Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains
Abstract
:1. Introduction
2. Results
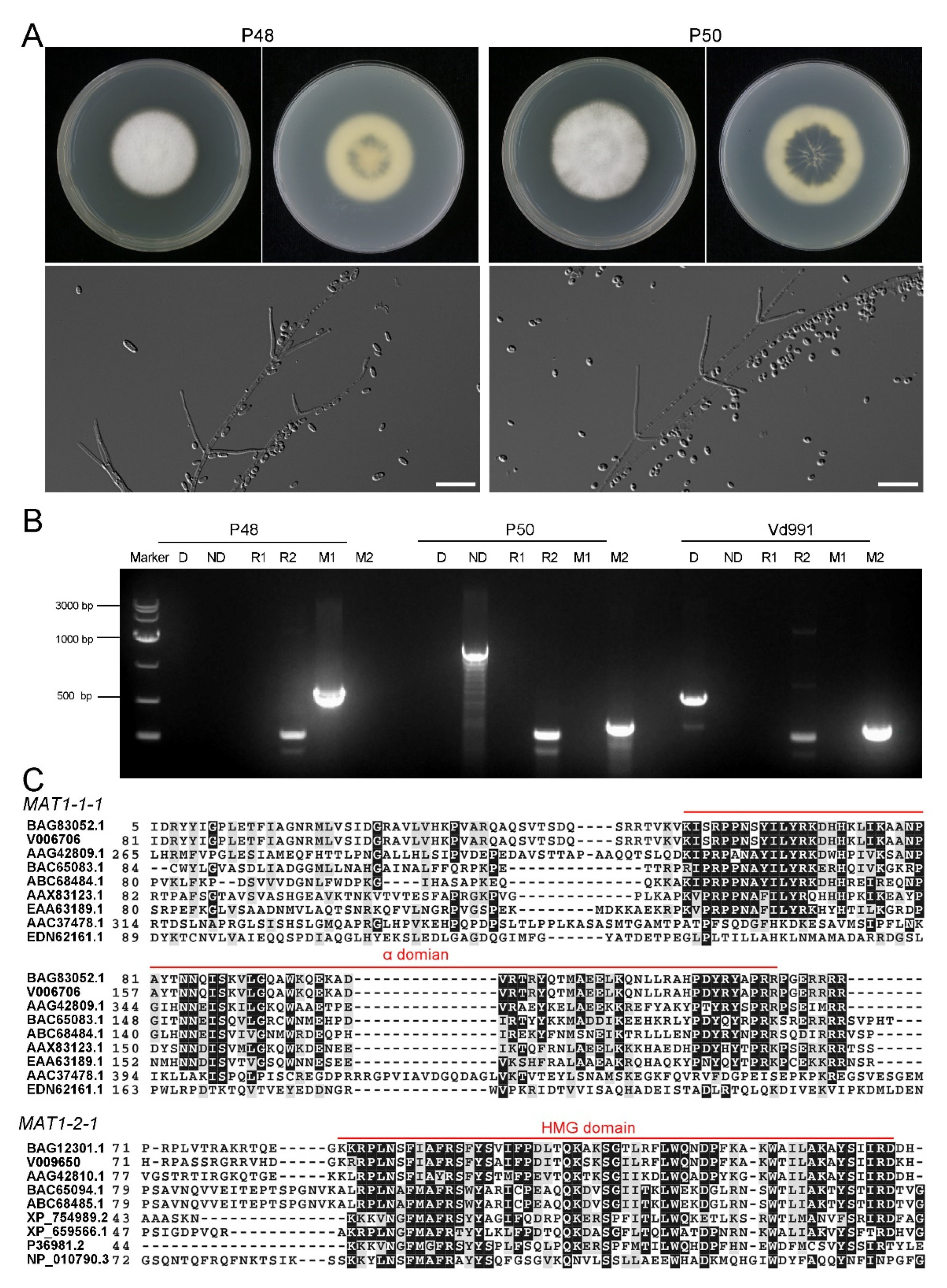
2.1. Characteristics of V. dahliae MAT1-1 Strain P48 and MAT1-2 Strain P50
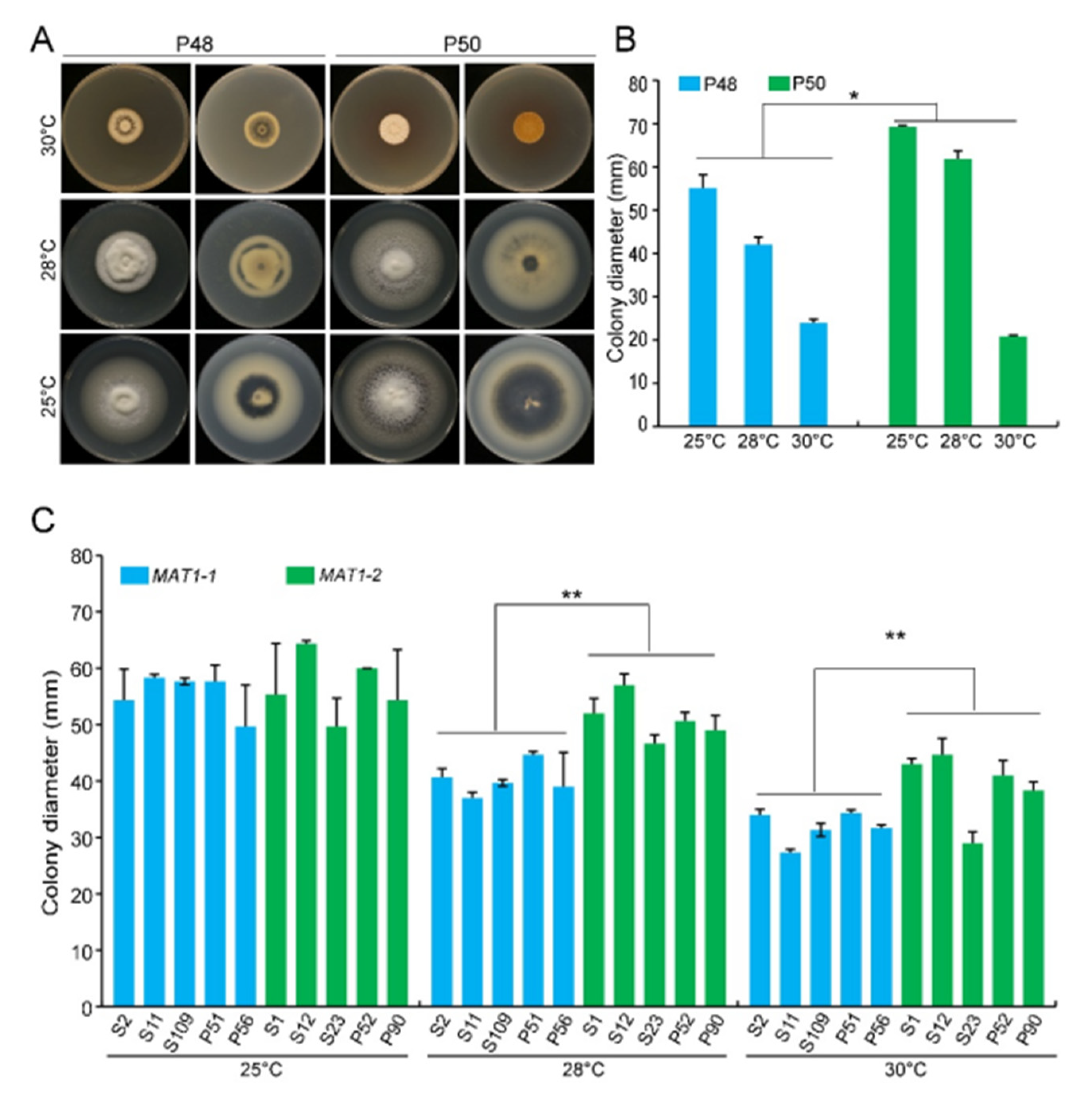
2.2. Growth of MAT1-1 and MAT1-2 Strains under Different Culture Conditions
2.2.1. Temperature
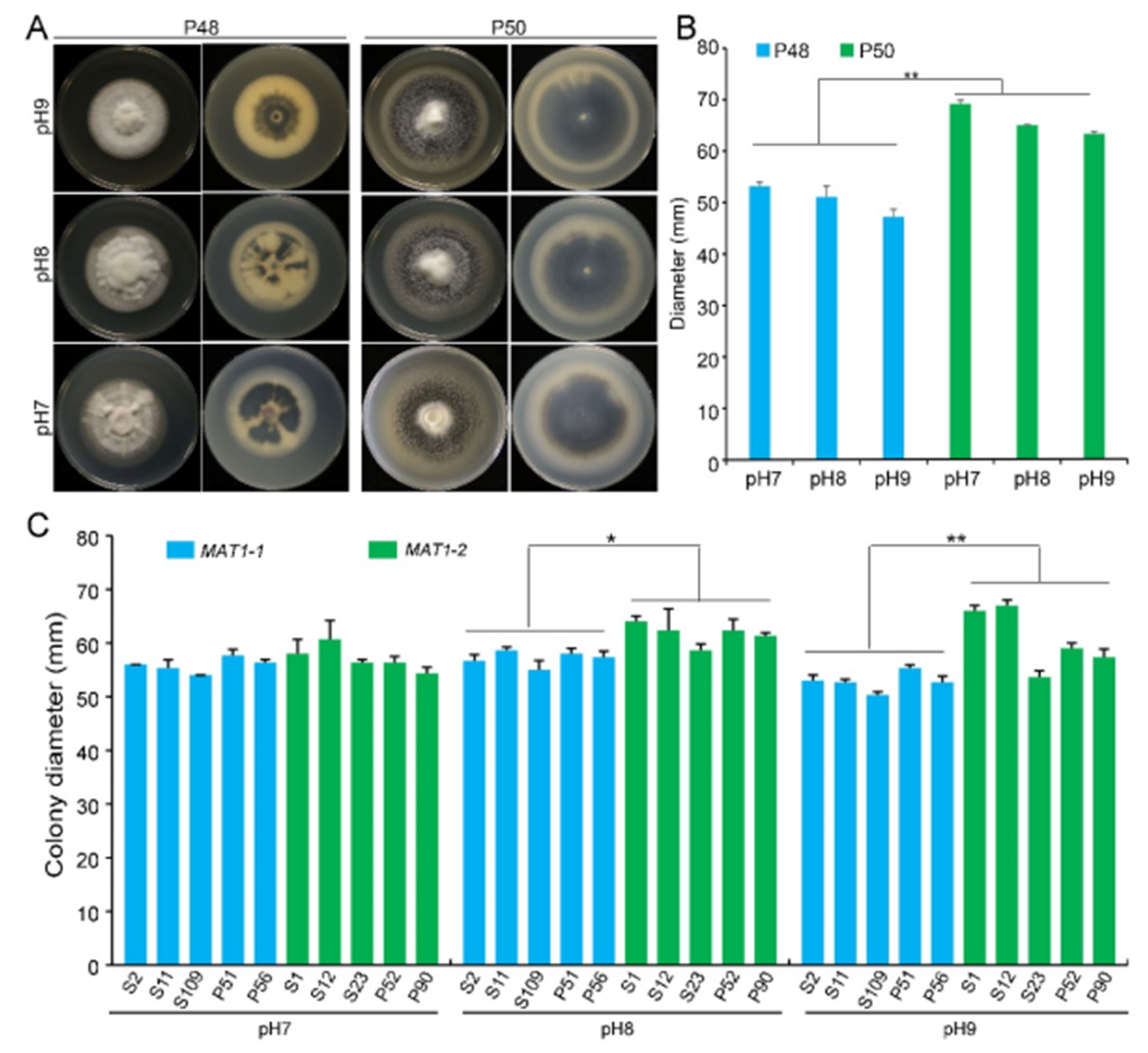
2.2.2. pH
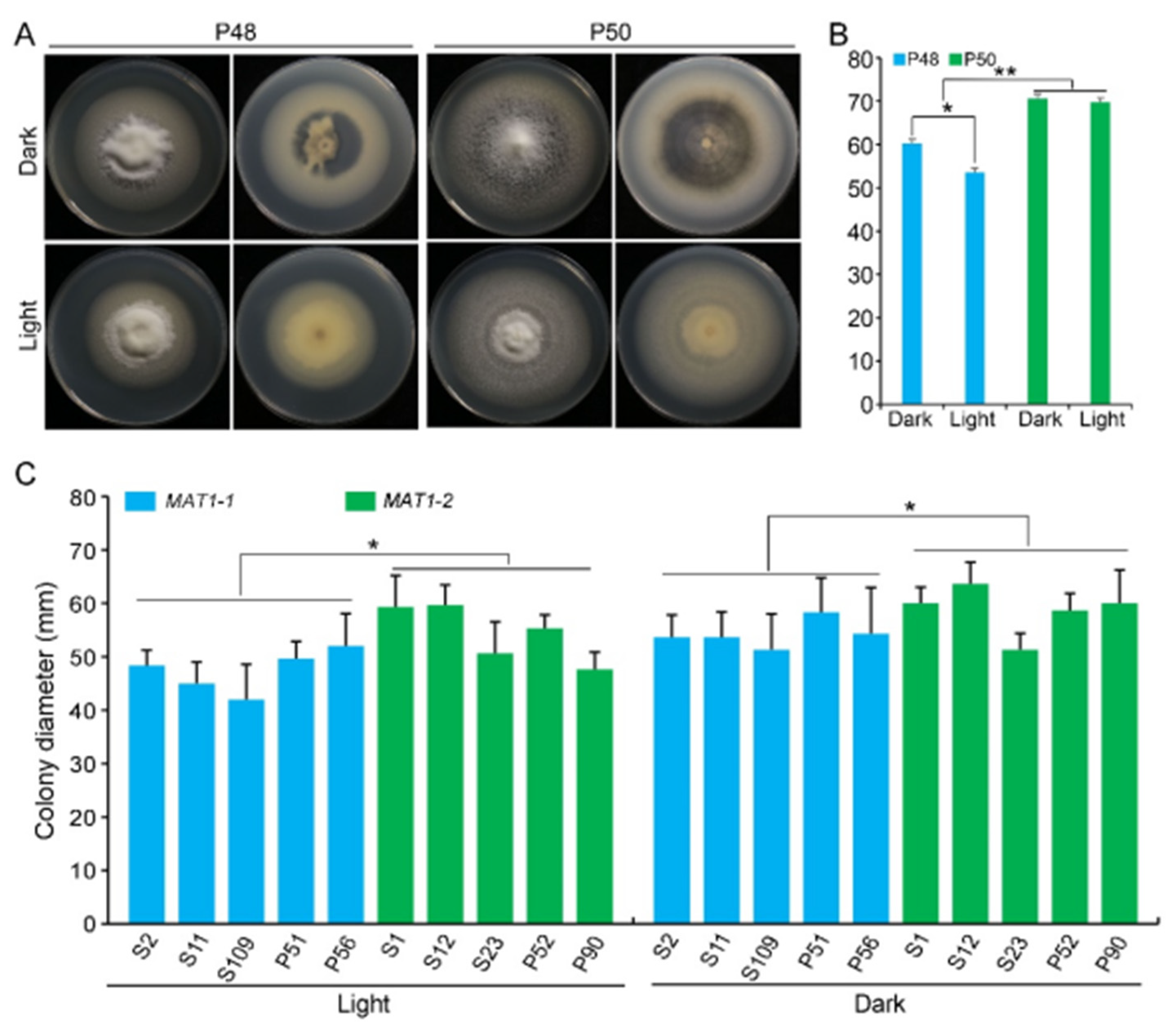
2.2.3. Light/Dark
2.2.4. Carbon Source
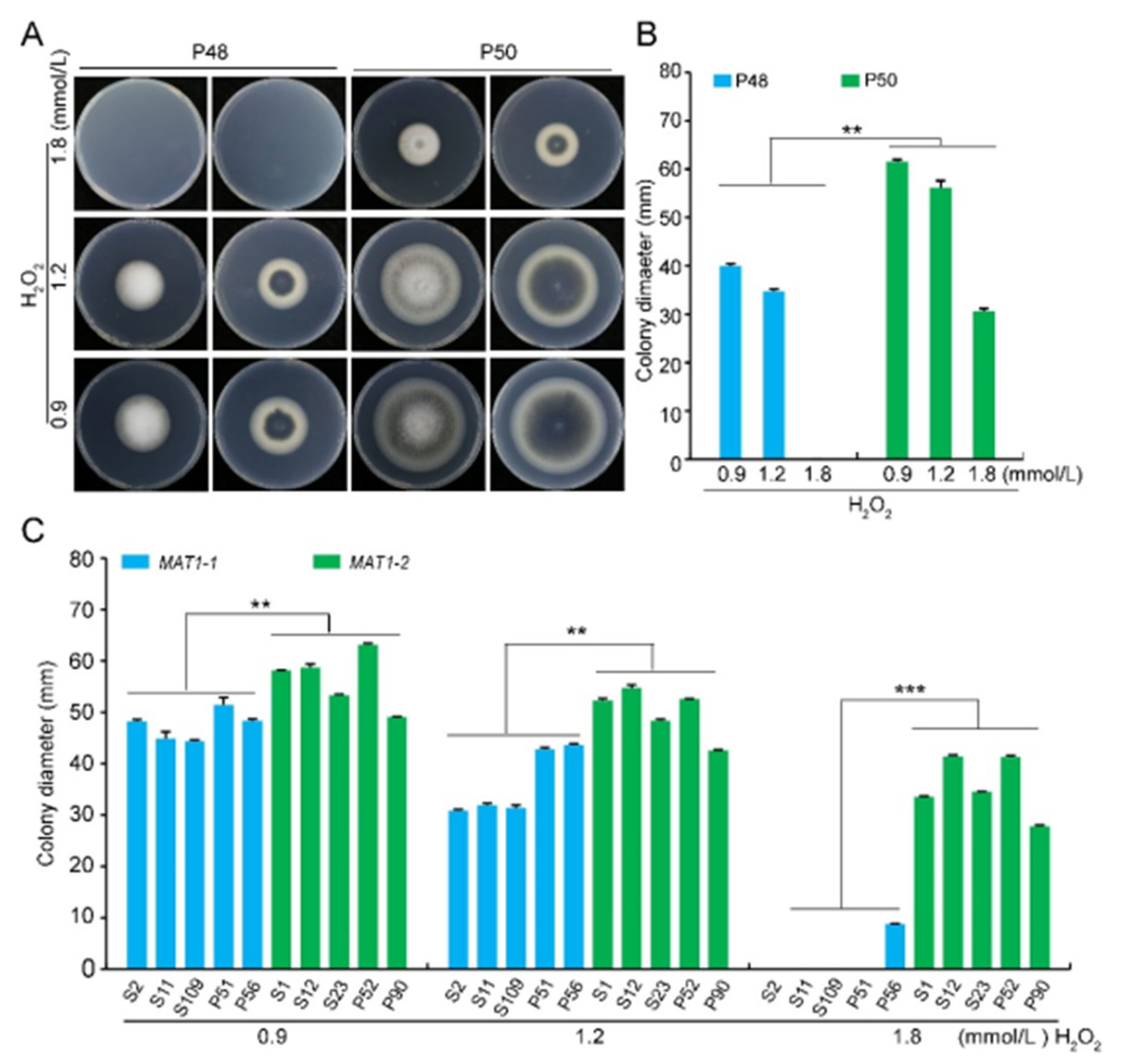
2.3. Stress Tolerance (Osmotic Stress, Oxidative Stress, Cell Wall Integrity Stress)
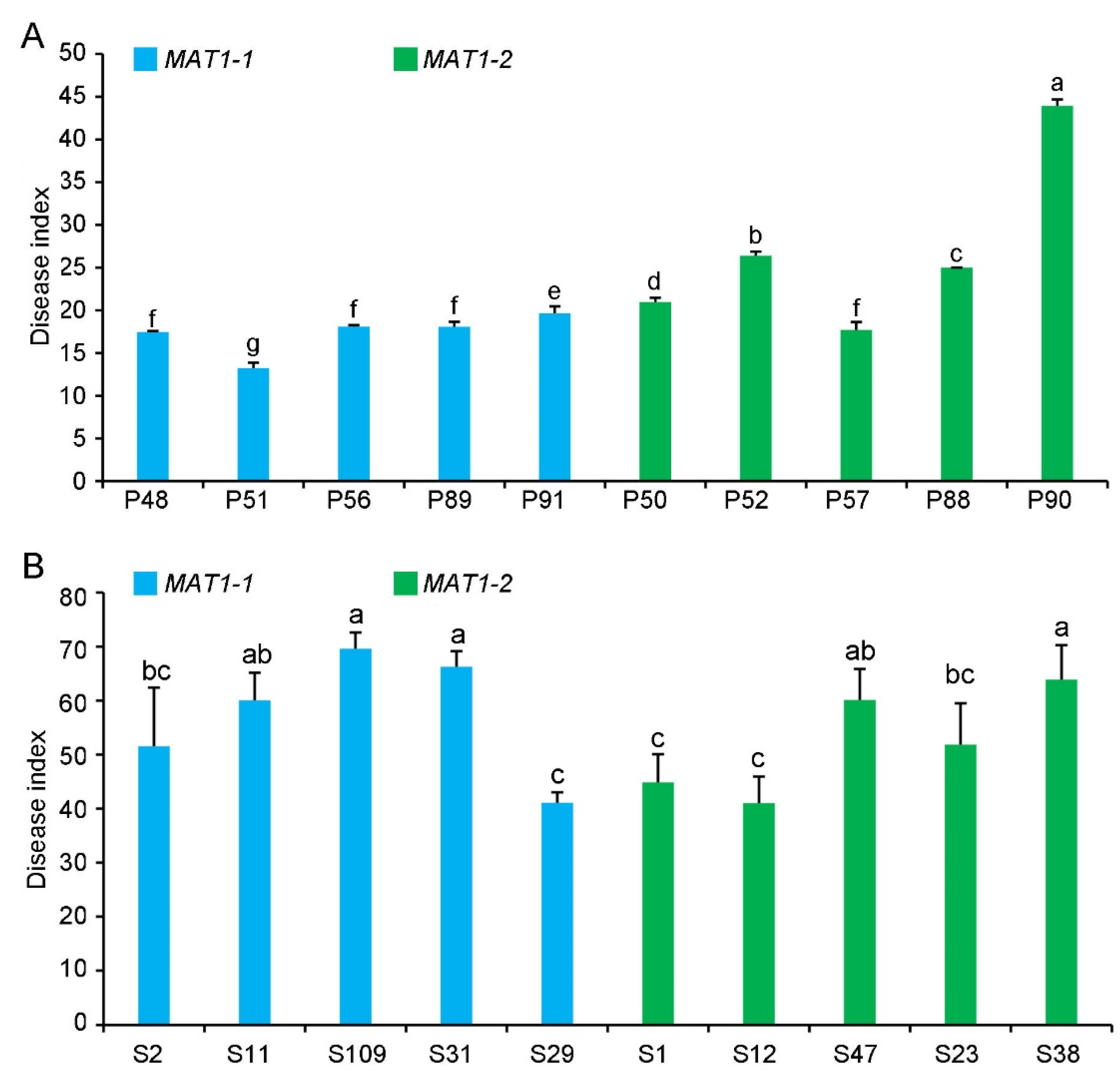
2.4. Pathogenicity of MAT1-1 and MAT1-2 Strains on Their Original Hosts
3. Discussion
4. Materials and Methods
4.1. Growth of V. dahliae Strains under Various Environmental Factors
4.2. DNA Extraction and Genotype Identification Assays of V. dahliae Strains
4.3. Gene Cloning and Bioinformatics Analysis
4.4. Virulence of MAT1-1 and MAT1-2 Strains
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Normark, B.B.; Judson, O.P.; Moran, N.A. Genomic signatures of ancient asexual lineages. Biol. J. Linn. Soc. 2003, 79, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Gorelick, R.; Carpinone, J. Origin and maintenance of sex: The evolutionary joys of self sex. Biol. J. Linn. Soc. 2009, 98, 707–728. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Kirk, P.M.; Sutton, B.C.; Pegler, D.N. Ainsworth & Bisby’s Dictionary of the Fungi, 8th ed.; CABI: Wallingford, UK, 1995. [Google Scholar]
- Taylor, J.W.; Geiser, D.M.; Burt, A.; Koufopanou, V. The evolutionary biology and population genetics underlying fungal strain typing. Clin. Microbiol. Rev. 1999, 12, 126–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, D.R. The Fungal Holomorph: An Overview. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CABI: Wallingford, UK, 1993; pp. 15–25. [Google Scholar]
- Felsenstein, J. The evolutionary advantage of recombination. Genetics 1974, 78, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Burt, A. Perspective: Sex, recombination, and the efficacy of selection—Was Weismann right? Evolution 2000, 54, 337–351. [Google Scholar] [PubMed]
- McDonald, B.A.; Linde, C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu. Rev. Phytopathol. 2002, 40, 349–379. [Google Scholar] [CrossRef] [Green Version]
- Heitman, J. Sexual reproduction and the evolution of microbial pathogens. Curr. Biol. 2006, 16, 711–725. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, S.; Kamoun, S. Genome evolution in filamentous plant pathogens: Why bigger can be better. Nat. Rev. Microbiol. 2012, 10, 417–430. [Google Scholar] [CrossRef]
- Kück, U.; Pöggeler, S. Cryptic sex in fungi. Fungal Biol. Rev. 2009, 23, 86–90. [Google Scholar] [CrossRef]
- Dyer, P.S.; Inderbitzin, P.; Debuchy, R. Mating-Type Structure, Function, Regulation and Evolution in the Pezizomycotina. In Growth, Differentiation and Sexuality, The Mycota I, 3rd ed.; Wendland, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 351–385. [Google Scholar]
- Hull, C.M.; Raisner, R.M.; Johnson, A.D. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 2000, 289, 307–310. [Google Scholar] [CrossRef]
- Magee, B.B.; Magee, P.T. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 2000, 289, 310–313. [Google Scholar] [CrossRef]
- Horn, B.W.; Moore, G.G.; Carbone, I. Sexual reproduction in Aspergillus flavus. Mycologia 2009, 101, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Horn, B.W.; Ramirez-Prado, J.H.; Carbone, I. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 2009, 46, 169–175. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, C.M.; Fuller, H.; Dyer, P.S. Discovery of a sexual cycle in th opportunistic fungal pathogen Aspergillus fumigatus. Nature 2009, 457, 471–474. [Google Scholar] [CrossRef]
- Seidl, V.; Seibel, C.; Kubicek, C.P.; Schmoll, M. Sexual development in the industrial workhorse Trichoderma reesei. Proc. Natl. Acad. Sci. USA 2009, 106, 13909–13914. [Google Scholar] [CrossRef] [Green Version]
- Böhm, J.; Hoff, B.; O’Gorman, C.M.; Wolfers, S.; Klix, V.; Binger, D.; Zadra, I.; Kürnsteiner, H.; Pöggeler, S.; Dyer, P.S.; et al. Sexual reproduction and mating-type-mediated strain development in the penicillin-producing fungus Penicillium chrysogenum. Proc. Natl. Acad. Sci. USA 2013, 110, 1476–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swilaiman, S.S. Sexual Potential and Population Biology of Fungal Aspergillus and Penicillium Species. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Ropars, J.; López-Villavicencio, M.; Dupont, J.; Snirc, A.; Gillot, G.; Coton, M.; Jany, J.L.; Coton, E.; Giraud, T. Induction of sexual reproduction and genetic diversity in the cheese fungus Penicillium roqueforti. Evol. Appl. 2014, 7, 433–441. [Google Scholar] [CrossRef]
- Dyer, P.S.; Kück, U. Sex and the Imperfect Fungi. Microbiol. Spectr. 2017, 5, 43–2017. [Google Scholar] [CrossRef] [PubMed]
- Debuchy, R.; Turgeon, B.G. Mating-Type Structure, Evolution, and Function in Euascomycetes. In The Mycota Volume I: Growth, Differentiation and Sexuality; Springer: Berlin, Germany, 2006; pp. 293–323. [Google Scholar]
- Ni, M.; Feretzaki, M.; Sun, S.; Wang, X.; Heitman, J. Sex in fungi. Annu. Rev. Genet. 2011, 45, 405–430. [Google Scholar] [CrossRef]
- Wada, R.; Maruyama, J.; Yamaguchi, H.; Yamamoto, N.; Wagu, Y.; Paoletti, M.; Archer, D.B.; Dyer, P.S.; Kitamoto, K. Presence and functionality of mating type genes in the supposedly asexual filamentous fungus Aspergillus oryzae. Appl. Environ. Microbiol. 2012, 78, 2819–2829. [Google Scholar] [CrossRef] [Green Version]
- Böhm, J. Mating-Type Genes and the Sexual Cycle of the Penicillin Producer Penicillium chrysogenum. Ph.D. Thesis, Ruhr-University Bochum, Bochum, Germany, 2014. [Google Scholar]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Valled, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [Green Version]
- Bolek, Y.; El-Zik, K.M.; Papper, A.E.; Bell, A.A.; Magill, C.W.; Thaxton, P.M.; Reddy, O.U.K. Mapping of Verticillium wilt resistance genes in cotton. Plant Sci. 2005, 168, 1581–1590. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atallah, Z.K.; Hayes, R.J.; Subbarao, K.V. Fifteen years of Verticillium wilt of lettuce in America’s Salad Bowl: A tale of immigration, subjugation and abatement. Plant Dis. 2011, 95, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Usami, T.; Itoh, M.; Amemiya, Y. Mating type gene MAT1-2-1 is common among Japanese isolates of Verticillium dahliae. Physiol. Mol. Plant Pathol. 2008, 73, 133–137. [Google Scholar] [CrossRef]
- Atallah, Z.K.; Maruthachalam, K.; du Toit, L.; Koike, S.T.; Davis, R.M.; Klosterman, S.J.; Hayes, R.J.; Subbarao, K.V. Population analyses of the vascular plant pathogen Verticillium dahliae detect recombination and transcontinental gene flow. Fungal Genet. Biol. 2010, 47, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V.; Litvintseva, A. The ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS ONE 2011, 6, e18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, D.P.G.; Gurung, S.; Hu, X.; Inderbitzin, P.; Subbarao, K.V. Maintenance of sex-related genes and the co-occurrence of both mating types in Verticillium dahliae. PLoS ONE 2014, 9, e112145. [Google Scholar] [CrossRef] [Green Version]
- Bhat, R.G.; Smith, R.G.; Koike, S.T.; Wu, B.M.; Subbarao, K.V. Characterization of Verticillium dahliae isolates and wilt epidemics in pepper. Plant Dis. 2003, 87, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Joaquim, T.R.; Rowe, R.C. Vegetative compatibility and virulence of strains of Verticillium dahliae from soil and potato plants. Phytopathology 1991, 81, 552–558. [Google Scholar] [CrossRef]
- Milgroom, M.G.; Jimenez-Gasco, M.M.; Olivares, G.C.; Drott, M.T.; Jimenez-Diaz, R.M. Recombination between clonal lineages of the asexual fungus Verticillium dahliae detected by genotyping by sequencing. PLoS ONE 2014, 9, e106740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amen, J.; Shoemaker, P.B. Histopathology of resistant and susceptible tomato cultivars inoculated with Verticillium dahliae races 1 and 2. Phytopathology 1985, 75, 1361–1362. [Google Scholar]
- Vallad, G.E.; Qin, Q.M.; Grube, R.; Hayes, R.J.; Subbarao, K.V. Characterization of race-specific interactions among isolates of Verticillium dahliae pathogenic on lettuce. Phytopathology 2006, 96, 1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usami, T.; Momma, N.; Kikuchi, S.; Watanabe, H.; Hayashi, A.; Mizukawa, M.; Yoshino, K.; Ohmori, Y. Race 2 of Verticillium dahliae infecting tomato in japan can be split into two races with differential pathogenicity on resistant rootstocks. Plant Pathol. 2017, 66, 230–238. [Google Scholar] [CrossRef]
- Schnathorst, W.C.; Mathre, D.E. Host range and differentiation of a severe form of Verticillium albo-atrum in cotton. Phytopathology 1966, 56, 1155–1161. [Google Scholar]
- Klosterman, S.J.; Subbarao, K.V.; Kang, S.; Veronese, P.; Gold, S.E.; Thomma, B.P.; Chen, Z.; Henrissat, B.; Lee, H.; Park, J.; et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 2011, 7, e1002137. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, R.; Bolton, M.D.; Kombrink, A.; van den Berg, G.C.M.; Yadeta, K.A.; Thomma, B.P. Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res. 2013, 23, 1271–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Wu, C.; Hao, C.; Huang, P.; Liu, H.; Bian, Z.; Xu, J.R. Sexual specific functions of Tub1 beta-tubulins require stage-specific RNA processing and expression in Fusarium graminearum. Environ. Microbiol. 2018, 20, 4009–4021. [Google Scholar] [CrossRef] [PubMed]
- Inderbitzin, P.; Bostock, R.M.; Davis, R.M.; Usami, T.; Platt, H.W.; Subbarao, K.V. Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS ONE 2011, 6, e28341. [Google Scholar] [CrossRef]
- de Jonge, R.; Esse, H.P.V.; Maruthachalam, K.; Bolton, M.D.; Santhanam, P.; Saber, M.K.; Zhang, Z.; Usami, T.; Lievens, B.; Subbarao, K.V.; et al. Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl. Acad. Sci. USA 2012, 109, 5110–5115. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Artés, E.; García-Pedrajas, M.D.; Bejarano-Alcázar, J.; Jiménez-Díaz, R.M. Differentiation of cotton-defoliating and nondefoliating pathotypes of Verticillium dahliae by RAPD and specific PCR analyses. Eur. J. Plant Pathol. 2000, 106, 507–517. [Google Scholar] [CrossRef]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI: Wallingford, UK, 2001. [Google Scholar]
- Isaac, I. A comparative study of pathogenic isolates of Verticillium. Trans. Br. Mycol. Soc. 1949, 32, 137–157. [Google Scholar] [CrossRef]
- Malca, I.; Erwin, D.C.; Moje, W.; Jones, B. Effects of pH and carbon and nitrogen sources on the growth of Verticillium albo-atrum. Phytopathology 1966, 56, 401–406. [Google Scholar]
- Cooper, R.M.; Wood, R.K.S. Regulation of synthesis of cell-wall degrading enzymes by Verticillium albo-atrum and Fusarium oxysporum f.sp lycopersici. Physiol. Plant Pathol. 1975, 5, 135–156. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.; Hu, X.; Shang, W.; Shen, R.; Guo, C.; Guo, Q.; Subbarao, K.V. Verticillium wilt caused by V. dahliae and V. nonalfalfae in potato. Plant Dis. 2018, 102, 1958–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaux, A.L.; Sackston, W.B. Taxonomy of Verticillium species causing wilt of horticultural crops in Quebec. Can. J. Bot. Rev. Can. Bot. 1966, 44, 803–811. [Google Scholar] [CrossRef]
- Brinkerhoff, L.A. The influence of temperature, aeration, and soil microflora on microsclerotial development of Verticillium albo-atrum in abscised cotton leaves. Phytopathology 1969, 59, 805–808. [Google Scholar]
- Wang, Y.; Hu, X.; Fang, Y.; Anchieta, A.; Goldman, P.H.; Hernandez, G.; Klosterman, S.J. Transcription factor VdCmr1 is required for pigment production, protection from UV irradiation, and regulates expression of melanin biosynthetic genes in Verticillium dahliae. Microbiology 2018, 164, 685–696. [Google Scholar] [CrossRef]
- Pethybridge, G.H. The Verticillium disease of the potato. Sci. Proc. R. Dublin Soc. 1916, 15, 63–92. [Google Scholar]
- Caroselli, N.E.; Mahadevan, A.; Mozumder, B.G. The effect of light quality on the growth and microsclerotial production of Verticillium albo-atrum. Plant Dis. Rep. 1964, 48, 484–486. [Google Scholar]
- Kaiser, W.J. Influences of light on the production of microsclerotia by Verticillium albo-atrum. Phytopathology 1962, 52, 362. [Google Scholar]
- Kaiser, W.J. Effects of light on growth and sporulation of the Verticillium wilt fungus. Phytopathology 1964, 54, 765–770. [Google Scholar]
- Duressa, D.; Anchieta, A.; Chen, D.; Klimes, A.; Garcia-Pedrajas, M.D.; Dobinson, K.F.; Klosterman, S.J. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genom. 2013, 14, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, D.; Wang, Y.; Ma, J.; Klosterman, S.J.; Xiao, S.; Tian, C. Deep mRNA sequencing reveals stage-specific transcriptome alterations during microsclerotia development in the smoke tree vascular wilt pathogen, Verticillium dahliae. BMC Genom. 2014, 15, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Díaz, R.M.; Millar, R.L. Sporulation on infected tissues, and presence of airborne Verticillium albo-atrum in alfalfa fields in New York. Plant Pathol. 1988, 37, 64–70. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, H.; Du, X.; Wang, S.; Ma, X.W.; Nürnberger, T.; Guo, H.S.; Hua, C. The Verticillium-specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytol. 2017, 215, 368–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromm, J. Xylem Development in Trees: From Cambial Divisions to Mature Wood Cells; Springer: Berlin/Heidelberg, Germany, 2013; Volume 20, pp. 3–39. [Google Scholar]
- Zhang, D.D.; Wang, X.Y.; Chen, J.Y.; Kong, Z.Q.; Gui, Y.J.; Li, N.Y.; Bao, Y.M.; Dai, X.F. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 2016, 6, 27979. [Google Scholar] [CrossRef] [Green Version]
- Herth, W. Calcofluor white and Congo red inhibit chitin microfibril assembly of Poterioochromonas evidence for a gap between polymerization and microfibril formation. J. Cell Biol. 1980, 87, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.J. Specificity in the interaction of direct dyes with polysaccharides. Carbohydr. Res. 1980, 81, 271–287. [Google Scholar] [CrossRef]
- Vaknin, Y.; Shadkchan, Y.; Levdansky, E.; Morozov, M.; Romano, J.; Osherov, N. The three Aspergillus fumigatus CFEM-domain GPI-anchored protein (CfmA-C) affect cell-wall stability but do not play a role in fungal virulence. Fungal Genet. Biol. 2014, 63, 55–64. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Li, N.; Connelly, C.; Johnston, M. Functional profiling of the Saccharomyces cerevisiae genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef]
- Sabnam, N.; Barman, S. WISH, a novel CFEM GPCR is indispensable for surface sensing, asexual and pathogenic differentiation in rice blast fungus. Fungal Genet. Biol. 2017, 105, 37–51. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, J.Y.; Wang, J.L.; Li, L.; Xiao, H.L.; Adam, S.M.; Dai, X.F. Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae. Gene 2013, 529, 307–316. [Google Scholar] [CrossRef]
- Hu, X.P.; Gurung, S.; Short, D.P.G.; Sandoya, G.V.; Shang, W.J.; Hayes, R.J.; Davis, R.M.; Subbarao, K.V. Nondefoliating and defoliating strains from cotton correlate with races 1 and 2 of Verticillium dahliae. Plant Dis. 2015, 99, 1713–1720. [Google Scholar] [CrossRef] [Green Version]
- Usami, T.; Itoh, M.; Amemiya, Y. Asexual fungus Verticillium dahliae is potentially heterothallic. J. Gen. Plant Pathol. 2009, 75, 422–427. [Google Scholar] [CrossRef]
- Xiao, C.L.; Subbarao, K.V.; Schulbach, K.F.; Koike, S.T. Effects of crop rotation and irrigation on Verticillium dahliae microsclerotia in soil and wilt in cauliflower. Phytopathology 1998, 88, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkher, H.; Hadrami, A.E.; Rashid, K.Y.; Adam, L.R.; Daayf, F. Cross-pathogenicity of Verticillium dahliae between potato and sunflower. Eur. J. Plant Pathol. 2009, 124, 505–519. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Y.-D.; Zhang, D.-D.; Zhang, Y.-Y.; Wang, D.; Song, J.; Zhang, J.; Li, R.; Kong, Z.-Q.; Klosterman, S.J.; et al. Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains. Int. J. Mol. Sci. 2021, 22, 7148. https://doi.org/10.3390/ijms22137148
Liu L, Zhang Y-D, Zhang D-D, Zhang Y-Y, Wang D, Song J, Zhang J, Li R, Kong Z-Q, Klosterman SJ, et al. Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains. International Journal of Molecular Sciences. 2021; 22(13):7148. https://doi.org/10.3390/ijms22137148
Chicago/Turabian StyleLiu, Lin, Ya-Duo Zhang, Dan-Dan Zhang, Yuan-Yuan Zhang, Dan Wang, Jian Song, Jian Zhang, Ran Li, Zhi-Qiang Kong, Steven J. Klosterman, and et al. 2021. "Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains" International Journal of Molecular Sciences 22, no. 13: 7148. https://doi.org/10.3390/ijms22137148
APA StyleLiu, L., Zhang, Y.-D., Zhang, D.-D., Zhang, Y.-Y., Wang, D., Song, J., Zhang, J., Li, R., Kong, Z.-Q., Klosterman, S. J., Dai, X.-F., Subbarao, K. V., Zhao, J., & Chen, J.-Y. (2021). Biological Characteristics of Verticillium dahliae MAT1-1 and MAT1-2 Strains. International Journal of Molecular Sciences, 22(13), 7148. https://doi.org/10.3390/ijms22137148






