Synthesis of pH and Glucose Responsive Silk Fibroin Hydrogels
Abstract
:1. Introduction
2. Results
2.1. Cationic Modification of Silk Fibroin from Bombyx Mori
2.2. Morphology of CSF Hydrogel
2.3. Aggregation Structure of CSF Hydrogel
2.4. Mechanical Properties of CSF Hydrogels
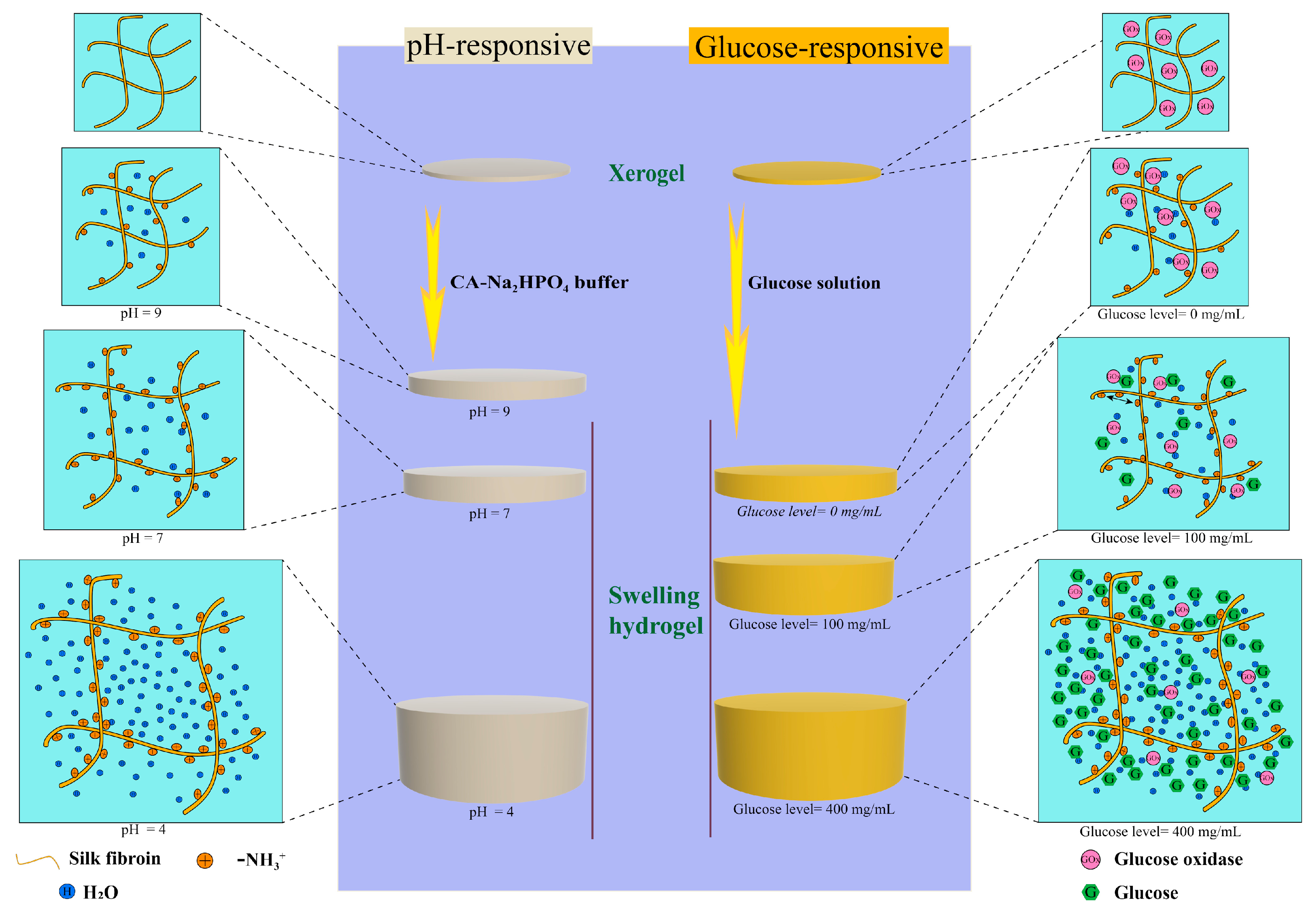
2.5. pH Sensitive Swelling Properties of CSF Hydrogels
2.6. Properties of Glucose-Responsive CSF Hydrogels
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Preparation of Silk Fibroin Solution
4.3. Preparation of CSF
4.4. Preparation of Hydrogels
4.5. Characterization of CSF
4.5.1. Zeta Potential
4.5.2. Isoelectric Point
4.5.3. Gel Electrophoresis Test
4.6. Structural Measurement of CSF
4.6.1. Fourier Transform Infrared Absorption Spectroscopy
4.6.2. Crystal Structure of CSF
4.7. Morphology of Hydrogels
4.8. Mechanical Properties of The Hydrogels
4.8.1. Compressive Mechanical Properties
4.8.2. Hydrogel Compression Resilience Test
4.9. pH Sensitive Swelling Properties
4.10. Performance of Glucose-Responsive Hydrogel
4.10.1. Response of Internal pH of Hydrogel to External Glucose Concentration
4.10.2. Glucose Responsive Properties
- (1)
- The response of xerogel internal pH to external glucose concentration.
- (2)
- The response of xerogel swelling degree to glucose concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SF | Silk Fibroin |
| CSF | Cationic Silk Fibroin |
| GOx | glucose oxidase |
| ε-PLL | ε-Poly-(L-lysine) |
| NHS | N-hydroxysuccinimide |
| EDC | 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide |
| MES | morpholine ethylsulfonic acid |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| SEM | Scanning electron microscope |
| pI | isoelectric point |
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.H.; Fan, G.H.; Liu, Y.; Liu, Z.B.; Xiang, J.; Wang, Y.M.; Song, B.; Gu, X.Y.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Cure, E.; Cumhur Cure, M. COVID-19 may affect the endocrine pancreas by activating Na+/H+ exchanger 2 and increasing lactate levels. J. Endocrinol. Investig. 2020, 43, 1167–1168. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Morris, E.; Goyder, C.; Kinton, J.; Perring, J.; Nunan, D.; Mahtani, K.; Buse, J.B.; Del Prato, S.; Ji, L.; et al. Diabetes and COVID-19: Risks, Management, and Learnings From Other National Disasters. Diabetes Care 2020, 43, 1695. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1alpha/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Kahkoska, A.R.; Buse, J.B.; Langer, R.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020, 4, 499–506. [Google Scholar] [CrossRef]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Del. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef]
- Ravaine, V.; Ancla, C.; Catargi, B. Chemically controlled closed-loop insulin delivery. J. Control. Release 2008, 132, 2–11. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Yan, J.; Kahkoska, A.R.; Gu, Z. Advances in bioresponsive closed-loop drug delivery systems. Int. J. Pharm. 2018, 544, 350–357. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Yu, J.; Kahkoska, A.R.; Buse, J.B.; Gu, Z. Glucose-Responsive Insulin and Delivery Systems: Innovation and Translation. Adv. Mater. 2020, 32, 1902004. [Google Scholar] [CrossRef]
- Cabane, E.; Zhang, X.; Langowska, K.; Palivan, C.G.; Meier, W. Stimuli-Responsive Polymers and Their Applications in Nanomedicine. Biointerphases 2012, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Liarou, E.; Varlas, S.; Skoulas, D.; Tsimblouli, C.; Sereti, E.; Dimas, K.; Iatrou, H. Smart polymersomes and hydrogels from polypeptide-based polymer systems through α-amino acid N-carboxyanhydride ring-opening polymerization. From chemistry to biomedical applications. Prog. Polym. Sci. 2018, 83, 28–78. [Google Scholar] [CrossRef]
- Cangialosi, A.; Yoon, C.; Liu, J.; Huang, Q.; Guo, J.; Nguyen, T.D.; Gracias, D.H.; Schulman, R. DNA sequence–directed shape change of photopatterned hydrogels via high-degree swelling. Science 2017, 357, 1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Ye, Y.; Sun, W.; Yu, J.; Wang, J.; Lawrence, D.S.; Buse, J.B.; Gu, Z. Red Blood Cells for Glucose-Responsive Insulin Delivery. Adv. Mater. 2017, 29, 1606617. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A. Glucose-Controlled Insulin-Delivery System–Semi-Synthetic Insulin Bound to Lectin. Science 1979, 206, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wu, M.; Lin, S.; Nargund, R.P.; Mu, J. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight 2018, 3, e97476. [Google Scholar] [CrossRef]
- Kataoka, K.; Miyazaki, H.; Bunya, M.; Okano, T.; Sakurai, Y. Totally Synthetic Polymer Gels Responding to External Glucose Concentration: Their Preparation and Application to On−Off Regulation of Insulin Release. J. Am. Chem. Soc. 1998, 120, 12694–12695. [Google Scholar] [CrossRef]
- Chou, D.H.-C.; Webber, M.J.; Tang, B.C.; Lin, A.B.; Thapa, L.S.; Deng, D.; Truong, J.V.; Cortinas, A.B.; Langer, R.; Anderson, D.G. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc. Natl. Acad. Sci. USA 2015, 112, 2401–2406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Z.; Aimetti, A.A.; Wang, Q.; Dang, T.T.; Zhang, Y.L.; Veiseh, O.; Cheng, H.; Langer, R.S.; Anderson, D.G. Injectable Nano-Network for Glucose-Mediated Insulin Delivery. ACS Nano 2013, 7, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2019, 5, 61–81. [Google Scholar] [CrossRef]
- Omenetto, F.G.; Kaplan, D.L. New Opportunities for an Ancient Material. Science 2010, 329, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.L.; Sun, M.Y.; Hu, X.Q.; Ren, B.; Cheng, J.; Li, C.X.; Duan, X.N.; Fu, X.; Zhang, J.Y.; Chen, H.F.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 7. [Google Scholar] [CrossRef]
- Cai, Z.X.; Mo, X.M.; Zhang, K.H.; Fan, L.P.; Yin, A.L.; He, C.L.; Wang, H.S. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Zhu, M.; Zhao, L.; Kuang, D.; Kundu, S.C.; Lu, S. Insulin-Loaded Silk Fibroin Microneedles as Sustained Release System. ACS Biomater. Sci. Eng. 2019, 5, 1887–1894. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Kundu, S.C.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.S.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.Q.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Ohgo, K.; Ishida, T.; Taddei, P.; Monti, P.; Kishore, R. Possible implications of serine and tyrosine residues and intermolecular interactions on the appearance of silk I structure of Bombyx mori silk fibroin-derived synthetic peptides: High-resolution C-13 cross-polarization/magic-angle spinning NMR study. Biomacromolecules 2005, 6, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.R.; Loh, X.J. Stimuli-Responsive Cationic Hydrogels in Drug Delivery Applications. Gels 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; Li, J.; Qu, J.; Sheng, W.; Yang, J.; Li, M. Cationized Bombyx mori silk fibroin as a delivery carrier of the VEGF165–Ang-1 coexpression plasmid for dermal tissue regeneration. J. Mater. Chem. B 2019, 7, 80–94. [Google Scholar] [CrossRef]
- Luo, H.; Chen, Y.C.; Niu, L.X.; Liang, A.H.; Yang, J.C.; Li, M.Z. Hepatoma Cell-Targeted Cationized Silk Fibroin as a Carrier for the Inhibitor of Growth 4-Interleukin-24 Double Gene Plasmid. J. Biomed. Nanotechnol. 2019, 15, 1622–1635. [Google Scholar] [CrossRef]
- Hua, J.C.; Li, Z.; Xia, W.; Yang, N.; Gong, J.X.; Zhang, J.F.; Qiao, C.S. Preparation and properties of EDC/NHS mediated crosslinking poly (gamma-glutamic acid)/epsilon-polylysine hydrogels. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Kahar, P.; Iwata, T.; Hiraki, J.; Park, E.Y.; Okabe, M. Enhancement of ε-polylysine production by Streptomyces albulus strain 410 using pH control. J. Biosci. Bioeng. 2001, 91, 190–194. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Chen, W.; Xu, T.; Yun, S.; Xu, Z.; Xu, Z.; Sato, T.; Chi, B.; Xu, H. A Biomimetic Mussel-Inspired ε-Poly-l-lysine Hydrogel with Robust Tissue-Anchor and Anti-Infection Capacity. Adv. Funct. Mater. 2017, 27, 1604894. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Ding, M.; He, W.; Li, J.; Li, J.; Tan, H. Antibacterial and Biocompatible Cross-Linked Waterborne Polyurethanes Containing Gemini Quaternary Ammonium Salts. Biomacromolecules 2018, 19, 279–287. [Google Scholar] [CrossRef]
- Francoia, J.P.; Vial, L. Everything You Always Wanted to Know about Poly-L-lysine Dendrigrafts (But Were Afraid to Ask). Chem. Eur. J. 2018, 24, 2806–2814. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Liu, L.; Li, X.; Zhou, R.T.; Yan, S.J.; Li, C.S.; Luan, S.F.; Yin, J.H.; Shi, H.C. Fabrication of polylysine based antibacterial coating for catheters by facile electrostatic interaction. Chem. Eng. J. 2019, 360, 1030–1041. [Google Scholar] [CrossRef]
- Lopez-Silva, T.L.; Leach, D.G.; Li, I.C.; Wang, X.R.; Hartgerink, J.D. Self-Assembling Multidomain Peptides: Design and Characterization of Neutral Peptide-Based Materials with pH and Ionic Strength Independent Self-Assembly. ACS Biomater. Sci. Eng. 2019, 5, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Chen, G.; Wang, Y.; Ci, T.; Li, H.; Liu, X.; Zhou, D.; Kahkoska, A.R.; Zhou, Z.; et al. Injectable Biodegradable Polymeric Complex for Glucose-Responsive Insulin Delivery. ACS Nano 2021, 15, 4294–4304. [Google Scholar] [CrossRef]
- Li, X.; Fu, M.; Wu, J.; Zhang, C.Y.; Deng, X.; Dhinakar, A.; Huang, W.L.; Qian, H.; Ge, L. pH-sensitive peptide hydrogel for glucose-responsive insulin delivery. Acta Biomater. 2017, 51, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Jianhua, W.; Yang, Q.; Cheng, N.; Tao, X.; Zhang, Z.; Sun, X.; Zhang, Q. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C 2016, 61, 705–711. [Google Scholar]
- Gupta, S.K.; Kumar, M.; Guldhe, A.; Ansari, F.A.; Rawat, I.; Kanney, K.; Bux, F. Design and development of polyamine polymer for harvesting microalgae for biofuels production. Energy Convers. Manag. 2014, 85, 537–544. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Hasturk, O.; Choi, J.; Montero, M.M.; Descoteaux, M.L.; Laubach, I.A.; Kaplan, D.L. Sugar Functionalization of Silks with Pathway-Controlled Substitution and Properties. Adv. Biol. 2021. [Google Scholar] [CrossRef]
- Zhou, B.; He, M.; Wang, P.; Fu, H.; Yu, Y.; Wang, Q.; Fan, X. Synthesis of silk fibroin-g-PAA composite using H2O2-HRP and characterization of the in situ biomimetic mineralization behavior. Mater. Sci. Eng. C 2017, 81, 291–302. [Google Scholar] [CrossRef]
- Kuang, D.; Wu, F.; Yin, Z.; Zhu, T.; Xing, T.; Kundu, S.C.; Lu, S. Silk Fibroin/Polyvinyl Pyrrolidone Interpenetrating Polymer Network Hydrogels. Polymers 2018, 10, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, D.; Jiang, F.; Wu, F.; Kaur, K.; Ghosh, S.; Kundu, S.C.; Lu, S. Highly elastomeric photocurable silk hydrogels. Int. J. Biol. Macromol. 2019, 134, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Jiang, F.; Wu, F.; Yin, Z.; Kaur, K.; Chakraborty, J.; Ghosh, S.; Lu, S. Photocurable silk fibroin- polyvinylpyrrolidone hydrogel. Materialia 2020, 9, 100525. [Google Scholar] [CrossRef]
- Serban, M.A.; Kaplan, D.L. pH-Sensitive Ionomeric Particles Obtained via Chemical Conjugation of Silk with Poly(amino acid)s. Biomacromolecules 2010, 11, 3406–3412. [Google Scholar] [CrossRef] [Green Version]
- Hasturk, O.; Sahoo, J.K.; Kaplan, D.L. Synthesis and Characterization of Silk Ionomers for Layer-by-Layer Electrostatic Deposition on Individual Mammalian Cells. Biomacromolecules 2020, 21, 2829–2843. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Z.; Zhong, J.; Shi, Z.; Mao, Y.; Tao, T.H. Body-Integrated, Enzyme-Triggered Degradable, Silk-Based Mechanical Sensors for Customized Health/Fitness Monitoring and In Situ Treatment. Adv. Sci. 2020, 7, 1903802. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, K.; Zhao, M.; Tao, X.; Hu, X.; Lu, S. Tunable High-Molecular-Weight Silk Fibroin Polypeptide Materials: Fabrication and Self-Assembly Mechanism. ACS Appl. Bio Mater. 2020, 3, 3248–3259. [Google Scholar] [CrossRef]
- Chao, P.-H.G.; Yodmuang, S.; Wang, X.; Sun, L.; Kaplan, D.L.; Vunjak-Novakovic, G. Silk hydrogel for cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 95, 84–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Zhang, X.; Tan, G.; Tian, L.; Liu, D.; Liu, Y.; Yang, X.; Pan, W. A novel pH-induced thermosensitive hydrogel composed of carboxymethyl chitosan and poloxamer cross-linked by glutaraldehyde for ophthalmic drug delivery. Carbohydr. Polym. 2017, 155, 208–217. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Jiang, F.; Cheng, K.; Qi, Z.; Yadavalli, V.K.; Lu, S. Synthesis of pH and Glucose Responsive Silk Fibroin Hydrogels. Int. J. Mol. Sci. 2021, 22, 7107. https://doi.org/10.3390/ijms22137107
Tao X, Jiang F, Cheng K, Qi Z, Yadavalli VK, Lu S. Synthesis of pH and Glucose Responsive Silk Fibroin Hydrogels. International Journal of Molecular Sciences. 2021; 22(13):7107. https://doi.org/10.3390/ijms22137107
Chicago/Turabian StyleTao, Xiaosheng, Fujian Jiang, Kang Cheng, Zhenzhen Qi, Vamsi K. Yadavalli, and Shenzhou Lu. 2021. "Synthesis of pH and Glucose Responsive Silk Fibroin Hydrogels" International Journal of Molecular Sciences 22, no. 13: 7107. https://doi.org/10.3390/ijms22137107





