Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis
Abstract
:1. Introduction
2. Results
2.1. Study Cohort Description
2.2. B-HIPE Clinical Results: Physical Activity and Diet Adherence
2.3. B-HIPE Impact on Immune and Inflammatory Responses: T Lymphocyte Functional Subpopulations, Microbial Translocation, and Gut Permeability
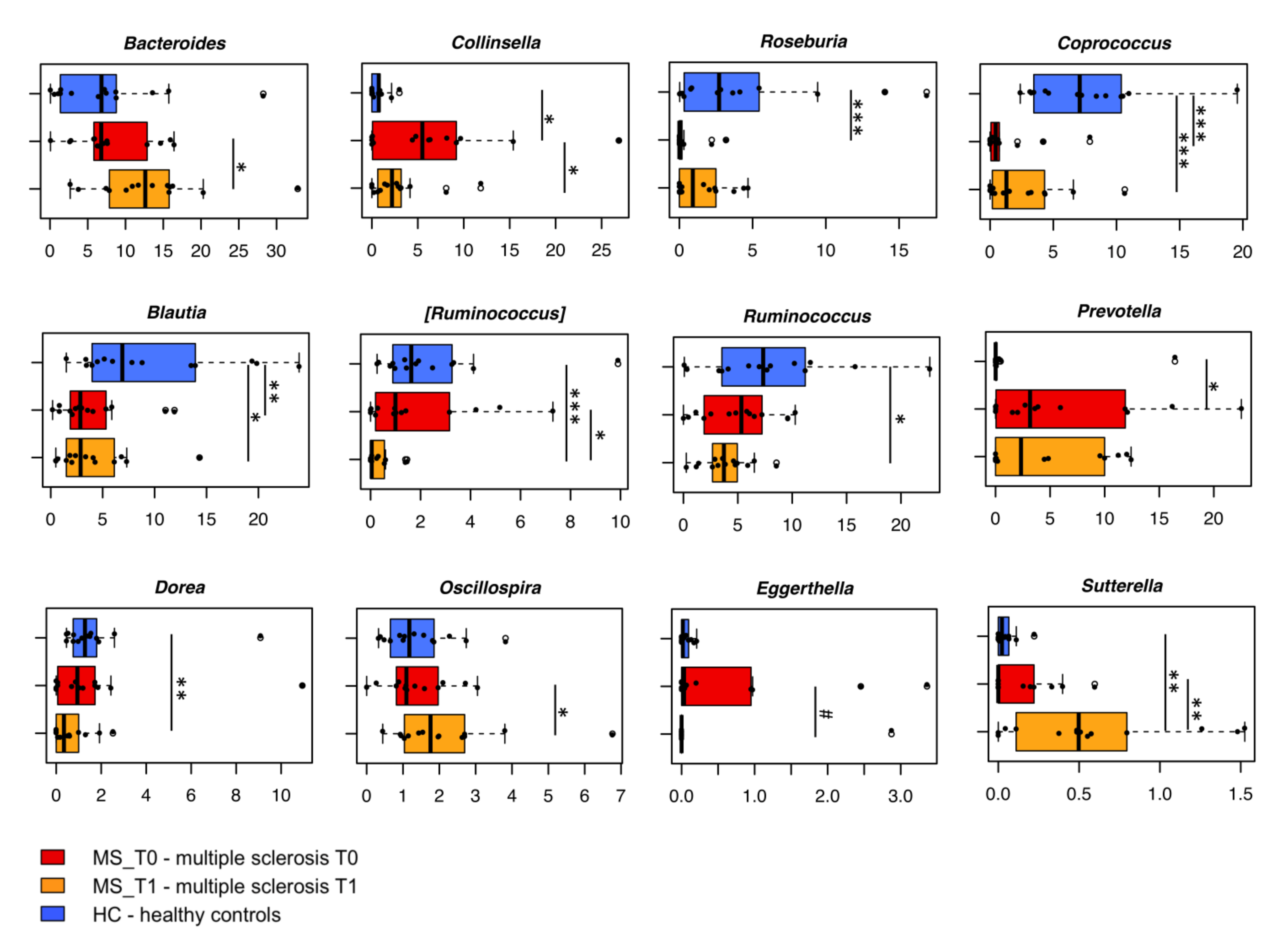
2.4. B-HIPE Impact on the Gut Microbiota
2.4.1. Gut Microbiota Dysbiosis at Baseline
2.4.2. B-HIPE-Related Modulation of the Gut Microbiota
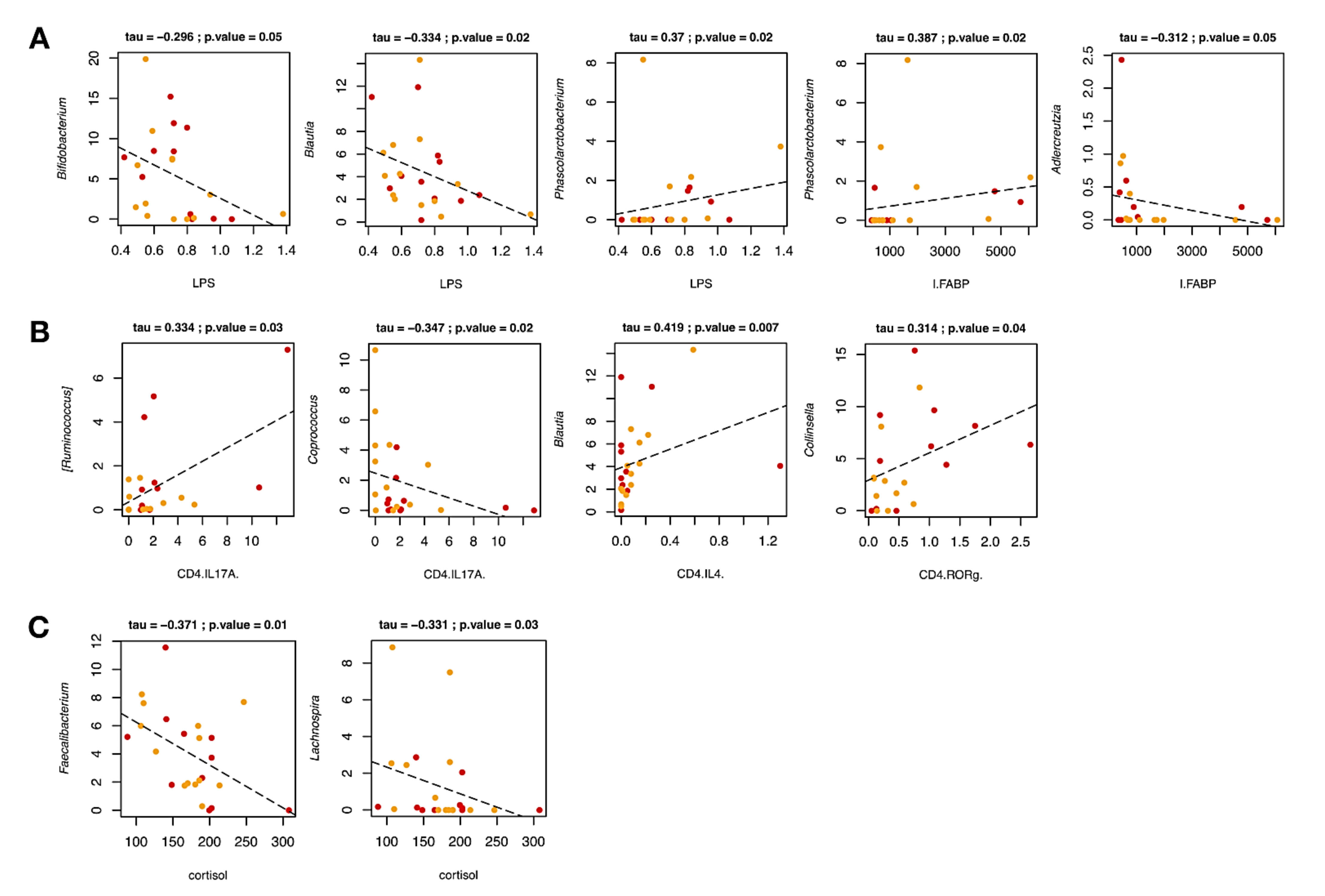
2.4.3. Correlations between Gut Microbiota Profiles and Host Covariates
3. Discussion
4. Materials and Methods
4.1. Subject Enrollment and Rehabilitation Program
- (a)
- Neuromotor rehabilitation in individual and group sessions adapted to each participant’s specific needs and functional limitations;
- (b)
- Recommended diet mainly based on the Mediterranean diet principles, including fresh fruits and vegetables, whole grain products, legumes, nuts and seeds, fish, eggs, and a small amount of poultry and dairy products. On the contrary, red meat, processed meat, alcoholic and sweet drinks were excluded;
- (c)
- Sailing course proposed with equipped single- and double-seated monohulls designed to accommodate disabled sailors;
- (d)
- Mindfulness, through group sessions taking place in the late afternoon hours on a quiet beach, with participation extended to all staff members.
4.2. Assessment of Clinical and Nutritional Variables
- (a)
- An exercise test used to assess aerobic capacity and endurance (six-Minute Walking Test, 6MWT) [75]; the body sway and dynamics of walking were recorded by an instrumental assessment with actigraphs;
- (b)
- A questionnaire to measure fatigue (Modified Fatigue Impact Scale, 5-item version, MFIS-5) [76];
- (c)
- A Food Frequency Questionnaire (FFQ), administered during a face-to-face interview with a team of professional nutritionists to evaluate the adherence to the diet.
4.3. Serum/Blood Analysis
4.3.1. Blood Sample Collection and Cell Separation
4.3.2. Serum
4.3.3. Intracellular Cytokine or Transcription Factor Staining in PBMCs
4.3.4. Flow-Cytometry Analysis
4.3.5. Microbial Translocation and Intestinal Barrier Function
4.3.6. Cortisol Detection
4.4. Gut Microbiota Analysis: DNA Extraction and Sequencing
4.5. Bioinformatics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Howard, J.; Trevick, S.; Younger, D.S. Epidemiology of multiple sclerosis. Neurol. Clin. 2016, 34, 919–939. [Google Scholar] [CrossRef]
- Li, H.; Hou, X.; Liang, Y.; Xu, F.; Zhang, X.; Cui, P.; Xing, G.; Wang, X.; Jiang, W. Gene-based tests of a genome-wide association study dataset highlight novel multiple sclerosis risk genes. Front. Neurosci. 2021, 15, 614528. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Rev. Neurother. 2013, 13 (Suppl. 12), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, P.; Mowry, E.M. Gut microbiome and multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2014, 14, 492. [Google Scholar] [CrossRef]
- Shahi, S.K.; Freedman, S.N.; Murra, A.C.; Zarei, K.; Sompallae, R.; Gibson-Corley, K.N.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front. Immunol. 2019, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, A.; Kantsjo, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.; Quattrochi, B.; Everett, C.; Hong, B.Y.; Cervantes, J. Gut commensals, dysbiosis, and immune response imbalance in the pathogenesis of multiple sclerosis. Mult. Scler. 2021, 27, 807–811. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasen, C.; Tauhid, S.; Chu, R.; et al. Gut microbiome in progressive multiple sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in circulating fatty acid are associated with gut microbiota dysbiosis and inflammation in multiple sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Sterlin, D.; Larsen, M.; Fadlallah, J.; Parizot, C.; Vignes, M.; Autaa, G.; Dorgham, K.; Juste, C.; Lepage, P.; Aboab, J.; et al. Perturbed microbiota/immune homeostasis in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e997. [Google Scholar] [CrossRef] [PubMed]
- Buscarinu, M.C.; Fornasiero, A.; Romano, S.; Ferraldeschi, M.; Mechelli, R.; Renie, R.; Morena, E.; Romano, C.; Pellicciari, G.; Landi, A.C.; et al. The contribution of gut barrier changes to multiple sclerosis pathophysiology. Front. Immunol. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Metz, L.M.; Yong, V.W. Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome. World J. Gastroenterol. 2018, 24, 4217–4223. [Google Scholar] [CrossRef]
- Grigg, J.B.; Sonnenberg, G.F. Host-microbiota interactions shape local and systemic inflammatory diseases. J. Immunol. 2017, 198, 564–571. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, F.; John, N.A.; Brownlee, W.J. Disease-modifying therapies for multiple sclerosis. BMJ 2018, 363, k4674. [Google Scholar] [CrossRef] [PubMed]
- Mendozzi, L.; Tovo, A.; Grosso, C.; Rovaris, M.; Rossi, V.; d’Arma, A.; Garegnani, M.; Margaritella, N.; Barbarito, N.; Meotti, M.; et al. Changing lifestyle of persons with multiple sclerosis: Development, feasibility and preliminary results of a novel high-impact collaborative intervention in leisure environments. Int. J. Phys. Med. Rehabil. 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef] [Green Version]
- Cancello, R.; Turroni, S.; Rampelli, S.; Cattaldo, S.; Candela, M.; Cattani, L.; Mai, S.; Vietti, R.; Scacchi, M.; Brigidi, P.; et al. Effect of short-term dietary intervention and probiotic mix supplementation on the gut microbiota of elderly obese women. Nutrients 2019, 11, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saresella, M.; Mendozzi, L.; Rossi, V.; Mazzali, F.; Piancone, F.; LaRosa, F.; Marventano, I.; Caputo, D.; Felis, G.E.; Clerici, M. Immunological and clinical effect of diet modulation of the gut microbiome in multiple sclerosis patients: A pilot study. Front. Immunol. 2017, 8, 1391. [Google Scholar] [CrossRef]
- Alpizar-Rodriguez, D.; Lesker, T.R.; Gronow, A.; Gilbert, B.; Raemy, E.; Lamacchia, C.; Gabay, C.; Finckh, A.; Strowig, T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Codarri, L.; Gyulveszi, G.; Tosevski, V.; Hesske, L.; Fontana, A.; Magnenat, L.; Suter, T.; Becher, B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011, 12, 560–567. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [Green Version]
- Kunkl, M.; Frascolla, S.; Amormino, C.; Volpe, E.; Tuosto, L. T helper cells: The modulators of inflammation in multiple sclerosis. Cells 2020, 9, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; US Network of Pediatric MS Centers. Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Lagier, J.C.; Chaudet, H.; Raoult, D. Bacterial cocktail to treat Clostridium difficile infection: Primum non nocere. Clin. Infect. Dis. 2018, 67, 1799. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Buscarinu, M.C.; Cerasoli, B.; Annibali, V.; Policano, C.; Lionetto, L.; Capi, M.; Mechelli, R.; Romano, S.; Fornasiero, A.; Mattei, G.; et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult. Scler. 2017, 23, 442–446. [Google Scholar] [CrossRef]
- Mizuno, M.; Noto, D.; Kaga, N.; Chiba, A.; Miyake, S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE 2017, 12, e0173032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatric Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Iljazovic, A.; Amend, L.; Galvez, E.J.C.; de Oliveira, R.; Strowig, T. Modulation of inflammatory responses by gastrointestinal Prevotella spp.—From associations to functional studies. Int. J. Med. Microbiol. 2021, 311, 151472. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.M.; Adebayo, A.S.; Bowyer, R.C.E.; Freidin, M.B.; Finckh, A.; Strowig, T.; Lesker, T.R.; Alpizar-Rodriguez, D.; Gilbert, B.; Kirkham, B.; et al. Associations between gut microbiota and genetic risk for rheumatoid arthritis in the absence of disease: A cross-sectional study. Lancet Rheumatol. 2020, 2, e418–e427. [Google Scholar] [CrossRef]
- Calcinotto, A.; Brevi, A.; Chesi, M.; Ferrarese, R.; Garcia Perez, L.; Grioni, M.; Kumar, S.; Garbitt, V.M.; Sharik, M.E.; Henderson, K.J.; et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 2018, 9, 4832. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, J.; Cai, Z.; Zhou, K.; Chang, L.; Bai, Y.; Ma, Y. Prevotella induces the production of Th17 cells in the colon of mice. J. Immunol. Res. 2020, 2020, 9607328. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Mangalam, A.K.; Murray, J. Microbial monotherapy with Prevotella histicola for patients with multiple sclerosis. Expert Rev. Neurother. 2019, 19, 45–53. [Google Scholar] [CrossRef]
- Brenner, P.; Piehl, F. Fatigue and depression in multiple sclerosis: Pharmacological and non-pharmacological interventions. Acta Neurol. Scand. 2016, 134 (Suppl. 200), 47–54. [Google Scholar] [CrossRef]
- Chen, H.; Shen, L.; Liu, Y.; Ma, X.; Long, L.; Ma, X.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; et al. Strength exercise confers protection in central nervous system autoimmunity by altering the gut microbiota. Front. Immunol. 2021, 12, 628629. [Google Scholar]
- Jakimovski, D.; Weinstock-Guttman, B.; Gandhi, S.; Guan, Y.; Hagemeier, J.; Ramasamy, D.P.; Fuchs, T.A.; Browne, R.W.; Bergsland, N.; Dwyer, M.G.; et al. Dietary and lifestyle factors in multiple sclerosis progression: Results from a 5-year longitudinal MRI study. J. Neurol. 2019, 266, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [Green Version]
- Bitarafan, S.; Harirchian, M.H.; Nafissi, S.; Sahraian, M.A.; Togha, M.; Siassi, F.; Saedisomeolia, A.; Alipour, E.; Mohammadpour, N.; Chamary, M.; et al. Dietary intake of nutrients and its correlation with fatigue in multiple sclerosis patients. Iran. J. Neurol. 2014, 13, 28–32. [Google Scholar] [PubMed]
- Tapsell, L.C. Dietary behaviour changes to improve nutritional quality and health outcomes. Chronic Dis. Transl. Med. 2017, 3, 154–158. [Google Scholar] [CrossRef]
- Branton, W.G.; Lu, J.Q.; Surette, M.G.; Holt, R.A.; Lind, J.; Laman, J.D.; Power, C. Brain microbiota disruption within inflammatory demyelinating lesions in multiple sclerosis. Sci. Rep. 2016, 6, 37344. [Google Scholar] [CrossRef] [Green Version]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.W.; et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef] [Green Version]
- Schepici, G.; Silvestro, S.; Bramanti, P.; Mazzon, E. The gut microbiota in multiple sclerosis: An overview of clinical trials. Cell Transplant. 2019, 28, 1507–1527. [Google Scholar] [CrossRef] [Green Version]
- Grootjans, J.; Thuijls, G.; Verdam, F.; Derikx, J.P.; Lenaerts, K.; Buurman, W.A. Non-invasive assessment of barrier integrity and function of the human gut. World J. Gastrointest. Surg. 2010, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Marques, C.; Pestana, D.; Santoalha, M.; Carvalho, D.; Freitas, P.; Calhau, C. The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr. Metab. 2016, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Fuke, N.; Nagata, N.; Suganuma, H.; Ota, T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients 2019, 11, 2277. [Google Scholar] [CrossRef] [Green Version]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.A.O.; Zhang, J.; Nagarkatti, P.; Nagarkatti, M. The role of gut microbiota in shaping the relapse-remitting and chronic-progressive forms of multiple sclerosis in mouse models. Sci. Rep. 2019, 9, 6923. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Ciric, B.; Marek, R.; Sadhukhan, S.; Caruso, M.L.; Shafagh, J.; Fitzgerald, D.C.; Shindler, K.S.; Rostami, A. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J. Neuroinflammation 2009, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Elain, G.; Jeanneau, K.; Rutkowska, A.; Mir, A.K.; Dev, K.K. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 2014, 62, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Altuntas, C.Z.; Gulen, M.F.; Liu, C.; Giltiay, N.; Qin, H.; Liu, L.; Qian, W.; Ransohoff, R.M.; Bergmann, C.; et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 2010, 32, 414–425. [Google Scholar] [CrossRef] [Green Version]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; DiCorleto, P.E.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef] [Green Version]
- Paintlia, M.K.; Paintlia, A.S.; Singh, A.K.; Singh, I. Synergistic activity of interleukin-17 and tumor necrosis factor-α enhances oxidative stress-mediated oligodendrocyte apoptosis. J. Neurochem. 2011, 116, 508–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulamea, A.O. Role of oligodendrocyte dysfunction in demyelination, remyelination and neurodegeneration in multiple sclerosis. Adv. Exp. Med. Biol. 2017, 958, 91–127. [Google Scholar] [PubMed]
- Deckx, N.; Lee, W.P.; Berneman, Z.N.; Cools, N. Neuroendocrine immunoregulation in multiple sclerosis. Clin. Dev. Immunol. 2013, 2013, 705232. [Google Scholar] [CrossRef]
- Ortega, V.A.; Mercer, E.M.; Giesbrecht, G.F.; Arrieta, M.C. Evolutionary significance of the neuroendocrine stress axis on vertebrate immunity and the influence of the microbiome on early-life stress regulation and health outcomes. Front. Microbiol. 2021, 12, 634539. [Google Scholar] [CrossRef]
- Butland, R.J.; Pang, J.; Gross, E.R.; Woodcock, A.A.; Geddes, D.M. Two-, six-, and 12-minute walking tests in respiratory disease. Br. Med. J. Clin. Res. Ed. 1982, 284, 1607–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, E. Modified fatigue impact scale—5-item version (MFIS-5). Occup. Med. 2016, 66, 256–257. [Google Scholar] [CrossRef] [Green Version]
- Schroder, H.; Fito, M.; Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Lamuela-Raventos, R.; Ros, E.; Salaverria, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut microbiota and extreme longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Masella, A.P.; Bartram, A.K.; Truszkowski, J.M.; Brown, D.G.; Neufeld, J.D. PANDAseq: Paired-end assembler for illumina sequences. BMC Bioinform. 2012, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Crittenden, A.N.; Venema, K.; Marlowe, F.W.; Henry, A.G. Assessing digestibility of Hadza tubers using a dynamic in-vitro model. Am. J. Phys. Anthropol. 2015, 158, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Culhane, A.C.; Thioulouse, J.; Perriere, G.; Higgins, D.G. MADE4: An R package for multivariate analysis of gene expression data. Bioinformatics 2005, 21, 2789–2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]

| Demographic Characteristics | |
|---|---|
| N | 14 |
| Sex (M/F) | 7/7 |
| Age (years) (average ± SD (min–max)) | 49.43 ± 9.08 (36–69) |
| MS course (RR/SP) | 10/4 |
| Disease duration (years) (average ± SD (min–max)) | 19.25 ± 5.40 (7–28) |
| EDSS score (average ± SD (min–max)) | 5.3 ± 1.66 (2–8) |
| Variable | T0 | T1 | p-Value |
|---|---|---|---|
| MFIS-5 | 45.00 (10–95) | 23.60 (0–60) | 0.009 |
| FFQ | 14.50 (1–22) | 33.86 (28–38) | 0.001 |
| 6MWT-meters | 191.07 (15–380) | 260.77 (60–460) | 0.002 |
| 6MWT-bs/s | 0.71 (0.30–1.04) | 0.74 (0.49–1.04) | ns |
| 6MWT-di | 2.04 (0.14–7.56) | 3.43 (0.06–11.26) | 0.046 |
| Subpopulation | T0 | T1 | p-Value |
|---|---|---|---|
| CD4+/CD25+/FOXP3+ | 0.1 | 0.03 | ns |
| (0.05–0.17) | (0.05–0.17) | ||
| CD4+/IFN-γ+ | 0.18 | 0.04 | 0.0004 |
| (0.1–0.5) | (0.0–0.8) | ||
| CD4+/Tbet+ | 0.07 | 0.03 | ns |
| (0.0–0.1) | (0.02–0.1) | ||
| CD4+/IL-17+ | 2.0 | 0.5 | 0.02 |
| (1.4–2.3) | (0.0–1.7) | ||
| CD4+/ROR-γ+ | 0.7 | 0.3 | 0.01 |
| (0.2–1.5) | (0.1–0.6) | ||
| CD4+/IL-4+ | 0.0 | 0.08 | ns |
| (0.0–0.03) | (0.01–0.1) | ||
| CD4+/IL-10+ | 0.2 | 0.03 | ns |
| (0.02–0.2) | (0.0–0.2) | ||
| CD4+/GATA3+ | 0.4 | 0.8 | ns |
| (0.2–1.5) | (0.2–1.4) | ||
| CD4+/TGF-β | 0.9 | 0.5 | ns |
| (0.4–1.3) | (0.3–0.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, M.; Mendozzi, L.; D’Amico, F.; Saresella, M.; Rampelli, S.; Piancone, F.; La Rosa, F.; Marventano, I.; Clerici, M.; d’Arma, A.; et al. Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis. Int. J. Mol. Sci. 2021, 22, 7173. https://doi.org/10.3390/ijms22137173
Barone M, Mendozzi L, D’Amico F, Saresella M, Rampelli S, Piancone F, La Rosa F, Marventano I, Clerici M, d’Arma A, et al. Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis. International Journal of Molecular Sciences. 2021; 22(13):7173. https://doi.org/10.3390/ijms22137173
Chicago/Turabian StyleBarone, Monica, Laura Mendozzi, Federica D’Amico, Marina Saresella, Simone Rampelli, Federica Piancone, Francesca La Rosa, Ivana Marventano, Mario Clerici, Alessia d’Arma, and et al. 2021. "Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis" International Journal of Molecular Sciences 22, no. 13: 7173. https://doi.org/10.3390/ijms22137173
APA StyleBarone, M., Mendozzi, L., D’Amico, F., Saresella, M., Rampelli, S., Piancone, F., La Rosa, F., Marventano, I., Clerici, M., d’Arma, A., Pugnetti, L., Rossi, V., Candela, M., Brigidi, P., & Turroni, S. (2021). Influence of a High-Impact Multidimensional Rehabilitation Program on the Gut Microbiota of Patients with Multiple Sclerosis. International Journal of Molecular Sciences, 22(13), 7173. https://doi.org/10.3390/ijms22137173











