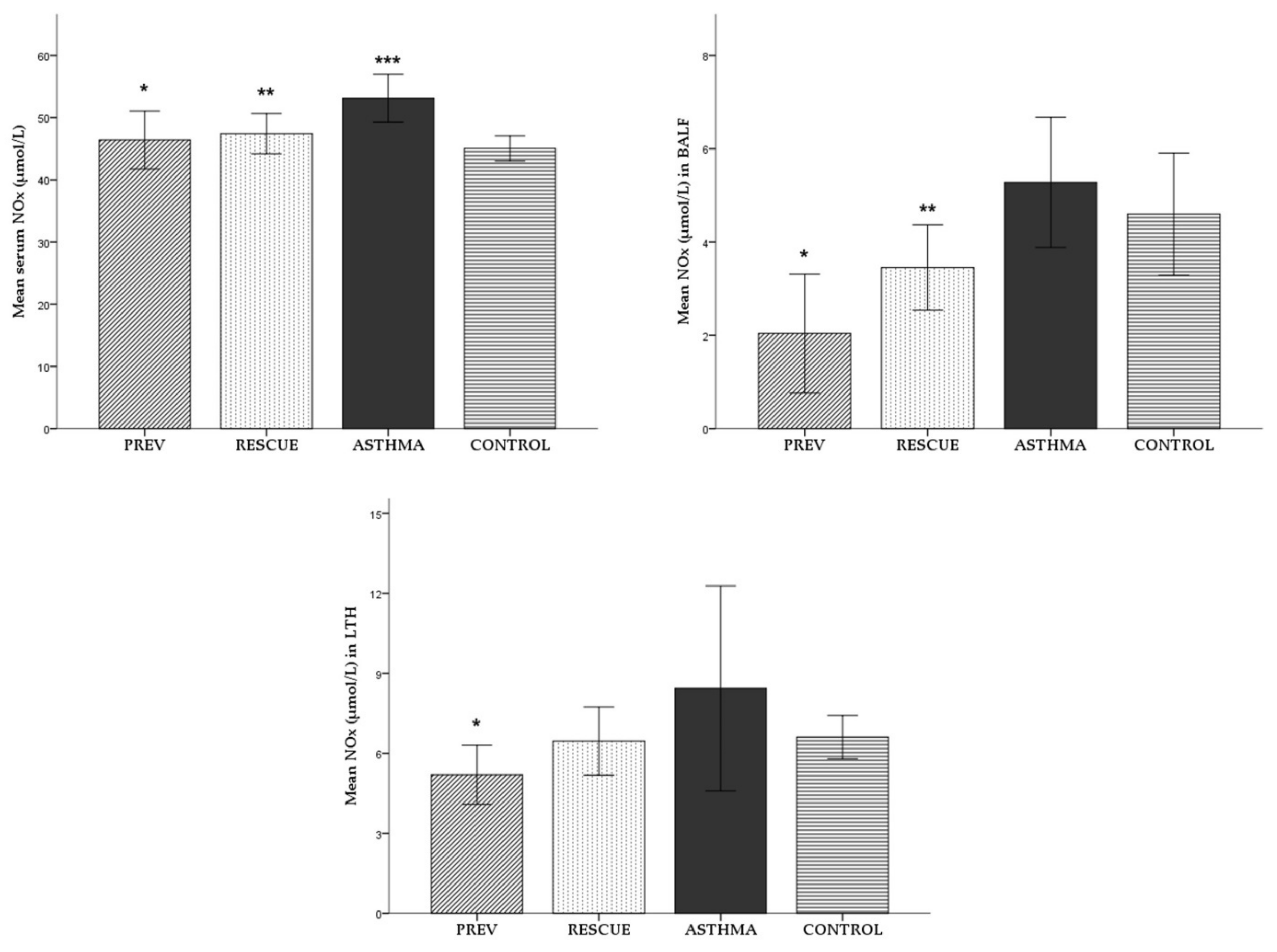
2.2.2. Nitric Oxide
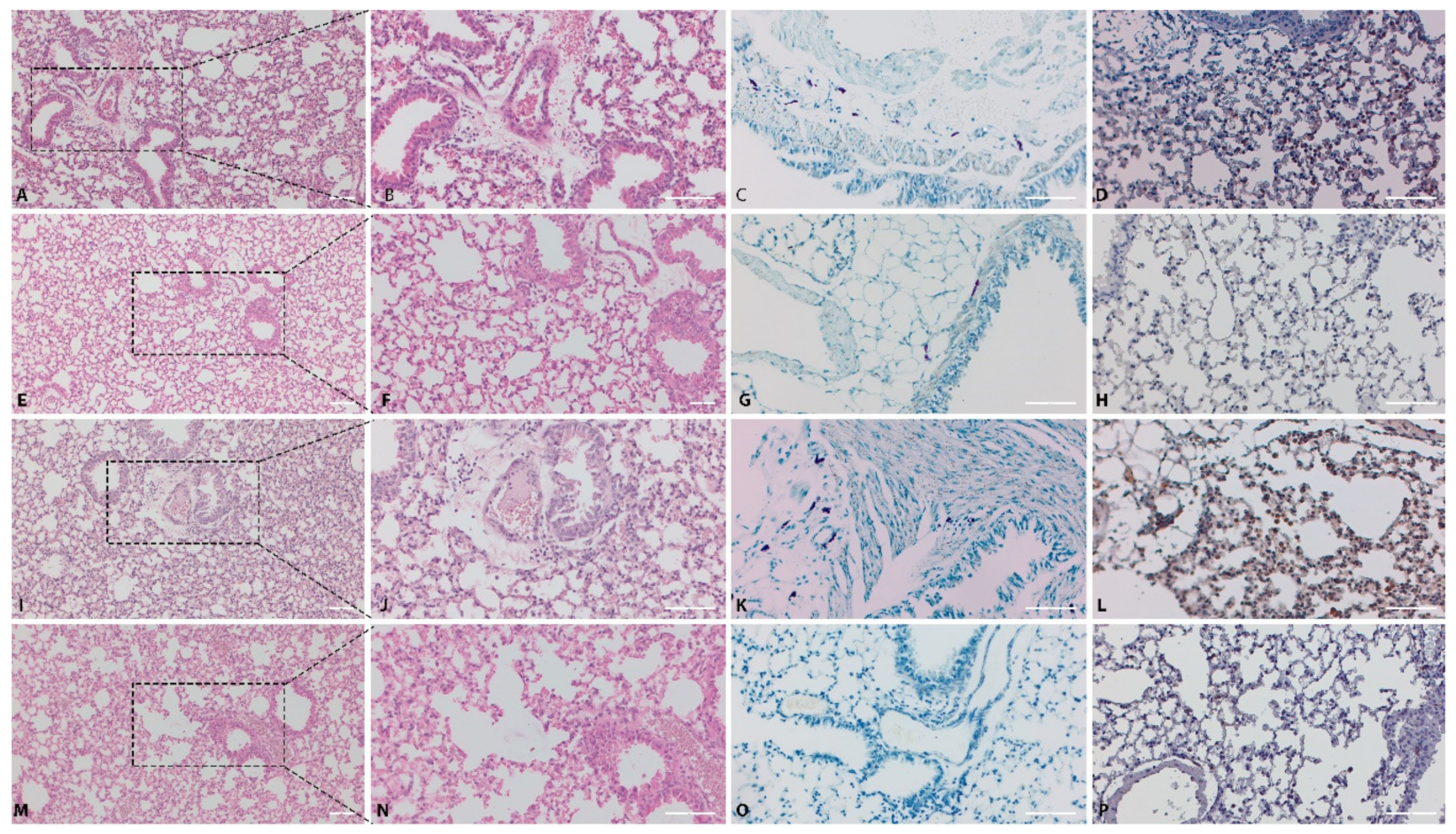
2.3. Pathology
3. Discussion
4. Materials and Methods
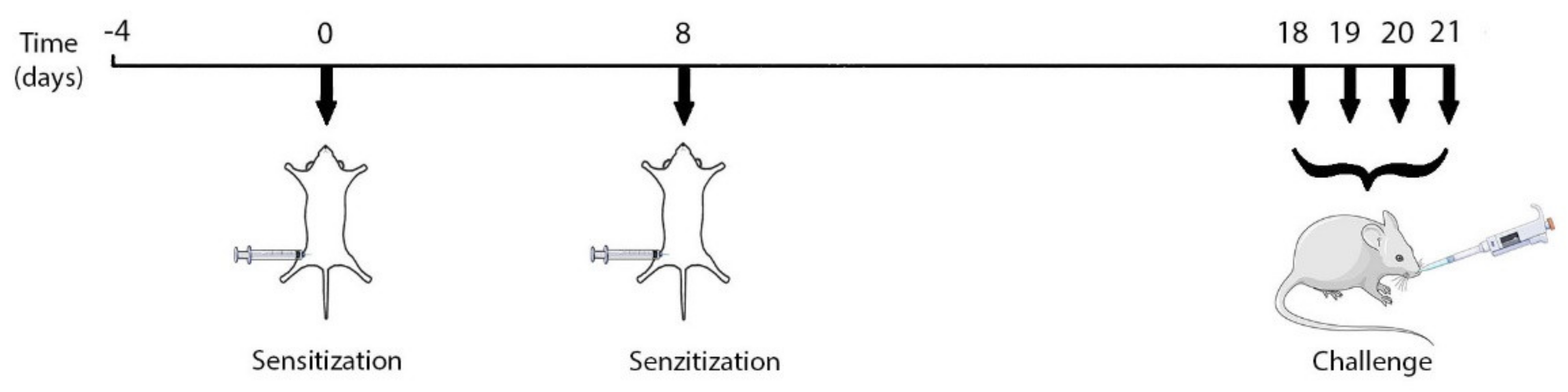
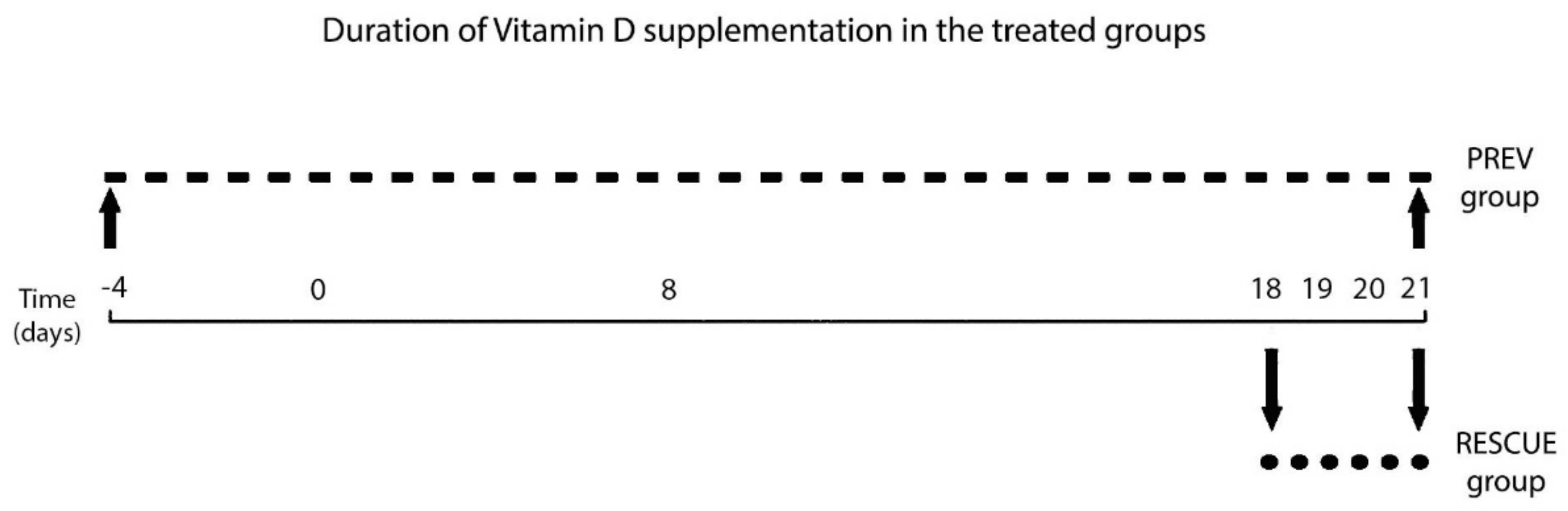
4.1. Experimental Design
4.1.1. Animal Subjects
4.1.2. Experimental Protocol: Ovalbumin-Mediated Asthma Inflammation
4.1.3. Experimental Intervention: VD Treatment Regimen
4.1.4 Harvesting of Biological Samples
4.2. Assessment of Oxidative Stress
4.3. Assessment of Inflammation
4.4. Pathology Analysis
4.4.1. Histopathology and Immunohistochemistry
4.4.2. Immunohistochemical Analysis of NF-kB
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Schadt, E.E. Systems biology of asthma and allergic diseases: A multiscale approach. J. Allergy Clin. Immunol. 2015, 135, 31–42. [Google Scholar] [CrossRef] [Green Version]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.-P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef] [Green Version]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef]
- Serebrisky, D.; Wiznia, A. Pediatric Asthma: A Global Epidemic. Ann. Glob. Health 2019, 85, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mallol, J.; Crane, J.; von Mutius, E.; Odhiambo, J.; Keil, U.; Stewart, A. The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergol. Immunopathol. 2013, 41, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2020. Available online: https://ginasthma.org/ (accessed on 16 March 2021).
- Raedler, D.; Schaub, B. Immune mechanisms and development of childhood asthma. Lancet Respir. Med. 2014, 2, 647–656. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Redox Control of Asthma: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedl, M.A.; Nel, A.E. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chamitava, L.; Cazzoletti, L.; Ferrari, M.; Garcia-Larsen, V.; Jalil, A.; Degan, P.; Fois, A.G.; Zinellu, E.; Fois, S.S.; Pasini, A.M.F.; et al. Biomarkers of Oxidative Stress and Inflammation in Chronic Airway Diseases. Int. J. Mol. Sci. 2020, 21, 4339. [Google Scholar] [CrossRef]
- Kirkham, P.; Rahman, I. Oxidative stress in asthma and COPD: Antioxidants as a therapeutic strategy. Pharmacol. Ther. 2006, 111, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative Stress in Asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Korn, S.; Hübner, M.; Jung, M.; Blettner, M.; Buhl, R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir. Res. 2013, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Thacher, T.D.; Clarke, B.L. Vitamin D Insufficiency. Mayo Clin. Proc. 2011, 86, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feketea, G.; Bocsan, C.I.; Stanciu, L.A.; Buzoianu, A.D.; Zdrenghea, M.T. The Role of Vitamin D Deficiency in Children With Recurrent Wheezing—Clinical Significance. Front. Pediatr. 2020, 8, 344. [Google Scholar] [CrossRef]
- Chinellato, I.; Piazza, M.; Sandri, M.; Paiola, G.; Tezza, G.; Boner, A.L. Correlation between vitamin D serum levels and passive smoking exposure in children with asthma. Allergy Asthma Proc. 2018, 39, 8–14. [Google Scholar] [CrossRef]
- Szentpetery, S.E.; Han, Y.-Y.; Brehm, J.M.; Acosta-Pérez, E.; Forno, E.; Boutaoui, N.; Canino, G.; Alcorn, J.F.; Celedón, J.C. Vitamin D insufficiency, plasma cytokines, and severe asthma exacerbations in school-aged children. J. Allergy Clin. Immunol. Pract. 2018, 6, 289–291.e2. [Google Scholar] [CrossRef] [PubMed]
- Brumpton, B.M.; Langhammer, A.; Henriksen, A.H.; Camargo, C.A.; Chen, Y.; Romundstad, P.R.; Mai, X.-M. Vitamin D and Lung Function Decline in Adults with Asthma. Am. J. Epidemiol. 2016, 183, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goleva, E.; Searing, D.A.; Jackson, L.P.; Richers, B.N.; Leung, D.Y. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J. Allergy Clin. Immunol. 2012, 129, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef]
- Solidoro, P.; Bellocchia, M.; Aredano, I.; Mattei, A.; Pivetta, E.; Patrucco, F.; Boita, M.; De Blasio, F.; Brussino, L.; Rolla, G.; et al. Asthmatic Patients with Vitamin D Deficiency have Decreased Exacerbations after Vitamin Replacement. Nutrients 2017, 9, 1234. [Google Scholar] [CrossRef] [Green Version]
- Sluyter, J.D.; Camargo, J.C.A.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.-T.; Scragg, R. Effect of Monthly, High-Dose, Long-Term Vitamin D on Lung Function: A Randomized Controlled Trial. Nutrients 2017, 9, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.-J.; Dai, R.-J. Serum levels of vitamin A and 25-hydroxyvitamin D3 (25OHD3) as reflectors of pulmonary function and quality of life (QOL) in children with stable asthma. Medicine 2018, 97, e9830. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Cates, C.J.; Urashima, M.; Jensen, M.; Griffiths, A.P.; Nurmatov, U.; Sheikh, A.; Griffiths, C.J. Vitamin D for the Management of Asthma (Review): Summary of Findings for the Main Comparison 2016, No. 9. Available online: https://doi.org/10.1002/14651858.CD011511.pub2.www.cochranelibrary.com (accessed on 17 March 2021).
- Hall, S.C.; Agrawal, D.K.; Science, T. Vitamin D and Bronchial Asthma: An Overview of the Last Five Years. Clin Ther. 2018, 39, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Thakur, C.; Dm, J.K.; Kumar, P.; Goyal, J.P.; Singh, K.; Gupta, A. Vitamin-D supplementation as an adjunct to standard treatment of asthma in children: A randomized controlled trial (ViDASTA Trial). Pediatr. Pulmonol. 2021, 56, 1427–1433. [Google Scholar] [CrossRef]
- Litonjua, A.A. Vitamin D and childhood asthma: Causation and contribution to disease activity. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Bourgoin-Heck, M.; Amat, F. Clinical phenotypes in asthma during childhood. Clin. Exp. Allergy 2017, 47, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Loza, M.J.; Djukanovic, R.; Chung, K.F.; Horowitz, D.; Ma, K.; Branigan, P.; Barnathan, E.S.; Susulic, V.S.; ADEPT (Airways Disease Endotyping for Personalized Therapeutics); U-BIOPRED (Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome Consortium) Investigators; et al. Validated and longitudinally stable asthma phenotypes based on cluster analysis of the ADEPT study. Respir. Res. 2016, 17, 1–21. [Google Scholar] [CrossRef]
- Kianmehr, M.; Ghorani, V.; Boskabady, M.H. Animal Model of Asthma, Various Methods and Measured Parameters: A Methodological Review. Iran J. Allergy Asthma Immunol. 2016, 15, 445–465. [Google Scholar]
- Yu, Q.; Chen, Z. Establishment of different experimental asthma models in mice. Exp. Ther. Med. 2018, 15, 2492–2498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltby, S.; Tay, H.L.; Yang, M.; Foster, P.S. Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation. Respirology 2017, 22, 874–885. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Zhao, J.; Cantorna, M.T. Invariant NKT Cell Defects in Vitamin D Receptor Knockout Mice Prevents Experimental Lung Inflammation. J. Immunol. 2011, 187, 4907–4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Hajiagha, A.M.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Wolsk, H.M.; Chawes, B.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Alansari, K.; Davidson, B.L.; Yousef, K.I.; Mohamed, A.N.H.; Alattar, I. Rapid vs. Maintenance Vitamin D Supplementation in Deficient Children With Asthma to Prevent Exacerbations. Chest 2017, 152, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Ducharme, F.M.; Alos, N.; Mailhot, G.; Mâsse, B.; White, J.H.; Sadatsafavi, M.; Khamessan, A.; Tse, S.M.; Alizadehfar, R.; et al. Vitamin D in the prevention of exacerbations of asthma in preschoolers (DIVA): Protocol for a multicentre randomised placebo-controlled triple-blind trial. BMJ Open 2019, 9, e033075. [Google Scholar] [CrossRef] [Green Version]
- Sikorska-Szaflik, H.; Sozańska, B. The Role of Vitamin D in Respiratory Allergies Prevention. Why the Effect Is so Difficult to Disentangle? Nutrients 2020, 12, 1801. [Google Scholar] [CrossRef]
- Forno, E.; Bacharier, L.B.; Phipatanakul, W.; Guilbert, T.W.; Cabana, M.D.; Ross, K.; Covar, R.; Gern, J.E.; Rosser, F.J.; Blatter, J.; et al. Effect of Vitamin D3 Supplementation on Severe Asthma Exacerbations in Children With Asthma and Low Vitamin D Levels. JAMA 2020, 324, 752. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Zeyrek, D.; Çakmak, A.; Atas, A.; Kocyigit, A.; Erel, O. DNA damage in children with asthma bronchiale and its association with oxidative and antioxidative measurements. Pediatr. Allergy Immunol. 2009, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Dilek, F.; Gedik, A.H.; Ozkaya, E.; Kocyigit, A.; Yazici, M.; Kesgin, S.; Cakir, E. Effect of Montelukast Monotherapy on Oxidative Stress Parameters and DNA Damage in Children with Asthma. Int. Arch. Allergy Immunol. 2015, 167, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Emin, O.; Hasan, A.; Rusen, D. Plasma paraoxonase, oxidative status level, and their relationship with asthma control test in children with asthma. Allergol. Immunopathol. 2015, 43, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Igde, M.; Baran, P.; Oksuz, B.G.; Topcuoğlu, S.; Karatekin, G. Association between the oxidative status, Vitamin D levels and respiratory function in asthmatic children. Niger. J. Clin. Pract. 2018, 21, 63–68. [Google Scholar] [CrossRef]
- Kianian, F.; Karimian, S.M.; Kadkhodaee, M.; Takzaree, N.; Seifi, B.; Adeli, S.; Harati, E.; Sadeghipour, H.R. Combination of ascorbic acid and calcitriol attenuates chronic asthma disease by reductions in oxidative stress and inflammation. Respir. Physiol. Neurobiol. 2019, 270, 103265. [Google Scholar] [CrossRef]
- Wei-Hong, T.; Min-Chang, G.; Zhen, X.; Jie, S. Pharmacological and Pharmacokinetic Studies with Vitamin D-loaded Nanoemulsions in Asthma Model. Inflammation 2013, 37, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.; Luo, G.; Yang, X.; Cheng, Y.; Zhang, Y.; Wang, X.; Wang, X.; Xie, T.; Li, G.; Liu, Z.; et al. 25-Hydroxyvitamin D3-Deficiency Enhances Oxidative Stress and Corticosteroid Resistance in Severe Asthma Exacerbation. PLoS ONE 2014, 9, e111599. [Google Scholar] [CrossRef]
- Dut, R.; Dizdar, E.A.; Birben, E.; Sackesen, C.; Soyer, O.U.; Besler, T.; Kalayci, O. Oxidative stress and its determinants in the airways of children with asthma. Allergy 2008, 63, 1605–1609. [Google Scholar] [CrossRef]
- Antus, B. Oxidative Stress Markers in Sputum. Oxidative Med. Cell. Longev. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ben Anes, A.; Ben Nasr, H.; Fetoui, H.; Bchir, S.; Chahdoura, H.; Yacoub, S.; Garrouch, A.; Benzarti, M.; Tabka, Z.; Chahed, K. Alteration in systemic markers of oxidative and antioxidative status in Tunisian patients with asthma: Relationships with clinical severity and airflow limitation. J. Asthma 2015, 53, 1–11. [Google Scholar] [CrossRef]
- Ercan, H.; Birben, E.; Dizdar, E.A.; Keskin, O.; Karaaslan, C.; Soyer, O.U.; Dut, R.; Sackesen, C.; Besler, T.; Kalayci, O. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J. Allergy Clin. Immunol. 2006, 118, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Fang, L.-W.; Liou, C.-J. Phloretin Attenuates Allergic Airway Inflammation and Oxidative Stress in Asthmatic Mice. Front. Immunol. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ge, A.; Zhu, W.; Liu, Y.-N.; Ji, N.-F.; Zha, W.-J.; Zhang, J.-X.; Zeng, X.-N.; Huang, M. Morin Attenuates Ovalbumin-Induced Airway Inflammation by Modulating Oxidative Stress-Responsive MAPK Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abdеlaziz, R.R.; Еlmahdy, M.K.; Suddеk, G.M. Flavocoxid attenuates airway inflammation in ovalbumin-induced mouse asthma model. Chem. Interact. 2018, 292, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, H.; Sun, X.; Ren, L. The protective role of vitamin D3 in a murine model of asthma via the suppression of TGF-β/Smad signaling and activation of the Nrf2/HO-1 pathway. Mol. Med. Rep. 2016, 14, 2389–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erzurum, S.C. New Insights in Oxidant Biology in Asthma. Ann. Am. Thorac. Soc. 2016, 13, S35–S39. [Google Scholar] [CrossRef]
- Comhair, S.A.; Bhathena, P.R.; Dweik, R.A.; Kavuru, M.; Erzurum, S.C. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet 2000, 355, 624. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Fernando, Y.; Wickramasinghe, P.; De Silva, U.; Alahakoon, M.; Anuradha, K.W.D.A.; Handunnetti, S. Differences in serum markers of oxidative stress in well controlled and poorly controlled asthma in Sri Lankan children: A pilot study. Allergy Asthma Clin. Immunol. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Katsoulis, K.; Kontakiotis, T.; Leonardopoulos, I.; Kotsovili, A.; Legakis, I.N.; Patakas, D. Serum Total Antioxidant Status in Severe Exacerbation of Asthma: Correlation with the Severity of the Disease. J. Asthma 2003, 40, 847–854. [Google Scholar] [CrossRef]
- De Raeve, H.R.; Thunnissen, F.B.; Kaneko, F.T.; Guo, F.H.; Lewis, M.; Kavuru, M.S.; Secic, M.; Thomassen, M.J.; Erzurum, S.C. Decreased Cu,Zn-SOD activity in asthmatic airway epithelium: Correction by inhaled corticosteroid in vivo. Am. J. Physiol. Cell. Mol. Physiol. 1997, 272, L148–L154. [Google Scholar] [CrossRef]
- Larsen, G.L.; White, C.W.; Takeda, K.; Loader, J.E.; Nguyen, D.D.H.; Joetham, A.; Groner, Y.; Gelfand, E.W.; Gary, L.; White, C.W.; et al. Mice That Overexpress Cu/Zn Superoxide Dismutase Are Resistant to Allergen-Induced Changes in Airway Control. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2000, 279, L350–L359. [Google Scholar] [CrossRef]
- Corradi, M.; Folesani, G.; Andreoli, R.; Manini, P.; Bodini, A.; Piacentini, G.; Carraro, S.; Zanconato, S.; Baraldi, E. Aldehydes and Glutathione in Exhaled Breath Condensate of Children with Asthma Exacerbation. Am. J. Respir. Crit. Care Med. 2003, 167, 395–399. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free. Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Donaldson, K.; Rahman, I.; MacNee, W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am. J. Respir. Crit. Care Med. 1994, 149, 1518–1525. [Google Scholar] [CrossRef]
- Cross, C.E.; Van Der Vliet, A.; O’Neill, C.A.; Louie, S.; Halliwell, B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994, 102, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilek, F.; Ozkaya, E.; Kocyigit, A.; Yazici, M.; Guler, E.M.; Dundaroz, M.R. Plasma total thiol pool in children with asthma: Modulation during montelukast monotherapy. Int. J. Immunopathol. Pharmacol. 2016, 29, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, A.; Siddiqui, N.; Alharbi, N.O.; Alharbi, M.M.; Imam, F.; Sayed-Ahmed, M.M. Glutathione modulation during sensitization as well as challenge phase regulates airway reactivity and inflammation in mouse model of allergic asthma. Biochimie 2014, 103, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.E.; Lu, H.; Mann, E.; Chen, Y.-H.; Ho, T.-R.; Cousins, D.; Corrigan, C.; Kelly, F.J.; Mudway, I.S.; Hawrylowicz, C.M. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE 2018, 13, e0200040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, G.-S.; Zhang, C.; Cheng, B.-H.; Lee, C.-H. Mechanisms of Action of Vitamin D as Supplemental Therapy for Pneumocystis Pneumonia. Antimicrob. Agents Chemother. 2017, 61, e01226-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renauld, J.-C. New insights into the role of cytokines in asthma. J. Clin. Pathol. 2001, 54, 577–589. [Google Scholar] [CrossRef] [Green Version]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Jiang, L.; Diaz, P.T.; Best, T.M.; Stimpfl, J.N.; He, F.; Zuo, L. Molecular characterization of redox mechanisms in allergic asthma. Ann. Allergy Asthma Immunol. 2014, 113, 137–142. [Google Scholar] [CrossRef]
- Walch, L.; Massade, L.; Dufilho, M.; Brunet, A.; Rendu, F. Pro-atherogenic effect of interleukin-4 in endothelial cells: Modulation of oxidative stress, nitric oxide and monocyte chemoattractant protein-1 expression. Atherosclerosis 2006, 187, 285–291. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, J.; Ma, Z.; Ma, S. Anti-asthmatic effects of matrine in a mouse model of allergic asthma. Fitoterapia 2014, 94, 183–189. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Kwon, O.-K.; Oh, S.-R.; Lee, H.-K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Preventive and therapeutic effects of vitamin D in a mouse model of allergic asthma. Asian Pac. J. Allergy Immunol. 2018, 37, 130–137. [CrossRef]
- Liu, J.; Dong, Y.-Q.; Yin, J.; Yao, J.; Shen, J.; Sheng, G.-J.; Li, K.; Lv, H.-F.; Fang, X.; Wu, W.-F. Meta-analysis of vitamin D and lung function in patients with asthma. Respir. Res. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, T.; Gupta, G.K.; Agrawal, D.K. Vitamin D Supplementation Reduces Airway Hyperresponsiveness and Allergic Airway Inflammation in a Murine Model. Clin. Exp. Allergy 2013, 43, 672–683. [Google Scholar] [CrossRef]
- Mazahery, H.; Von Hurst, P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantano, C.; Ather, J.L.; Alcorn, J.F.; Poynter, M.E.; Brown, A.L.; Guala, A.S.; Beuschel, S.L.; Allen, G.B.; Whittaker, L.A.; Bevelander, M.; et al. Nuclear Factor-κB Activation in Airway Epithelium Induces Inflammation and Hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2008, 177, 959–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yi, M.; Jin, R.; Feng, X.; Ma, L.; Wang, Y.; Shan, Y.; Yang, Z.; Zhao, B. Correlation between oxidative stress and NF-κB signaling pathway in the obesity-asthma mice. Mol. Biol. Rep. 2020, 47, 3735–3744. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The Dual Role of Inducible Nitric Oxide Synthase in Myocardial Ischemia/Reperfusion Injury: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.H.; Comhair, S.A.A.; Zheng, S.; Dweik, R.A.; Eissa, N.T.; Thomassen, M.J.; Calhoun, W.; Erzurum, S.C. Molecular Mechanisms of Increased Nitric Oxide (NO) in Asthma: Evidence for Transcriptional and Post-Translational Regulation of NO Synthesis. J. Immunol. 2000, 164, 5970–5980. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.H.; Uetani, K.; Haque, S.J.; Williams, B.R.; Dweik, R.A.; Thunnissen, F.B.; Calhoun, W.; Erzurum, S.C. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J. Clin. Investig. 1997, 100, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweik, R.A.; Comhair, S.A.A.; Gaston, B.; Thunnissen, E.; Farver, C.; Thomassen, M.J.; Kavuru, M.; Hammel, J.; Abu-Soud, H.M.; Erzurum, S.C. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. USA 2001, 98, 2622–2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silkoff, P.E.; Carlson, M.; Bourke, T.; Katial, R.; Ögren, E.; Szefler, S.J. The Aerocrine exhaled nitric oxide monitoring system NIOX is cleared by the US Food and Drug Administration for monitoring therapy in asthma. J. Allergy Clin. Immunol. 2004, 114, 1241–1256. [Google Scholar] [CrossRef]
- Seldeen, K.L.; Pang, M.; Rodríguez-Gonzalez, M.; Hernández, M.; Sheridan, Z.; Yu, P.; Troen, B.R. A mouse model of vitamin D insufficiency: Is there a relationship between 25(OH) vitamin D levels and obesity? Nutr. Metab. 2017, 14, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, D.A.J.; Van Beek, J.; Ferwerda, H.; Brugman, A.M.; Van Der Klis, F.R.M.; Van Der Heiden, H.J.; Muskiet, F.A.J. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br. J. Nutr. 1998, 79, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [Green Version]
- Daubeuf, F.; Frossard, N. Acute Asthma Models to Ovalbumin in the Mouse. Curr. Protoc. Mouse Biol. 2013, 3, 31–37. [Google Scholar] [CrossRef]
- Levin-Arama, M.; Abraham, L.; Waner, T.; Harmelin, A.; Steinberg, D.M.; Lahav, T.; Harlev, M. Subcutaneous Compared with Intraperitoneal Ketamine–Xylazine for Anesthesia of Mice. J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 794–800. [Google Scholar]
- Limjunyawong, N.; Mock, J.; Mitzner, W. Instillation and Fixation Methods Useful in Mouse Lung Cancer Research. J. Vis. Exp. 2015, 102, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Reddy, A.T.; Lakshmi, S.P.; Reddy, R.C. Murine Model of Allergen Induced Asthma. J. Vis. Exp. 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Draper, H.; Squires, E.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free. Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of Thiol Compounds and Lipid Peroxidative Products in Plasma of Patients with Copd. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kittel, B.; Ruehl-Fehlert, C.; Morawietz, G.; Klapwijk, J.; Elwell, M.R.; Lenz, B.; O’Sullivan, M.G.; Roth, D.R.; Wadsworth, P.F. Revised guides for organ sampling and trimming in rats and mice—Part 2: A joint publication of the RITA)and NACAD)groups. Exp. Toxicol. Pathol. 2004, 55, 413–431. [Google Scholar] [CrossRef]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of Inflammation and Airway Hyperresponsiveness in Immune-sensitized Mice by Dominant-Negative Phosphoinositide 3-Kinase–TAT. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarnegar, B.J.; Flynn, R.A.; Shen, Y.; Do, B.; Chang, H.Y.; Khavari, P.A. irCLIP platform for efficient characterization of protein–RNA interactions. Nat. Methods 2016, 13, 489–492. [Google Scholar] [CrossRef]
- Yukawa, M.; Fujimori, T.; Maeda, S.; Tabuchi, M.; Nagasako, K. Comparative clinicopathological and immunohistochemical study of ras and p53 in flat and polypoid type colorectal tumours. Gut 1994, 35, 1258–1261. [Google Scholar] [CrossRef] [Green Version]
- Charalambous, M.P.; Maihöfner, C.; Bhambra, U.; Lightfoot, T.; Gooderham, N.J.; The Colorectal Cancer Study Group. Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-κB and IκB kinase-α in human colorectal cancer epithelial cells. Br. J. Cancer 2003, 88, 1598–1604. [Google Scholar] [CrossRef]
- Winter, J.C.F. Using the Student’s t-test with Extremely Small Sample Sizes. Pract. Assess. Res. Eval. 2013, 18, 1–12. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam-Bonci, T.-I.; Bonci, E.-A.; Pârvu, A.-E.; Herdean, A.-I.; Moț, A.; Taulescu, M.; Ungur, A.; Pop, R.-M.; Bocșan, C.; Irimie, A. Vitamin D Supplementation: Oxidative Stress Modulation in a Mouse Model of Ovalbumin-Induced Acute Asthmatic Airway Inflammation. Int. J. Mol. Sci. 2021, 22, 7089. https://doi.org/10.3390/ijms22137089
Adam-Bonci T-I, Bonci E-A, Pârvu A-E, Herdean A-I, Moț A, Taulescu M, Ungur A, Pop R-M, Bocșan C, Irimie A. Vitamin D Supplementation: Oxidative Stress Modulation in a Mouse Model of Ovalbumin-Induced Acute Asthmatic Airway Inflammation. International Journal of Molecular Sciences. 2021; 22(13):7089. https://doi.org/10.3390/ijms22137089
Chicago/Turabian StyleAdam-Bonci, Teodora-Irina, Eduard-Alexandru Bonci, Alina-Elena Pârvu, Andrei-Ioan Herdean, Augustin Moț, Marian Taulescu, Andrei Ungur, Raluca-Maria Pop, Corina Bocșan, and Alexandru Irimie. 2021. "Vitamin D Supplementation: Oxidative Stress Modulation in a Mouse Model of Ovalbumin-Induced Acute Asthmatic Airway Inflammation" International Journal of Molecular Sciences 22, no. 13: 7089. https://doi.org/10.3390/ijms22137089





