Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis
Abstract
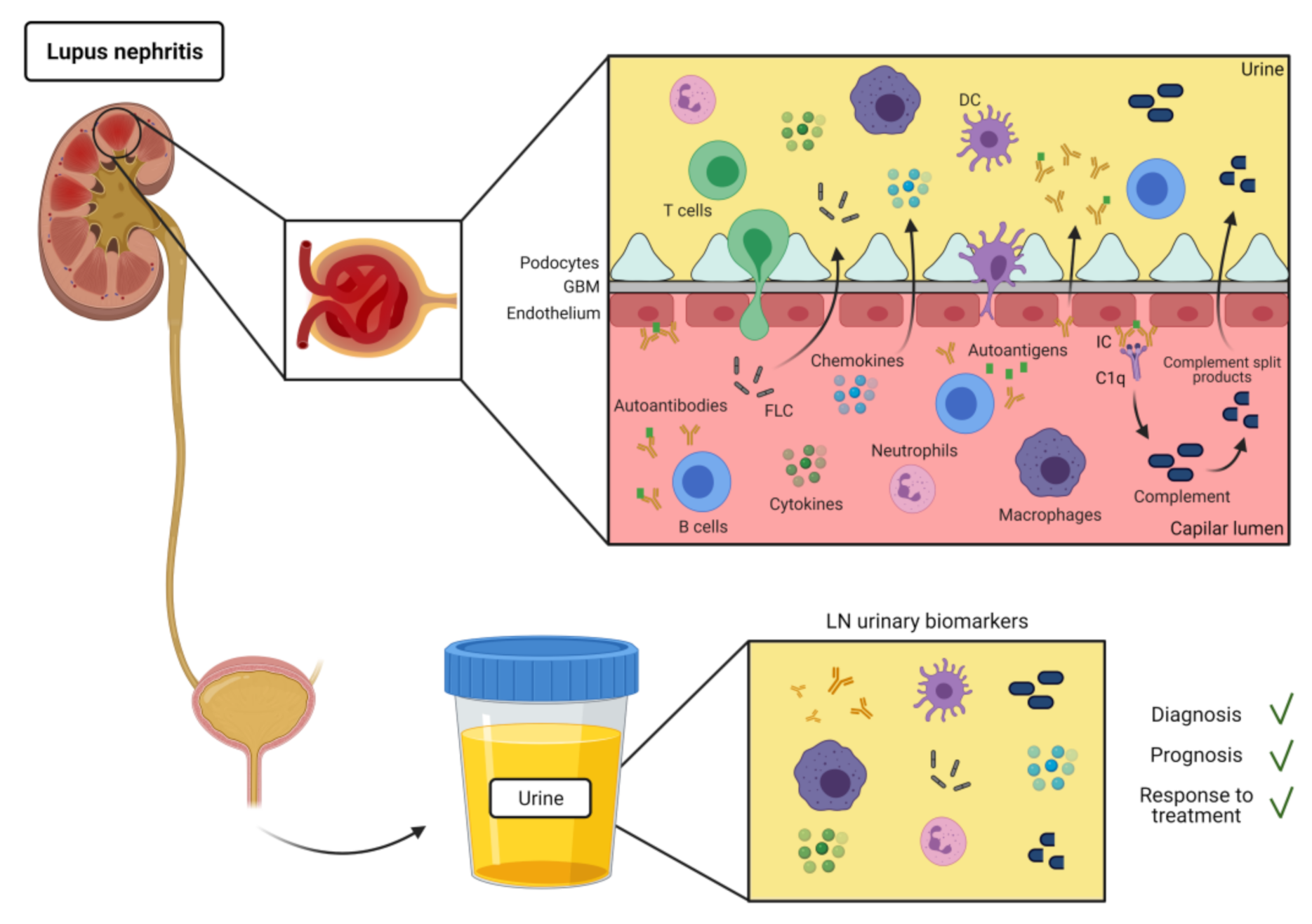
1. Introduction
2. Autoantibodies
| Urinary Biomarker Class | Diagnostic Value | Prognostic Utility | Response to Treatment |
|---|---|---|---|
| Autoantibodies | Anti-RNAPI, anti-dsDNA, anti-La, and anti-ribosomal P, levels correlated with disease activity [35] | ||
| FLC | FLC discriminate patients with severe forms of LN [36,37] | FLC increase before the onset of acute SLE relapses and reach normal values after remission [38,39,40] | λ and κ FLC decrease after treatment [37] |
| Complement components | C3d levels correlate with SLEDAI discriminate between active LN and inactive LN or non-renal SLE [41,42,43] | C3d decreased levels can predict treatment response at 6 months and non-response or flare [43] | C3d levels fall after therapy [43] |
| Soluble immune mediators | IL-6 higher in active LN [44] corroborated by renal biopsy [45] | No differences between active or inactive LN [46]. | Decreased significantly after treatment [47] |
| MCP-1 correlates with LN activity [48]. Higher in patients with inactive LN (Meta-analysis) [49]. Increased also in serum of SLE patients [50] MCP-1, KIM-1, and NGAL higher in patients with active LN [51] | |||
| IP-10 positively correlated with renal SLEDAI but not significantly higher in LN [52] | |||
| EGF lower in patients with active LN [53] | Decreased overtime in adverse long-term kidney damage [53] | ||
| VCAM-1 higher in active renal disease [54]. Presence of LN, clinical and histological activity indexes severe renal lesions [55,56] VCAM-1 and ALCAM elevated in active LN [57,58] ICAM-1 elevated in SLE patients [59] VCAM-1, cystatin C, and KIM-1 discrimination between proliferative versus membranous LN. Non-specific for SLE [60] NGAL; higher in LN than in non-LN patients [61] | Increased in active LN [56]. It may indicate patients at increased risk for long-term renal function loss [57]. ALCAM levels correlated positively with activity index [58] | Effective LN therapy reduced uVCAM-1 levels over the time [56] | |
| Leukocytes | Monocytes/macrophages in proliferative LN [62,63,64] Higher eosinophils numbers in LN [65] CD4+CD25-Foxp3+ T cells in active LN [66] CD4+ and CD8+ T cells in active LN [67,68] Th17 associated with less severe disease [69] pDC and PB/PC in severe LN [70,71] | Lower numbers of CD8+ T cells in remission [67,68] | |
| Soluble leukocyte marker | sCD163 in active LN [72,73], mostly in proliferative classes [74] sCD11b correlates with histological activity [75] T-bet mRNA in higher in active LN [76,77] | sCD163 increases from 6 months before flare [78] Higher T-bet mRNA gives higher risk of severe flare [77] | sCD163 decreases after treatment in drug responders [73,78] sCD11b decreases with clinical remission [75] |
3. Free Light Chains
4. Complement
5. Soluble Immune Mediators
6. Cell-Associated Biomarkers
7. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morimoto, A.M.; Flesher, D.T.; Yang, J.; Wolslegel, K.; Wang, X.; Brady, A.; Abbas, A.R.; Quarmby, V.; Wakshull, E.; Richardson, B.; et al. Association of endogenous anti-interferon-alpha autoantibodies with decreased interferon-pathway and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 2407–2415. [Google Scholar] [CrossRef] [PubMed]
- Mok, C.C. Biomarkers for lupus nephritis: A critical appraisal. J. Biomed. Biotechnol. 2010, 2010, 638413. [Google Scholar] [CrossRef] [PubMed]
- Aragon, C.C.; Tafur, R.A.; Suarez-Avellaneda, A.; Martinez, M.T.; Salas, A.L.; Tobon, G.J. Urinary biomarkers in lupus nephritis. J. Transl. Autoimmun. 2020, 3, 100042. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, M.; Gatto, M.; Binda, V.; Doria, A.; Moroni, G. Lupus nephritis: Clinical presentations and outcomes in the 21st century. Rheumatology 2020, 59 (Suppl. 5), v39–v51. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Anders, H.J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 2013, 24, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 2020, 110, 102374. [Google Scholar] [CrossRef]
- Kalantari, S.; Chashmniam, S.; Nafar, M.; Zakeri, Z.; Parvin, M. Metabolomics approach reveals urine biomarkers and pathways associated with the pathogenesis of lupus nephritis. Iran J. Basic Med. Sci. 2019, 22, 1288–1295. [Google Scholar]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Murphy, G.; Lisnevskaia, L.; Isenberg, D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: Challenges to treatment. Lancet 2013, 382, 809–818. [Google Scholar] [CrossRef]
- Ligtenberg, G.; Arends, S.; Stegeman, C.A.; de Leeuw, K. Predictors of renal flares and long-term renal outcome in patients with lupus nephritis: Results from daily clinical practice. Clin. Exp. Rheumatol. 2021. [Google Scholar] [PubMed]
- Soliman, S.; Mohan, C. Lupus nephritis biomarkers. Clin. Immunol. 2017, 185, 10–20. [Google Scholar] [CrossRef]
- Hahn, B.H.; McMahon, M.A.; Wilkinson, A.; Wallace, W.D.; Daikh, D.I.; Fitzgerald, J.D.; Karpouzas, G.A.; Merrill, J.T.; Wallace, D.J.; Yazdany, J.; et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 2012, 64, 797–808. [Google Scholar] [CrossRef]
- Bertsias, G.K.; Tektonidou, M.; Amoura, Z.; Aringer, M.; Bajema, I.; Berden, J.H.; Boletis, J.; Cervera, R.; Dorner, T.; Doria, A.; et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann. Rheum. Dis. 2012, 71, 1771–1782. [Google Scholar] [CrossRef]
- Caster, D.J.; Powell, D.W. Utilization of Biomarkers in Lupus Nephritis. Adv. Chronic Kidney Dis. 2019, 26, 351–359. [Google Scholar] [CrossRef]
- Chedid, A.; Rossi, G.M.; Peyronel, F.; Menez, S.; Atta, M.G.; Bagnasco, S.M.; Arend, L.J.; Rosenberg, A.Z.; Fine, D.M. Low-Level Proteinuria in Systemic Lupus Erythematosus. Kidney Int. Rep. 2020, 5, 2333–2340. [Google Scholar] [CrossRef]
- Ishizaki, J.; Saito, K.; Nawata, M.; Mizuno, Y.; Tokunaga, M.; Sawamukai, N.; Tamura, M.; Hirata, S.; Yamaoka, K.; Hasegawa, H.; et al. Low complements and high titre of anti-Sm antibody as predictors of histopathologically proven silent lupus nephritis without abnormal urinalysis in patients with systemic lupus erythematosus. Rheumatology 2015, 54, 405–412. [Google Scholar] [CrossRef]
- Moroni, G.; Depetri, F.; Ponticelli, C. Lupus nephritis: When and how often to biopsy and what does it mean? J. Autoimmun. 2016, 74, 27–40. [Google Scholar] [CrossRef]
- Abedini, A.; Zhu, Y.O.; Chatterjee, S.; Halasz, G.; Devalaraja-Narashimha, K.; Shrestha, R.; Balzer, M.S.; Park, J.; Zhou, T.; Ma, Z.; et al. Urinary Single-Cell Profiling Captures the Cellular Diversity of the Kidney. J. Am. Soc. Nephrol. 2021, 32, 614–627. [Google Scholar] [CrossRef]
- Mok, C.C.; Mohan, C. Urinary Biomarkers in Lupus Nephritis: Are We There Yet? Arthritis Rheumatol. 2021, 73, 194–196. [Google Scholar] [CrossRef]
- Rao, D.A.; Arazi, A.; Wofsy, D.; Diamond, B. Design and application of single-cell RNA sequencing to study kidney immune cells in lupus nephritis. Nat. Rev. Nephrol. 2020, 16, 238–250. [Google Scholar] [CrossRef]
- Capecchi, R.; Puxeddu, I.; Pratesi, F.; Migliorini, P. New biomarkers in SLE: From bench to bedside. Rheumatology 2020, 59 (Suppl. 5), v12–v18. [Google Scholar] [CrossRef]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies 2016, 5, 2. [Google Scholar] [CrossRef]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.H. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat. Rev. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Nowling, T.K.; Gilkeson, G.S. Mechanisms of tissue injury in lupus nephritis. Arthritis Res. Ther. 2011, 13, 250. [Google Scholar] [CrossRef]
- Yung, S.; Chan, T.M. Anti-dsDNA antibodies and resident renal cells–Their putative roles in pathogenesis of renal lesions in lupus nephritis. Clin. Immunol. 2017, 185, 40–50. [Google Scholar] [CrossRef]
- Tan, Y.; Song, D.; Wu, L.H.; Yu, F.; Zhao, M.H. Serum levels and renal deposition of C1q complement component and its antibodies reflect disease activity of lupus nephritis. BMC Nephrol. 2013, 14, 63. [Google Scholar] [CrossRef]
- Dieker, J.; Berden, J.H.; Bakker, M.; Briand, J.P.; Muller, S.; Voll, R.; Sjowall, C.; Herrmann, M.; Hilbrands, L.B.; van der Vlag, J. Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis. PLoS ONE 2016, 11, e0165373. [Google Scholar] [CrossRef]
- Rekvig, O.P. The dsDNA, Anti-dsDNA Antibody, and Lupus Nephritis: What We Agree on, What Must Be Done, and What the Best Strategy Forward Could Be. Front. Immunol. 2019, 10, 1104. [Google Scholar] [CrossRef]
- Dumestre-Perard, C.; Clavarino, G.; Colliard, S.; Cesbron, J.Y.; Thielens, N.M. Antibodies targeting circulating protective molecules in lupus nephritis: Interest as serological biomarkers. Autoimmun. Rev. 2018, 17, 890–899. [Google Scholar] [CrossRef]
- Howe, H.S.; Leung, B.P.L. Anti-Cytokine Autoantibodies in Systemic Lupus Erythematosus. Cells 2019, 9, 72. [Google Scholar] [CrossRef]
- Hanrotel-Saliou, C.; Segalen, I.; Le Meur, Y.; Youinou, P.; Renaudineau, Y. Glomerular antibodies in lupus nephritis. Clin. Rev. Allergy Immunol. 2011, 40, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Meryhew, N.L.; Messner, R.P.; Tan, E.M. Urinary excretion of antinuclear antibodies. J. Rheumatol. 1983, 10, 913–919. [Google Scholar] [PubMed]
- Picking, W.L.; Smith, C.; Petruci, R.; Scheffel, J.; Levich, J.D.; Stetler, D.A. Anti-RNA polymerase I antibodies in the urine of patients with systemic lupus erythematosus. J. Rheumatol. 1990, 17, 1308–1313. [Google Scholar] [PubMed]
- Sciascia, S.A.; Dickensheets, H.; Picking, W.; Robson, K.; Wang, D.; Ye, B.H.; Zhu, L.; Stetler, D.A. Autoantibodies and autoantigens in the urine of SLE patients. Autoimmunity 2004, 37, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Wu, T.H.; Sun, K.H.; Lin, W.M.; Yu, C.L. Increased excretion of soluble interleukin 2 receptors and free light chain immunoglobulins in the urine of patients with active lupus nephritis. Ann. Rheum. Dis. 1992, 51, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Gono, T.; Kawaguchi, Y.; Uchida, K.; Koseki, Y.; Katsumata, Y.; Kaneko, H.; Takagi, K.; Ichida, H.; Nitta, K.; et al. Urinary free light chain is a potential biomarker for ISN/RPS class III/IV lupus nephritis. Rheumatology 2013, 52, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zeron, P.; Retamozo, S.; Gandia, M.; Akasbi, M.; Perez-De-Lis, M.; Diaz-Lagares, C.; Bosch, X.; Bove, A.; Perez-Alvarez, R.; Soto-Cardenas, M.J.; et al. Monoclonal gammopathy related to Sjogren syndrome: A key marker of disease prognosis and outcomes. J. Autoimmun. 2012, 39, 43–48. [Google Scholar] [CrossRef]
- Hopper, J.E.; Sequeira, W.; Martellotto, J.; Papagiannes, E.; Perna, L.; Skosey, J.L. Clinical relapse in systemic lupus erythematosus: Correlation with antecedent elevation of urinary free light-chain immunoglobulin. J. Clin. Immunol. 1989, 9, 338–350. [Google Scholar] [CrossRef]
- Hopper, J.E.; Golbus, J.; Meyer, C.; Ferrer, G.A. Urine free light chains in SLE: Clonal markers of B-cell activity and potential link to in vivo secreted Ig. J. Clin. Immunol. 2000, 20, 123–137. [Google Scholar] [CrossRef]
- Manzi, S.; Rairie, J.E.; Carpenter, A.B.; Kelly, R.H.; Jagarlapudi, S.P.; Sereika, S.M.; Medsger, T.A., Jr.; Ramsey-Goldman, R. Sensitivity and specificity of plasma and urine complement split products as indicators of lupus disease activity. Arthritis Rheum. 1996, 39, 1178–1188. [Google Scholar] [CrossRef]
- Negi, V.S.; Aggarwal, A.; Dayal, R.; Naik, S.; Misra, R. Complement degradation product C3d in urine: Marker of lupus nephritis. J. Rheumatol. 2000, 27, 380–383. [Google Scholar]
- Ganguly, S.; Majumder, S.; Kumar, S.; Gupta, R.; Muhammed, H.; Shobha, V.; Aggarwal, A.; Misra, R. Urinary C3d is elevated in patients with active Lupus nephritis and a fall in its level after 3 months predicts response at 6 months on follow up. Lupus 2020, 29, 1800–1806. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Wu, T.H.; Yu, C.L.; Lu, J.Y.; Tsai, Y.Y. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron 2000, 85, 207–214. [Google Scholar] [CrossRef]
- El-Shereef, R.R.; Lotfi, A.; Abdel-Naeam, E.A.; Tawfik, H. Serum and Urinary Interleukin-6 in Assessment of Renal Activity in Egyptian Patients with Systemic Lupus Erythematosus. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2016, 9, 29–36. [Google Scholar] [CrossRef]
- Bona, N.; Pezzarini, E.; Balbi, B.; Daniele, S.M.; Rossi, M.F.; Monje, A.L.; Basiglio, C.L.; Pelusa, H.F.; Arriaga, S.M.M. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus 2020, 29, 311–323. [Google Scholar] [CrossRef]
- Iwano, M.; Dohi, K.; Hirata, E.; Kurumatani, N.; Horii, Y.; Shiiki, H.; Fukatsu, A.; Matsuda, T.; Hirano, T.; Kishimoto, T.; et al. Urinary levels of IL-6 in patients with active lupus nephritis. Clin. Nephrol. 1993, 40, 16–21. [Google Scholar]
- Rovin, B.H.; Zhang, X. Biomarkers for lupus nephritis: The quest continues. Clin. J. Am. Soc. Nephrol. 2009, 4, 1858–1865. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, G.G. Urinary MCP-1 as a biomarker for lupus nephritis: A meta-analysis. Z. Rheumatol. 2017, 76, 357–363. [Google Scholar] [CrossRef]
- Abozaid, M.A.; Ahmed, G.H.; Tawfik, N.M.; Sayed, S.K.; Ghandour, A.M.; Madkour, R.A. Serum and Urine Monocyte Chemoattractant Protein-1 as A Markers for Lupus Nephritis. Egypt J. Immunol. 2020, 27, 97–107. [Google Scholar]
- Ding, Y.; Nie, L.M.; Pang, Y.; Wu, W.J.; Tan, Y.; Yu, F.; Zhao, M.H. Composite urinary biomarkers to predict pathological tubulointerstitial lesions in lupus nephritis. Lupus 2018, 27, 1778–1789. [Google Scholar] [CrossRef]
- Puapatanakul, P.; Chansritrakul, S.; Susantitaphong, P.; Ueaphongsukkit, T.; Eiam-Ong, S.; Praditpornsilpa, K.; Kittanamongkolchai, W.; Avihingsanon, Y. Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4954. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Shapiro, J.P.; Zhang, X.L.; Cruz, C.; Zimmerman, G.; Mendez-Perez, R.A.; Cano-Verduzco, M.L.; Parikh, S.V.; Nagaraja, H.N.; Morales-Buenrostro, L.E.; et al. Association Between Urinary Epidermal Growth Factor and Renal Prognosis in Lupus Nephritis. Arthritis Rheumatol. 2021, 73, 244–254. [Google Scholar] [CrossRef]
- Mok, C.C.; Soliman, S.; Ho, L.Y.; Mohamed, F.A.; Mohamed, F.I.; Mohan, C. Urinary angiostatin, CXCL4 and VCAM-1 as biomarkers of lupus nephritis. Arthritis Res. Ther. 2018, 20, 6. [Google Scholar] [CrossRef]
- Gasparin, A.A.; Pamplona Bueno de Andrade, N.; Hax, V.; Tres, G.L.; Veronese, F.V.; Monticielo, O.A. Urinary biomarkers for lupus nephritis: The role of the vascular cell adhesion molecule-1. Lupus 2019, 28, 265–272. [Google Scholar] [CrossRef]
- Gasparin, A.A.; de Andrade, N.P.B.; Hax, V.; Palominos, P.E.; Siebert, M.; Marx, R.; Schaefer, P.G.; Veronese, F.V.; Monticielo, O.A. Urinary soluble VCAM-1 is a useful biomarker of disease activity and treatment response in lupus nephritis. BMC Rheumatol. 2020, 4, 67. [Google Scholar] [CrossRef]
- Parodis, I.; Gokaraju, S.; Zickert, A.; Vanarsa, K.; Zhang, T.; Habazi, D.; Botto, J.; Serdoura Alves, C.; Giannopoulos, P.; Larsson, A.; et al. ALCAM and VCAM-1 as urine biomarkers of activity and long-term renal outcome in systemic lupus erythematosus. Rheumatology 2020, 59, 2237–2249. [Google Scholar] [CrossRef]
- Ding, H.; Lin, C.; Cai, J.; Guo, Q.; Dai, M.; Mohan, C.; Shen, N. Urinary activated leukocyte cell adhesion molecule as a novel biomarker of lupus nephritis histology. Arthritis Res. Ther. 2020, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Guo Liu, R.N.; Cheng, Q.Y.; Zhou, H.Y.; Li, B.Z.; Ye, D.Q. Elevated Blood and Urinary ICAM-1 is a Biomarker for Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Immunol. Investig. 2020, 49, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, R.; Ding, H.; Tian, L.; Gao, T.; Bao, C. The utility of urinary biomarker panel in predicting renal pathology and treatment response in Chinese lupus nephritis patients. PLoS ONE 2020, 15, e0240942. [Google Scholar] [CrossRef] [PubMed]
- El Shahawy, M.S.; Hemida, M.H.; Abdel-Hafez, H.A.; El-Baz, T.Z.; Lotfy, A.M.; Emran, T.M. Urinary neutrophil gelatinase-associated lipocalin as a marker for disease activity in lupus nephritis. Scand. J. Clin. Lab. Investig. 2018, 78, 264–268. [Google Scholar] [CrossRef]
- Abdelati, A.A.; Eshak, N.Y.; Donia, H.M.; El-Girby, A.H. Urinary Cellular Profile as a Biomarker for Lupus Nephritis. J. Clin. Rheumatol. 2020. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.S.; Go, H.; Lim, J.S.; Oh, J.S.; Kim, Y.G.; Lee, C.K.; Yoo, B.; Hong, S. Clinical and histological significance of urinary CD11c(+) macrophages in lupus nephritis. Arthritis Res. Ther. 2020, 22, 173. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.H.; Jung, J.; Jeon, H.; Lee, S.; Lim, J.S.; Go, H.; Oh, J.S.; Kim, Y.G.; Lee, C.K.; et al. Immunological characteristics and possible pathogenic role of urinary CD11c+ macrophages in lupus nephritis. Rheumatology 2020, 59, 2135–2145. [Google Scholar] [CrossRef]
- Brito, T.N.; Vilar, M.J.; Almeida, J.B.; Faria, A.L.; Medeiros, S.D.; Medeiros, M.C.; Silva, E.M.; Silva, V.M.; Souza, L.B.; Arruda, L.K.; et al. Measuring eosinophiluria, urinary eosinophil cationic protein and urinary interleukin-5 in patients with Lupus Nephritis. Allergy Asthma Clin. Immunol. 2014, 10, 61. [Google Scholar] [CrossRef][Green Version]
- Bonelli, M.; Goschl, L.; Bluml, S.; Karonitsch, T.; Steiner, C.W.; Steiner, G.; Smolen, J.S.; Scheinecker, C. CD4(+)CD25(-)Foxp3(+) T cells: A marker for lupus nephritis? Arthritis Res. Ther. 2014, 16, R104. [Google Scholar] [CrossRef]
- Enghard, P.; Rieder, C.; Kopetschke, K.; Klocke, J.R.; Undeutsch, R.; Biesen, R.; Dragun, D.; Gollasch, M.; Schneider, U.; Aupperle, K.; et al. Urinary CD4 T cells identify SLE patients with proliferative lupus nephritis and can be used to monitor treatment response. Ann. Rheum. Dis. 2014, 73, 277–283. [Google Scholar] [CrossRef]
- Dolff, S.; Abdulahad, W.H.; Arends, S.; van Dijk, M.C.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. Urinary CD8+ T-cell counts discriminate between active and inactive lupus nephritis. Arthritis Res. Ther. 2013, 15, R36. [Google Scholar] [CrossRef]
- Mesquita, D., Jr.; Kirsztajn, G.M.; Franco, M.F.; Reis, L.A.; Perazzio, S.F.; Mesquita, F.V.; Ferreira, V.D.S.; Andrade, L.E.C.; de Souza, A.W.S. CD4(+) T helper cells and regulatory T cells in active lupus nephritis: An imbalance towards a predominant Th1 response? Clin. Exp. Immunol. 2018, 191, 50–59. [Google Scholar] [CrossRef]
- Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; Li, S.; et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019, 20, 902–914. [Google Scholar] [CrossRef]
- Scott, E.; Dooley, M.A.; Vilen, B.J.; Clarke, S.H. Immune cells and type 1 IFN in urine of SLE patients correlate with immunopathology in the kidney. Clin. Immunol. 2016, 168, 16–24. [Google Scholar] [CrossRef]
- Endo, N.; Tsuboi, N.; Furuhashi, K.; Shi, Y.; Du, Q.; Abe, T.; Hori, M.; Imaizumi, T.; Kim, H.; Katsuno, T.; et al. Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial. Transplant. 2016, 31, 2023–2033. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, A.; Aggarwal, A. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clin. Rheumatol. 2021, 40, 941–948. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.; Vanarsa, K.; Gidley, G.; Mok, C.C.; Petri, M.; Saxena, R.; Mohan, C. Association of Urine sCD163 With Proliferative Lupus Nephritis, Fibrinoid Necrosis, Cellular Crescents and Intrarenal M2 Macrophages. Front. Immunol. 2020, 11, 671. [Google Scholar] [CrossRef]
- Kitagawa, A.; Tsuboi, N.; Yokoe, Y.; Katsuno, T.; Ikeuchi, H.; Kajiyama, H.; Endo, N.; Sawa, Y.; Suwa, J.; Sugiyama, Y.; et al. Urinary levels of the leukocyte surface molecule CD11b associate with glomerular inflammation in lupus nephritis. Kidney Int. 2019, 95, 680–692. [Google Scholar] [CrossRef]
- Chan, R.W.; Lai, F.M.; Li, E.K.; Tam, L.S.; Chung, K.Y.; Chow, K.M.; Li, P.K.; Szeto, C.C. Urinary mononuclear cell and disease activity of systemic lupus erythematosus. Lupus 2006, 15, 262–267. [Google Scholar] [CrossRef]
- Chan, R.W.; Lai, F.M.; Li, E.K.; Tam, L.S.; Chow, K.M.; Li, P.K.; Szeto, C.C. Expression of T-bet, a type 1 T-helper cell transcription factor, in the urinary sediment of lupus patients predicts disease flare. Rheumatology 2007, 46, 44–48. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Zhang, X.L.; Cruz, C.; Cano-Verduzco, M.L.; Shapiro, J.P.; Nagaraja, H.N.; Morales-Buenrostro, L.E.; Rovin, B.H. Urinary Soluble CD163: A Novel Noninvasive Biomarker of Activity for Lupus Nephritis. J. Am. Soc. Nephrol. 2020, 31, 1335–1347. [Google Scholar] [CrossRef]
- Yamada, A.; Miyakawa, Y.; Kosaka, K. Entrapment of anti-DNA antibodies in the kidney of patients with systemic lupus erythematosus. Kidney Int. 1982, 22, 671–676. [Google Scholar] [CrossRef]
- Perez-Vazquez, M.E.; Cabiedes, J.; Cabral, A.R.; Alarcon-Segovia, D. Decrease in serum antiphospholipid antibody levels upon development of nephrotic syndrome in patients with systemic lupus erythematosus: Relationship to urinary loss of IgG and other factors. Am. J. Med. 1992, 92, 357–362. [Google Scholar] [CrossRef]
- Macanovic, M.; Hogarth, M.B.; Lachmann, P.J. Anti-DNA antibodies in the urine of lupus nephritis patients. Nephrol Dial. Transplant. 1999, 14, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehuda, A.; Rasooly, L.; Bar-Tana, R.; Breuer, G.; Tadmor, B.; Ulmansky, R.; Naparstek, Y. The urine of SLE patients contains antibodies that bind to the laminin component of the extracellular matrix. J. Autoimmun. 1995, 8, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.M.; Dao, K.H.; Han, B.K.; Kornu, R.; Lakhanpal, S.; Mobley, A.B.; Li, Q.Z.; Lian, Y.; Wu, T.; Reimold, A.M.; et al. SLE peripheral blood B cell, T cell and myeloid cell transcriptomes display unique profiles and each subset contributes to the interferon signature. PLoS ONE 2013, 8, e67003. [Google Scholar] [CrossRef] [PubMed]
- Chiche, L.; Jourde-Chiche, N.; Whalen, E.; Presnell, S.; Gersuk, V.; Dang, K.; Anguiano, E.; Quinn, C.; Burtey, S.; Berland, Y.; et al. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014, 66, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Cafiero, C.; Divella, C.; Sallustio, F.; Gigante, M.; Pontrelli, P.; De Palma, G.; Rossini, M.; Grandaliano, G.; Gesualdo, L. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 2015, 17, 72. [Google Scholar] [CrossRef]
- Harris, B.D.; Kuruganti, S.; Deshpande, A.; Goepfert, P.A.; Chatham, W.W.; Walter, M.R. Characterization of Type-I IFN subtype autoantibodies and activity in SLE serum and urine. Lupus 2020, 29, 1095–1105. [Google Scholar] [CrossRef]
- Mastroianni-Kirsztajn, G.; Nishida, S.K.; Pereira, A.B. Are urinary levels of free light chains of immunoglobulins useful markers for differentiating between systemic lupus erythematosus and infection? Nephron. Clin. Pract. 2008, 110, c258–c263. [Google Scholar] [CrossRef]
- Bramlage, C.P.; Froelich, B.; Wallbach, M.; Minguet, J.; Grupp, C.; Deutsch, C.; Bramlage, P.; Koziolek, M.; Muller, G.A. The significance and predictive value of free light chains in the urine of patients with chronic inflammatory rheumatic disease. Clin. Rheumatol. 2016, 35, 2939–2946. [Google Scholar] [CrossRef]
- Brebner, J.A.; Stockley, R.A. Polyclonal free light chains: A biomarker of inflammatory disease or treatment target? F1000 Med. Rep. 2013, 5, 4. [Google Scholar] [CrossRef]
- Napodano, C.; Pocino, K.; Rigante, D.; Stefanile, A.; Gulli, F.; Marino, M.; Basile, V.; Rapaccini, G.L.; Basile, U. Free light chains and autoimmunity. Autoimmun. Rev. 2019, 18, 484–492. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sequeira, W.; Kokebie, R.; Mikolaitis, R.A.; Fogg, L.; Finnegan, A.; Plaas, A.; Block, J.A.; Jolly, M. Serum free light chains as biomarkers for systemic lupus erythematosus disease activity. Arthritis Care Res. 2011, 63, 891–898. [Google Scholar] [CrossRef]
- Ye, Y.; Li, S.L.; Xie, M.; Jiang, P.; Liu, K.G.; Li, Y.J. Judging disease activity in rheumatoid arthritis by serum free kappa and lambda light chain levels. Kaohsiung J. Med. Sci. 2013, 29, 547–553. [Google Scholar] [CrossRef]
- Gottenberg, J.E.; Aucouturier, F.; Goetz, J.; Sordet, C.; Jahn, I.; Busson, M.; Cayuela, J.M.; Sibilia, J.; Mariette, X. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren’s syndrome. Ann. Rheum. Dis. 2007, 66, 23–27. [Google Scholar] [CrossRef]
- Epstein, W.V. Immunologic events preceding clinical exacerbation of systemic lupus erythematosus. Am. J. Med. 1973, 54, 631–636. [Google Scholar] [CrossRef]
- Cooper, A.; Bluestone, R. Free immunoglobulin light chains in connective tissue diseases. Ann. Rheum. Dis. 1968, 27, 537–543. [Google Scholar] [CrossRef]
- Aggarwal, R. Urinary free light chains: A potential biomarker in lupus nephritis. Rheumatology 2013, 52, 2106–2107. [Google Scholar] [CrossRef]
- Weinstein, A.; Alexander, R.V.; Zack, D.J. A Review of Complement Activation in SLE. Curr. Rheumatol. Rep. 2021, 23, 16. [Google Scholar] [CrossRef]
- Chen, M.; Daha, M.R.; Kallenberg, C.G. The complement system in systemic autoimmune disease. J. Autoimmun. 2010, 34, J276–J286. [Google Scholar] [CrossRef]
- Song, D.; Guo, W.Y.; Wang, F.M.; Li, Y.Z.; Song, Y.; Yu, F.; Zhao, M.H. Complement Alternative Pathways Activation in Patients with Lupus Nephritis. Am. J. Med. Sci. 2017, 353, 247–257. [Google Scholar] [CrossRef]
- Ueda, Y.; Nagasawa, K.; Tsukamoto, H.; Horiuchi, T.; Yoshizawa, S.; Tsuru, T.; Furugo, I.; Niho, Y. Urinary C4 excretion in systemic lupus erythematosus. Clin. Chim. Acta 1995, 243, 11–23. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.; Kim, M.; Lee, H.Y.; Kim, Y.; Kang, M.S.; Kim, J. Activation of the alternative complement pathway predicts renal outcome in patients with lupus nephritis. Lupus 2020, 29, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Troldborg, A.; Thiel, S.; Trendelenburg, M.; Friebus-Kardash, J.; Nehring, J.; Steffensen, R.; Hansen, S.W.K.; Laska, M.J.; Deleuran, B.; Jensenius, J.C.; et al. The Lectin Pathway of Complement Activation in Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2018, 45, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Monticielo, O.A.; Mucenic, T.; Xavier, R.M.; Brenol, J.C.; Chies, J.A. The role of mannose-binding lectin in systemic lupus erythematosus. Clin. Rheumatol. 2008, 27, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, D.J.; Hebert, L.A. The Complement System in Lupus Nephritis. Semin. Nephrol. 2015, 35, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Trattner, R.; Nilsson, S.C.; Bjork, A.; Zickert, A.; Blom, A.M.; Gunnarsson, I. Plasma C4d Correlates with C4d Deposition in Kidneys and With Treatment Response in Lupus Nephritis Patients. Front. Immunol. 2020, 11, 582737. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Manzi, S.; Danchenko, N.; Ahearn, J.M. New advances in measurement of complement activation: Lessons of systemic lupus erythematosus. Curr. Rheumatol. Rep. 2004, 6, 375–381. [Google Scholar] [CrossRef]
- Kusunoki, Y.; Akutsu, Y.; Itami, N.; Tochimaru, H.; Nagata, Y.; Takekoshi, Y.; Sagawa, A.; Kataoka, Y.; Nagasawa, S. Urinary excretion of terminal complement complexes in glomerular disease. Nephron 1991, 59, 27–32. [Google Scholar] [CrossRef]
- Gou, S.J.; Yuan, J.; Wang, C.; Zhao, M.H.; Chen, M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin. J. Am. Soc. Nephrol. 2013, 8, 1884–1891. [Google Scholar] [CrossRef]
- Kelly, R.H.; Carpenter, A.B.; Sudol, K.S.; Jagarlapudi, S.P.; Manzi, S. Complement C3 fragments in urine: Detection in systemic lupus erythematosus patients by western blotting. Appl. Theor. Electrophor. 1993, 3, 265–269. [Google Scholar]
- Mejia-Vilet, J.M.; Gomez-Ruiz, I.A.; Cruz, C.; Mendez-Perez, R.A.; Comunidad-Bonilla, R.A.; Uribe-Uribe, N.O.; Nunez-Alvarez, C.A.; Morales-Buenrostro, L.E. Alternative complement pathway activation in thrombotic microangiopathy associated with lupus nephritis. Clin. Rheumatol. 2020, 40. [Google Scholar] [CrossRef]
- Tamano, M.; Fuke, Y.; Endo, M.; Ohsawa, I.; Fujita, T.; Ohi, H. Urinary complement factor H in renal disease. Nephron 2002, 92, 705–707. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Linker-Israeli, M.; Deans, R.J.; Wallace, D.J.; Prehn, J.; Ozeri-Chen, T.; Klinenberg, J.R. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J. Immunol. 1991, 147, 117–123. [Google Scholar]
- Herrera-Esparza, R.; Barbosa-Cisneros, O.; Villalobos-Hurtado, R.; Avalos-Diaz, E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus 1998, 7, 154–158. [Google Scholar] [CrossRef]
- Rovin, B.H. The chemokine network in systemic lupus erythematous nephritis. Front. Biosci. 2008, 13, 904–922. [Google Scholar] [CrossRef]
- Kulkarni, O.; Anders, H.J. CCL2/MCP1: A novel target in systemic lupus erythematosus and lupus nephritis. Z. Rheumatol. 2008, 67, 220–224. [Google Scholar] [CrossRef]
- Mirioglu, S.; Cinar, S.; Yazici, H.; Ozluk, Y.; Kilicaslan, I.; Gul, A.; Ocal, L.; Inanc, M.; Artim-Esen, B. Serum and urine TNF-like weak inducer of apoptosis, monocyte chemoattractant protein-1 and neutrophil gelatinase-associated lipocalin as biomarkers of disease activity in patients with systemic lupus erythematosus. Lupus 2020, 29, 379–388. [Google Scholar] [CrossRef]
- Fernandez-Ochoa, A.; Brunius, C.; Borras-Linares, I.; Quirantes-Pine, R.; Cadiz-Gurrea, M.L.; Precisesads Clinical, C.; Alarcon Riquelme, M.E.; Segura-Carretero, A. Metabolic Disturbances in Urinary and Plasma Samples from Seven Different Systemic Autoimmune Diseases Detected by HPLC-ESI-QTOF-MS. J. Proteome Res. 2020, 19, 3220–3229. [Google Scholar] [CrossRef]
- Vincent, F.B.; Kandane-Rathnayake, R.; Hoi, A.Y.; Slavin, L.; Godsell, J.D.; Kitching, A.R.; Harris, J.; Nelson, C.L.; Jenkins, A.J.; Chrysostomou, A.; et al. Urinary B-cell-activating factor of the tumour necrosis factor family (BAFF) in systemic lupus erythematosus. Lupus 2018, 27, 2029–2040. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.M.; Mori, K.; Kalandadze, A.; Li, J.Y.; Paragas, N.; Nicholas, T.; Devarajan, P.; Barasch, J. Neutrophil gelatinase-associated lipocalin-mediated iron traffic in kidney epithelia. Curr. Opin. Nephrol. Hypertens. 2006, 15, 442–449. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark. Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Cost, N.G.; Noh, P.H.; Devarajan, P.; Ivancic, V.; Reddy, P.P.; Minevich, E.; Bennett, M.; Haffner, C.; Schulte, M.; DeFoor, W.R., Jr. Urinary NGAL levels correlate with differential renal function in patients with ureteropelvic junction obstruction undergoing pyeloplasty. J. Urol. 2013, 190 (Suppl. 4), 1462–1467. [Google Scholar] [CrossRef]
- Vanarsa, K.; Soomro, S.; Zhang, T.; Strachan, B.; Pedroza, C.; Nidhi, M.; Cicalese, P.; Gidley, C.; Dasari, S.; Mohan, S.; et al. Quantitative planar array screen of 1000 proteins uncovers novel urinary protein biomarkers of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1349–1361. [Google Scholar] [CrossRef]
- Bertolo, M.; Baumgart, S.; Durek, P.; Peddinghaus, A.; Mei, H.; Rose, T.; Enghard, P.; Grutzkau, A. Deep Phenotyping of Urinary Leukocytes by Mass Cytometry Reveals a Leukocyte Signature for Early and Non-Invasive Prediction of Response to Treatment in Active Lupus Nephritis. Front. Immunol. 2020, 11, 256. [Google Scholar] [CrossRef]
- Hotta, O.; Yusa, N.; Ooyama, M.; Unno, K.; Furuta, T.; Taguma, Y. Detection of urinary macrophages expressing the CD16 (Fc gamma RIII) molecule: A novel marker of acute inflammatory glomerular injury. Kidney Int. 1999, 55, 1927–1934. [Google Scholar] [CrossRef][Green Version]
- Maria, N.I.; Davidson, A. Renal Macrophages and Dendritic Cells in SLE Nephritis. Curr. Rheumatol. Rep. 2017, 19, 81. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, W.; Tang, W. Immune cell infiltration characteristics and related core genes in lupus nephritis: Results from bioinformatic analysis. BMC Immunol. 2019, 20, 37. [Google Scholar] [CrossRef]
- Kuriakose, J.; Redecke, V.; Guy, C.; Zhou, J.; Wu, R.; Ippagunta, S.K.; Tillman, H.; Walker, P.D.; Vogel, P.; Hacker, H. Patrolling monocytes promote the pathogenesis of early lupus-like glomerulonephritis. J. Clin. Investig. 2019, 129, 2251–2265. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Nakatani, K.; Iwano, M.; Asai, O.; Samejima, K.; Sakan, H.; Terada, M.; Harada, K.; Akai, Y.; Shiiki, H.; et al. Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am. J. Kidney Dis. 2007, 50, 47–58. [Google Scholar] [CrossRef]
- Barrera Garcia, A.; Gomez-Puerta, J.A.; Arias, L.F.; Burbano, C.; Restrepo, M.; Vanegas, A.L.; Munoz, C.H.; Rojas, M.; Gonzalez, L.A.; Vasquez, G. Infiltrating CD16(+) Are Associated with a Reduction in Peripheral CD14(+)CD16(++) Monocytes and Severe Forms of Lupus Nephritis. Autoimmune Dis. 2016, 2016, 9324315. [Google Scholar] [CrossRef] [PubMed]
- Moller, H.J.; Nielsen, M.J.; Maniecki, M.B.; Madsen, M.; Moestrup, S.K. Soluble macrophage-derived CD163: A homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 2010, 215, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.F.; Liu, C.H.; Wang, C.M. Significance of M2 macrophages in glomerulonephritis with crescents. Pathol. Res. Pract. 2017, 213, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Coxon, A.; Cullere, X.; Knight, S.; Sethi, S.; Wakelin, M.W.; Stavrakis, G.; Luscinskas, F.W.; Mayadas, T.N. Fc gamma RIII mediates neutrophil recruitment to immune complexes. a mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity 2001, 14, 693–704. [Google Scholar] [CrossRef]
- Tsuboi, N.; Asano, K.; Lauterbach, M.; Mayadas, T.N. Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 2008, 28, 833–846. [Google Scholar] [CrossRef]
- Nath, S.K.; Han, S.; Kim-Howard, X.; Kelly, J.A.; Viswanathan, P.; Gilkeson, G.S.; Chen, W.; Zhu, C.; McEver, R.P.; Kimberly, R.P.; et al. A nonsynonymous functional variant in integrin-alpha(M) (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat. Genet. 2008, 40, 152–154. [Google Scholar] [CrossRef]
- Kopetschke, K.; Klocke, J.; Griessbach, A.S.; Humrich, J.Y.; Biesen, R.; Dragun, D.; Burmester, G.R.; Enghard, P.; Riemekasten, G. The cellular signature of urinary immune cells in Lupus nephritis: New insights into potential biomarkers. Arthritis Res. Ther. 2015, 17, 94. [Google Scholar] [CrossRef]
- Dolff, S.; Abdulahad, W.H.; van Dijk, M.C.; Limburg, P.C.; Kallenberg, C.G.; Bijl, M. Urinary T cells in active lupus nephritis show an effector memory phenotype. Ann. Rheum. Dis. 2010, 69, 2034–2041. [Google Scholar] [CrossRef]
- Shakweer, M.M.; Behairy, M.; Elhefnawy, N.G.; Elsaid, T.W. Value of Foxp3 expressing T-regulatory cells in renal tissue in lupus nephritis; an immunohistochemical study. J. Nephropathol. 2016, 5, 105–110. [Google Scholar] [CrossRef][Green Version]
- Fiore, N.; Castellano, G.; Blasi, A.; Capobianco, C.; Loverre, A.; Montinaro, V.; Netti, S.; Torres, D.; Manno, C.; Grandaliano, G.; et al. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol. Immunol. 2008, 45, 259–265. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morell, M.; Pérez-Cózar, F.; Marañón, C. Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis. Int. J. Mol. Sci. 2021, 22, 7143. https://doi.org/10.3390/ijms22137143
Morell M, Pérez-Cózar F, Marañón C. Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis. International Journal of Molecular Sciences. 2021; 22(13):7143. https://doi.org/10.3390/ijms22137143
Chicago/Turabian StyleMorell, María, Francisco Pérez-Cózar, and Concepción Marañón. 2021. "Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis" International Journal of Molecular Sciences 22, no. 13: 7143. https://doi.org/10.3390/ijms22137143
APA StyleMorell, M., Pérez-Cózar, F., & Marañón, C. (2021). Immune-Related Urine Biomarkers for the Diagnosis of Lupus Nephritis. International Journal of Molecular Sciences, 22(13), 7143. https://doi.org/10.3390/ijms22137143






