Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review
Abstract
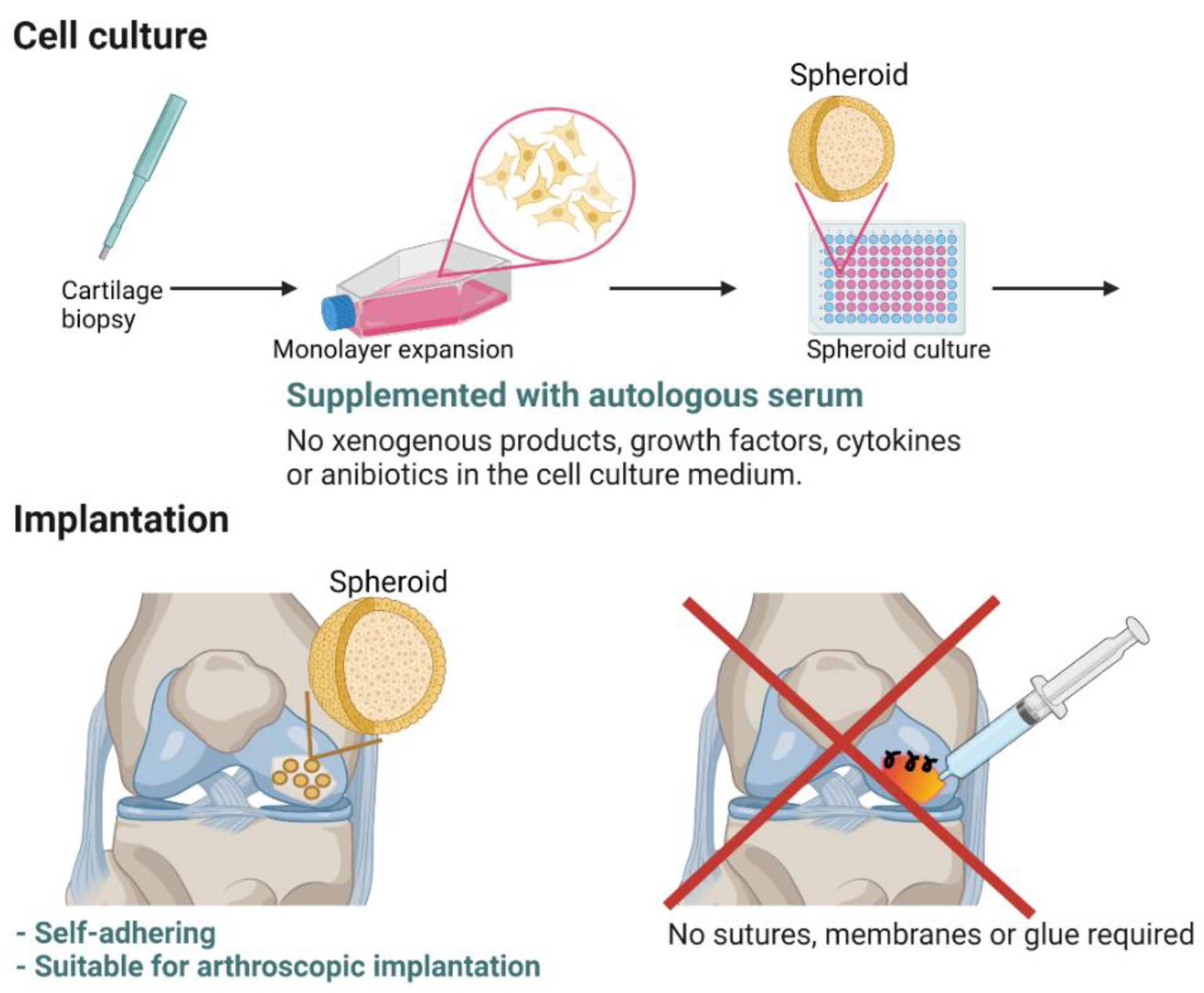
:1. Introduction
2. Methods
3. Results
3.1. Preclinical and Nonclinical Studies—From Concept to Advanced Therapy Medicinal Product
3.2. Clinical Studies
4. Discussion
4.1. Quality in Manufacturing
4.2. Clinical Use in the Knee
4.3. Usage in Hip and Shoulder
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic Science of Articular Cartilage and Osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef]
- Mandelbaum, B.R.; Browne, J.E.; Fu, F.; Micheli, L.; Mosely, J.B.; Erggelet, C.; Minas, T.; Peterson, L. Articular Cartilage Lesions of the Knee. Am. J. Sports Med. 1998, 26, 853–861. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Jones, K.J.; Kelley, B.; Arshi, A.; McAllister, D.R.; Fabricant, P.D. Comparative Effectiveness of Cartilage Repair With Respect to the Minimal Clinically Important Difference. Am. J. Sports Med. 2019, 47, 3284–3293. [Google Scholar] [CrossRef]
- Anderer, U.; Libera, J. In Vitro Engineering of Human Autogenous Cartilage. J. Bone Miner. Res. 2002, 17, 1420–1429. [Google Scholar] [CrossRef]
- Higgings, J. Cochrane Risk of Bias Tool—Appendix F. Available online: https://www.ncbi.nlm.nih.gov/books/NBK132494/bin/appf-fm1.pdf (accessed on 30 April 2021).
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Niemeyer, P.; Laute, V.; John, T.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Niks, M.; Fickert, S.; et al. The Effect of Cell Dose on the Early Magnetic Resonance Morphological Outcomes of Autologous Cell Implantation for Articular Cartilage Defects in the Knee. Am. J. Sports Med. 2016, 44, 2005–2014. [Google Scholar] [CrossRef]
- Becher, C.; Laute, V.; Fickert, S.; Zinser, W.; Niemeyer, P.; John, T.; Diehl, P.; Kolombe, T.; Siebold, R.; Fay, J. Safety of three different product doses in autologous chondrocyte implantation: Results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 2017, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, P.; Laute, V.; Zinser, W.; John, T.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Safety and efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology is independent of spheroid dose after 4 years. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1130–1143. [Google Scholar] [CrossRef]
- Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; Fickert, S. A Prospective, Randomized, Open-Label, Multicenter, Phase III Noninferiority Trial to Compare the Clinical Efficacy of Matrix-Associated Autologous Chondrocyte Implantation With Spheroid Technology Versus Arthroscopic Microfracture for Cartilage Defects of the Knee. Orthop. J. Sports Med. 2019, 7, 2325967119854442. [Google Scholar]
- Hoburg, A.; Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; et al. Matrix-Associated Autologous Chondrocyte Implantation with Spheroid Technology Is Superior to Arthroscopic Microfracture at 36 Months Regarding Activities of Daily Living and Sporting Activities after Treatment. Cartilage 2020. [Google Scholar] [CrossRef] [PubMed]
- Fickert, S.; Gerwien, P.; Helmert, B.; Schattenberg, T.; Weckbach, S.; Kaszkin-Bettag, M.; Lehmann, L.J. One-Year Clinical and Radiological Results of a Prospective, Investigator-Initiated Trial Examining a Novel, Purely Autologous 3-Dimensional Autologous Chondrocyte Transplantation Product in the Knee. Cartilage 2012, 3, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebold, R.; Karidakis, G.; Fernandez, F. Clinical outcome after medial patellofemoral ligament reconstruction and autologous chondrocyte implantation following recurrent patella dislocation. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2477–2483. [Google Scholar] [CrossRef]
- Siebold, R.; Karidakis, G.; Feil, S.; Fernandez, F. Second-look assessment after all-arthroscopic autologous chondrocyte implantation with spheroides at the knee joint. Knee Surg. Sports Traumatol. Arthrosc. 2015, 24, 1678–1685. [Google Scholar] [CrossRef]
- Siebold, R.; Suezer, F.; Schmitt, B.; Trattnig, S.; Essig, M. Good clinical and MRI outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg. Sports Traumatol. Arthrosc. 2017, 26, 831–839. [Google Scholar] [CrossRef]
- Hoburg, A.; Löer, I.; Körsmeier, K.; Siebold, R.; Niemeyer, P.; Fickert, S.; Ruhnau, K. Matrix-Associated Autologous Chondrocyte Implantation Is an Effective Treatment at Midterm Follow-up in Adolescents and Young Adults. Orthop. J. Sports Med. 2019, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grevenstein, D.; Mamilos, A.; Schmitt, V.H.; Niedermair, T.; Wagner, W.; Kirkpatrick, C.J.; Brochhausen, C. Excellent histological results in terms of articular cartilage regeneration after spheroid-based autologous chondrocyte implantation (ACI). Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 417–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Clinical outcome and success rates of ACI for cartilage defects of the patella: A subgroup analysis from a controlled randomized clinical phase II trial (CODIS study). Arch. Orthop. Trauma Surg. 2019, 140, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Nakamura, K.; Feil, S.; Siebold, M.; Kirsch, J.; Siebold, R. Good healing potential of patellar chondral defects after all-arthroscopic autologous chondrocyte implantation with spheroids: A second-look arthroscopic assessment. Knee Surg. Sports Traumatol. Arthrosc. 2021, 1–8. [Google Scholar] [CrossRef]
- Meyer, U.; Wiesmann, H.-P.; Libera, J.; Depprich, R.; Naujoks, C.; Handschel, J. Cartilage defect regeneration by ex vivo engineered autologous microtissue—Preliminary results. In Vivo 2012, 26, 251–257. [Google Scholar]
- Bartz, C.; Meixner, M.; Giesemann, P.; Roël, G.; Bulwin, G.-C.; Smink, J.J. An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. J. Transl. Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schedel, J.; Schubert, T.; Anders, S.; Neumann, E.; Schölmerich, J.; Hofstädter, F.; Grifka, J.; Müller-Ladner, U.; Libera, J. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int. J. Mol. Med. 2009, 23, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Zscharnack, M.; Krause, C.; Aust, G.; Thümmler, C.; Peinemann, F.; Keller, T.; Smink, J.J.; Holland, H.; Somerson, J.S.; Knauer, J.; et al. Preclinical good laboratory practice-compliant safety study to evaluate biodistribution and tumorigenicity of a cartilage advanced therapy medicinal product (ATMP). J. Transl. Med. 2015, 13, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallenborn, M.; Petters, O.; Rudolf, D.; Hantmann, H.; Richter, M.; Ahnert, P.; Rohani, L.; Smink, J.J.; Bulwin, G.C.; Krupp, W.; et al. Comprehensive high-resolution genomic profiling and cytogenetics of human chondrocyte cultures by GTG-banding, locus-specific FISH, SKY and SNP array. Eur. Cells Mater. 2018, 35, 225–241. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Beyls, E.; Declercq, H. Scaffold Free Microtissue Formation for Enhanced Cartilage Repair. Ann. Biomed. Eng. 2019, 48, 298–311. [Google Scholar] [CrossRef]
- Omelyanenko, N.P.; Karalkin, P.A.; Bulanova, E.A.; Koudan, E.V.; Parfenov, V.A.; Rodionov, S.A.; Knyazeva, A.D.; Kasyanovs, V.; Babichenko, I.I.; Chkadua, T.Z.; et al. Extracellular Matrix Determines Biomechanical Properties of Chondrospheres during Their Maturation In Vitro. Cartilage 2020, 11, 521–531. [Google Scholar] [CrossRef]
- Imabayashi, H.; Mori, T.; Gojo, S.; Kiyono, T.; Sugiyama, T.; Irie, R.; Isogai, T.; Hata, J.-I.; Toyama, Y.; Umezawa, A. Redifferentiation of dedifferentiated chondrocytes and chondrogenesis of human bone marrow stromal cells via chondrosphere formation with expression profiling by large-scale cDNA analysis. Exp. Cell Res. 2003, 288, 35–50. [Google Scholar] [CrossRef]
- Eschen, C.; Kaps, C.; Widuchowski, W.; Fickert, S.; Zinser, W.; Niemeyer, P.; Ro, G. Osteoarthritis and Cartilage Open Clinical Outcome Is Signi Fi Cantly Better with Spheroid-Based Autologous Chondrocyte Implantation Manufactured with More Stringent Cell Culture Criteria. Osteoarthr. Cartil. Open 2020, 2, 1–7. [Google Scholar]
- Gardner, R.M.; Sutherland, G.R.; Shaffer, L.G. Chromosome Abnormalities and Genetic Counseling; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Niemeyer, P.; Pestka, J.M.; Salzmann, G.M.; Südkamp, N.P.; Schmal, H. Influence of Cell Quality on Clinical Outcome After Autologous Chondrocyte Implantation. Am. J. Sports Med. 2011, 40, 556–561. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of Tumorigenicity in Mesenchymal Stromal Cell e Based Therapies—Bridging Scienti Fi c Observations and Regulatory Viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Rapko, S.; Parker, A.; Mortelliti, C.; Duguay, S. P192 Aggrecan gene expression as a potency marker for matrix-induced autologous chondroctye implantation (MACI). Osteoarthr. Cartil. 2007, 15, B136. [Google Scholar] [CrossRef] [Green Version]
- Dell’Accio, F.; De Bari, C.; Luyten, F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001, 44, 1608–1619. [Google Scholar] [CrossRef]
- Bravery, C.A.; Carmen, J.; Fong, T.; Oprea, W.; Hoogendoorn, K.H.; Woda, J.; Burger, S.R.; Rowley, J.A.; Bonyhadi, M.L.; Hof, W.V. Potency assay development for cellular therapy products: An ISCT∗ review of the requirements and experiences in the industry. Cytotherapy 2013, 15, 9–19.e9. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, J.; Saris, D.B.F.; Victor, J.; Almqvist, K.F.; Bellemans, J. Five-Year Outcome of Characterized Chondrocyte Implantation Versus Microfracture for Symptomatic Cartilage Defects of the Knee Early Treatment Matters. Am. J. Sports Med. 2011, 39, 2566–2574. [Google Scholar] [CrossRef]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.; on behalf of the SUMMIT Extension Study Group. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Gobbi, A.; Berruto, M.; Andriolo, L.; Ferrua, P.; Crespiatico, I.; Marcacci, M. Long-term Results After Hyaluronan-based MACT for the Treatment of Cartilage Lesions of the Patellofemoral Joint. Am. J. Sports Med. 2016, 44, 602–608. [Google Scholar] [CrossRef]
- Revell, C.M.; Athanasiou, K.A. Success Rates and Immunologic Responses of Autogenic, Allogenic, and Xenogenic Treatments to Repair Articular Cartilage Defects. Tissue Eng. Part B Rev. 2009, 15, 1–15. [Google Scholar] [CrossRef]
- Biant, L.C.; Simons, M.; Gillespie, T.; McNicholas, M.J. Cell Viability in Arthroscopic Versus Open Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 77–81. [Google Scholar] [CrossRef]
- Hunziker, E.; Stähli, A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthr. Cartil. 2008, 16, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheule, A.M.; Beierlein, W.; Wendel, H.P.; Eckstein, F.S.; Heinemann, M.K.; Ziemer, G. Fibrin sealant, aprotinin, and immune response in children undergoing operations for congenital heart disease. J. Thorac. Cardiovasc. Surg. 1998, 115, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.L.A.; Bacher, J.D.; Baron, J. The Role of the Resting Zone in Growth Plate Chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef]
- Bernstein, P.; Dong, M.; Graupher, S.; Corbeil, D.; Gelinsky, M.; Günther, K.-P.; Fickert, S. Sox9 expression of alginate-encapsulated chondrocytes is stimulated by low cell density. J. Biomed. Mater. Res. Part A 2009, 91, 910–918. [Google Scholar] [CrossRef]
- Francioli, S.E.; Candrian, C.; Martin, K.; Heberer, M.; Martin, I.; Barbero, A. Effect of Three-Dimensional Expansion and Cell Seeding Density on the Cartilage-Forming Capacity of Human Articular Chondrocytes in Type II Collagen Sponges. J. Biomed. Mater. Res. Part A 2010, 95, 924–931. [Google Scholar] [CrossRef]
- Mahmoudifar, N.; Doran, P.M. Effect of Seeding and Bioreactor Culture Conditions on the Development of Human Tissue-Engineered Cartilage. Tissue Eng. 2006, 12, 1675–1685. [Google Scholar] [CrossRef]
- Spahn, G.; Fritz, J.; Albrecht, D.; Hofmann, G.O.; Niemeyer, P. Characteristics and associated factors of Klee cartilage lesions: Preliminary baseline-data of more than 1000 patients from the German cartilage registry (KnorpelRegister DGOU). Arch. Orthop. Trauma Surg. 2016, 136, 805–810. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Gillogly, S.D.; Cole, B.J.; Farr, J.; Arnold, R.; Hussey, K.; Minas, T. Autologous Chondrocyte Implantation in the Patella: A Multicenter Experience. Am. J. Sports Med. 2014, 42, 1074–1081. [Google Scholar] [CrossRef]
- Gobbi, A.; Chaurasia, S.; Karnatzikos, G.; Nakamura, N. Matrix-Induced Autologous Chondrocyte Implantation versus Multipotent Stem Cells for the Treatment of Large Patellofemoral Chondral Lesions: A Nonrandomized Prospective Trial. Cartilage 2015, 6, 82–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Keudell, A.; Han, R.; Bryant, T.; Minas, T. Autologous Chondrocyte Implantation to Isolated Patella Cartilage Defects: Two- to 15-Year Follow-Up. Cartilage 2017, 8, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Ebert, J.R.; Schneider, A.; Fallon, M.; Wood, D.J.; Janes, G.C. A Comparison of 2-Year Outcomes in Patients Undergoing Tibiofemoral or Patellofemoral Matrix-Induced Autologous Chondrocyte Implantation. Am. J. Sports Med. 2017, 45, 3243–3253. [Google Scholar] [CrossRef] [Green Version]
- Krueger, D.R.; Gesslein, M.; Schuetz, M.; Perka, C.; Schroeder, J.H. Injectable autologous chondrocyte implantation (ACI) in acetabular cartilage defects—Three-year results. J. Hip Preserv. Surg. 2018, 5, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Thier, S.; Weiss, C.; Fickert, S. Arthroscopic autologous chondrocyte implantation in the hip for the treatment of full-thickness cartilage defects. SICOT-J 2017, 3, 72. [Google Scholar] [CrossRef] [Green Version]
- Körsmeier, K.; Claßen, T.; Kamminga, M.; Rekowski, J.; Jäger, M.; Landgraeber, S. Arthroscopic three-dimensional autologous chondrocyte transplantation using spheroids for the treatment of full-thickness cartilage defects of the hip joint. Knee Surg. Sports Traumatol. Arthrosc. 2014, 24, 2032–2037. [Google Scholar] [CrossRef]
- Fickert, S.; Schattenberg, T.; Nikš, M.; Weiss, C.; Thier, S. Feasibility of arthroscopic 3-dimensional, purely autologous chondrocyte transplantation for chondral defects of the hip: A case series. Arch. Orthop. Trauma Surg. 2014, 134, 971–978. [Google Scholar] [CrossRef]
- Boehm, E.; Minkus, M.; Scheibel, M. Autologous chondrocyte implantation for treatment of focal articular cartilage defects of the humeral head. J. Shoulder Elb. Surg. 2020, 29, 2–11. [Google Scholar] [CrossRef] [PubMed]


| Lead Author (Year) | Level of Evidence | Random Sequence Generation | Allocation Concealment | Selective Reporting | Other Sources of Bias | Blinding (Participants and Personnel) | Blinding (Outcome Assessment) | Incomplete Outcome Data |
|---|---|---|---|---|---|---|---|---|
| Niemeyer (2016) [10], Becher (2017) [11], Niemeyer (2020) [12] | 1b | Low | Low | Unclear | Unclear | High | High | Low |
| Niemeyer (2019) [13], Hoburg (2020) [14] | 1b | Low | Low | Unclear | Unclear | High | High | Low |
| Lead Author (Year) | Level of Evidence | MINORS Score a |
|---|---|---|
| Fickert (2012) [15] | 2b | 11 |
| Siebold (2014) [16] | 4 | 9 |
| Siebold (2016) [17] | 4 | 8 |
| Siebold (2018) [18] | 4 | 12 |
| Hoburg (2019) [19] | 3b | 19 * |
| Grevenstein (2020) [20] | 4 | 8 |
| Niemeyer (2020) [21] | 2b | 22 * |
| Sumida (2021) [22] | 3b | 8 |
| Lead Author (Year) | Patients (n) | Lesion Size (cm2) | Follow-Up (Months) | KOOS | MOCART | IKDC Subjective Score | Lysholm | Other | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Pain | Sympt | ADL | Sports | QOL | ||||||||
| Phase II; Niemeyer (2016) [10] | 75 | 5.0 ± 1.9 | 12 | 73.2 ± 17.6 | d 16.4 ± 20.2 § | d 12.9 ± 17.3 § | d 12.4 ± 17.9 § | d 17.1 ± 28.9 § | d 22.6 ± 24.4 § | 72.4 ± 13.0 | 68.0 ± 18.3 § | Safety | |
| Phase II; Becher (2017) [11] | 75 | 5.0 ± 1.9 | 36 | d 19.9 ± 16.3 § | d 18.2 ± 18.2 § | d 14.8 ± 17.5 § | d 13.6 ± 16.6 § | d 24.3 ± 26.6 § | d 28.9 ± 23.8 § | 75.2 ± 13.4 § | 73.2 ± 18.8 § | Safety | |
| Phase II; Niemeyer (2020) [12] | 75 | 5.0 ± 1.9 | 48 | d 20.1 ± 17.3 | d 18.8 ± 18.2 | d 14.0 ± 17.5 | d 14.2 ± 17.9 | d 23.2 ± 28.9 | d 30.1 ± 24.1 | 75.5 ± 13.1 | 74.6 ± 18.7 | IKDC knee examination, safety | |
| Phase III; Niemeyer (2019) [13] | 52 | 2.2 ± 0.7 | 12 | 78.7 ± 18.6 | 89.9 ± 16.5 $ | 71.6 ± 27.5 $ | 79 ± 14 | Macroscopic repair, histology, safety | |||||
| 24 | 81.5 ± 17.3 | 92.1 ± 13.0 $ | 74.4 ± 24.9 $ | 76 ± 16 | d 24.2 ± 16.9 | d 4.9 ± 4.3 m | |||||||
| Phase III; Hoburg (2020) [14] | 52 | 2.2 ± 0.7 | 36 | 83.2 ± 14.9 | 92.8 ± 12.0 | 79.2 ± 23.2 | Safety | ||||||
| Niemeyer (2020) [21] | Patella 45 * | 5.4 ± 1.6 | 60 | 82.6 ± 14.0 | 88.3 ± 14.4 | 87.6 ± 13.9 | 91.4 ± 10.0 | 76.0 ± 23.0 | 70.6 ± 21.5 | ||||
| Femur 28 * | 6.0 ± 1.7 | 81.9 ± 18.6 | |||||||||||
| Siebold (2014) [16] | 10 | 7.2 ± 3.5 | 24 | 74.4 ± 16.9 | 13.7 ± 1.8 m | 63.9 ± 22.1 | 74.1 ± 18.7 | Kujala | |||||
| Hoburg (2019) [19] | Adolescent 29 | 4.6 ± 2.4 | 63.3 | 82.6 ± 11.6 | 88.5 ± 10.4 | 83.1 ± 16.1 | 94.9 ± 7.4 | 78.6 ± 20.2 | 67.6 ± 17.2 | 74.7 ± 12.0 | 81.1 ± 17.7 | 21.0 ± 2.4 m | |
| Adult 42 * | 4.7 ± 1.2 | 48.4 | 84.6 ± 11.7 | 90.9 ± 8.9 | 91.5 ± 7.0 | 94.2 ± 7.9 | 77.7 ± 21.2 | 69.0 ± 22.3 | 77.2 ± 11.2 | 80.5 ± 15.2 | 22.3 ± 1.9 m | ||
| Fickert (2012) [15] | 37 | 4.4 (1.0–12.0) | 12 | 70 | 64 | 82.5 (34–100) | Tegner, VAS pain, SF-36, safety | ||||||
| Siebold (2016) [17] | 41 | 4.3 ± 3.4 | 34 ± 19.2 | 81 ± 12.9 | 76.8 ± 16.6 | 85.1 ± 14.9 | 55.3 ± 27.7 | 50.6 ± 23.8 | 63.0 ± 18.8 | 79.0 ± 18.0 | Tegner, macroscopic repair | ||
| Siebold (2018) [18] | 30 | 6 ± 3.1 | 34.8 ± 10.2 | 82.2 ± 16.1 | 81.7 ± 12.1 | 86.3 ± 15.6 | 71.0 ± 16.0 | 72.3 ± 16.9 | 60 ± 21 m | 84.2 ± 5.6 | 77.7 ± 14.6 | Tegner, EQ-VAS | |
| Sumida (2021) [22] | 30 | 4.4 ± 3.7 | 14.9 ± 16.3 | Macroscopic repair | |||||||||
| Grevenstein (2020) [20] | 5 | 5.5–16 | Histology | ||||||||||
| Score | Lead Author (Year) | Value | Patients (n) |
|---|---|---|---|
| Overall KOOS | Niemeyer (2020) [12] | 77.1 ± 18.6 | 73 |
| Hoburg (2020) [14] | 83.2 ± 14.9 | 48 | |
| Siebold (2014) [16] | 74.4 ± 16.9 | 10 | |
| Siebold (2016) [17] | 69.76 | 31 | |
| Siebold (2018) [18] | 78.7 | 30 | |
| Hoburg (2019) [19] * | 82.6 ± 11.6 | 29 | |
| Weighted average | 78.2 | 221 | |
| MOCART | Niemeyer (2020) [12] | 75.5 ± 13.1 | 69 |
| Fickert (2012) [15] | 70 | 14 | |
| Hoburg (2019) [19] * | 74.7 ± 12 | 29 | |
| Niemeyer 2016 [10] § | 76 ± 16 | 46 | |
| Weighted average | 75.0 | 158 | |
| IKDC | Niemeyer (2020) [12] | 74.6 ± 18.7 | 73 |
| Fickert (2012) [15] | 64 | 37 | |
| Siebold (2014) [16] | 63.9 ± 22.1 | 10 | |
| Siebold (2016) [10] | 63 ± 18.8 | 31 | |
| Siebold (2018) [18] | 84.2 ± 5.6 | 30 | |
| Hoburg (2019) [19] * | 81.1 ± 17.7 | 22 | |
| Weighted average | 72.5 | 203 | |
| Lysholm | Fickert (2012) [15] | 82.5 | 37 |
| Siebold (2014) [16] | 74.1 ± 18.7 | 10 | |
| Siebold (2016) [17] | 79 ± 18 | 31 | |
| Siebold (2018) [18] | 77.7 ± 14.6 | 30 | |
| Weighted average | 79.4 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonk, L.A.; Roël, G.; Hernigou, J.; Kaps, C.; Hernigou, P. Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7149. https://doi.org/10.3390/ijms22137149
Vonk LA, Roël G, Hernigou J, Kaps C, Hernigou P. Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review. International Journal of Molecular Sciences. 2021; 22(13):7149. https://doi.org/10.3390/ijms22137149
Chicago/Turabian StyleVonk, Lucienne Angela, Giulietta Roël, Jacques Hernigou, Christian Kaps, and Philippe Hernigou. 2021. "Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review" International Journal of Molecular Sciences 22, no. 13: 7149. https://doi.org/10.3390/ijms22137149
APA StyleVonk, L. A., Roël, G., Hernigou, J., Kaps, C., & Hernigou, P. (2021). Role of Matrix-Associated Autologous Chondrocyte Implantation with Spheroids in the Treatment of Large Chondral Defects in the Knee: A Systematic Review. International Journal of Molecular Sciences, 22(13), 7149. https://doi.org/10.3390/ijms22137149








