The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information
Abstract
1. Introduction
2. ApoE Expression in Selected Female and Male Diseases
2.1. Reproductive System Disorders
2.1.1. ApoE in Female Fertility
2.1.2. ApoE in Male Fertility
2.2. ApoE in Gynaecological Diseases Influencing Fertility
2.2.1. Endometriosis
2.2.2. Polycystic Ovary Syndrome
| Pathological Condition | Origin of the Tested Material | Observed Changes | References |
|---|---|---|---|
| Female Gynecological Diseases | |||
| Breast cancer |
|
| [46] |
|
| [47] | |
| Choriocarcinoma |
|
| [48] |
| Endometrial adenocarcinoma (ECa) |
|
| [49] |
| Endometrial hyperplasia (EH) |
|
| [50] |
| Ovarian cancer |
|
| [51] |
|
| [47] | |
|
| [52] | |
| Male Reproductive Tract Disorders | |||
| Prostate cancer |
|
| [47] |
|
| [53] | |
|
| [54] | |
|
| [55] | |
|
| [56] | |
|
| [57] | |
| Other Pathologies | |||
|
|
| [58,59] |
|
|
| [60] |
|
|
| [61] |
|
|
| [47] |
3. The Role of ApoE Glycosylation
3.1. ApoE Glycosylation Changes in Preeclampsia
3.2. ApoE Glycosylation Changes in Breast Cancer
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahley, R.W. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988, 240, 622–630. [Google Scholar] [CrossRef]
- Tudorache, I.F.; Trusca, V.G.; Gafencu, A.V. Apolipoprotein E- a multifunctional protein with implications in various pathologies as a result of its structural features. Comput. Struct. Biotechnol. J. 2017, 15, 359–365. [Google Scholar] [CrossRef]
- Linton, M.F.; Gish, R.; Hubl, S.T.; Bütler, E.; Esquivel, C.; Bry, W.I.; Boyles, J.K.; Wardell, M.R.; Young, S.G. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Investig. 1991, 88, 270–281. [Google Scholar] [CrossRef]
- Kockx, M.; Jessup, W.; Kritharides, L. Regulation of endogenous apolipoprotein E secretion by macrophages. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1060–1067. [Google Scholar] [CrossRef]
- Nelissen, K.; Mulder, M.; Smets, I.; Timmermans, S.; Smeets, K.; Ameloot, M.; Hendriks, J.J.A. Liver X receptors regulate cholesterol homeostasis in oligodendrocytes. J. Neurosci. Res. 2012, 90, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, I.B.; Wilhelmus, M.M.M.; Kox, M.; Veerhuis, R.; de Waal, R.M.W.; Verbeek, M.M. Apolipoprotein E protects cultured pericytes and astrocytes from D-Aβ1-40-mediated cell death. Brain Res. 2010, 1315, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Rebeck, G.W. APOE in the normal brain. Neurobiol. Dis. 2020, 136, 104724. [Google Scholar] [CrossRef]
- Flowers, S.A.; Grant, C.G.; Woods, R.J.; Rebeck, G.W. O-glycosylation on cerebrospinal fluid and plasma apolipoprotein E differs in the lipid-binding domain. Glycobiology 2020, 30, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Wardell, M.R.; Weisgraber, K.H.; Mahley, R.W.; Agard, D.A. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science 1991, 252, 1817–1822. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Wang, J. Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc. Natl. Acad. Sci. USA 2011, 108, 14813–14818. [Google Scholar] [CrossRef]
- Das, H.K.; McPherson, J.; Bruns, G.A.; Karathanasis, S.K.; Breslow, J.L. Isolation, characterization, and mapping to chromosome 19 of the human apolipoprotein E gene. J. Biol. Chem. 1985, 260, 6240–6247. [Google Scholar] [CrossRef]
- GeneCards Version 5.1. Available online: https://www.genecards.org (accessed on 8 March 2021).
- Tata, F.; Henry, I.; Markham, A.F.; Wallis, S.C.; Weil, D.; Grzeschik, K.H. Isolation and characterisation of a cDNA clone for human apolipoprotein CI and assignment of the gene to chromosome 19. Hum. Genet. 1985, 69, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.I.; Just, P.W.; Breslow, J.L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am. J. Hum. Genet. 1981, 33, 11–24. [Google Scholar] [PubMed]
- Weisgraber, K.H. Apolipoprotein E: Structure-function relationships. Adv. Protein Chem. 1994, 45, 249–302. [Google Scholar] [CrossRef]
- Bu, G. Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat. Rev. Neurosci. 2009, 10, 333–344. [Google Scholar] [CrossRef]
- Matsunaga, A.; Sasaki, J.; Moriyama, K.; Arakawa, F.; Takada, Y.; Nishi, K.; Hidaka, K.; Arakawa, K. Population frequency of apolipoprotein E5 (Glu3-->Lys) and E7 (Glu244-->Lys, Glu245-->Lys) variants in western Japan. Clin. Genet. 1995, 48, 93–99. [Google Scholar] [CrossRef]
- Wardell, M.R.; Brennan, S.O.; Janus, E.D.; Fraser, R.; Carrell, R.W. Apolipoprotein E2-Christchurch (136 Arg-Ser). New variant of human apolipoprotein E in a patient with type III hyperlipoproteinemia. J. Clin. Investig. 1987, 80, 483–490. [Google Scholar] [CrossRef]
- Matsunaga, A.; Saito, T. Apolipoprotein E mutations: A comparison between lipoprotein glo-merulopathy and type III hyperlipoproteinemia. Clin. Exp. Nephrol. 2014, 18, 220–224. [Google Scholar] [CrossRef]
- Arboleda-Velasquez, J.F.; Lopera, F.; O’Hare, M.; Delgado-Tirado, S.; Marino, C.; Chmielewska, N.; Saez-Torres, K.L.; Amarnani, D.; Schultz, A.P.; Sperling, R.A.; et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: A case report. Nat. Med. 2019, 25, 1680–1683. [Google Scholar] [CrossRef]
- Hernandez, I.; Gelpi, E.; Molina-Porcel, L.; Bernal, S.; Rodríguez-Santiago, B.; Dols-Icardo, O.; Ruiz, A.; Alcolea, D.; Boada, M.; Lleó, A.; et al. Heterozygous APOE Christchurch in familial Alzheimer’s disease without mutations in other Mendelian genes. Neuropathol. Appl. Neurobiol. 2021, 47, 579–582. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Mahley, R.W. Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1305–1315. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 57–89. [Google Scholar] [CrossRef]
- Cruchaga, C.; Kauwe, J.S.; Nowotny, P.; Bales, K.; Pickering, E.H.; Mayo, K.; Bertelsen, S.; Hinrichs, A.; Alzheimer’s Disease Neuroimaging Initiative; Fagan, A.M.; et al. Cerebrospinal fluid APOE levels: An endophenotype for genetic studies for Alzheimer’s disease. Hum. Mol. Genet. 2012, 21, 4558–4571. [Google Scholar] [CrossRef]
- Vincent-Viry, M.; Schiele, F.; Gueguen, R.; Bohnet, K.; Visvikis, S.; Siest, G. Biological variations and genetic reference values for apolipoprotein E serum concentrations: Results from the STANISLAS cohort study. Clin. Chem. 1998, 44, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E: Structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J. Lipid Res. 2009, 50, S183–S188. [Google Scholar] [CrossRef] [PubMed]
- Von Wald, T.; Monisova, Y.; Hacker, M.R.; WookYoo, S.; Penzias, A.S.; Reindollar, R.R.; Usheva, A. Age-related variations in follicular apolipoproteins may influence human oocyte maturation and fertility potential. Fertil. Steril. 2010, 93, 2354–2361. [Google Scholar] [CrossRef]
- Gerdes, L.U.; Gerdes, C.; Hansen, P.S.; Klausen, I.C.; Faergeman, O. Are men carrying the apolipoprotein epsilon 4- or epsilon 2 allele less fertile than epsilon 3 epsilon 3 genotypes? Hum. Genet. 1996, 98, 239–242. [Google Scholar] [CrossRef]
- Zerbinatti, C.V.; Mayer, L.P.; Audet, R.G.; Dyer, C.A. Apolipoprotein E is a putative autocrine regulator of the rat ovarian theca cell compartment. Biol. Reprod. 2001, 64, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Oriá, R.B.; de Almeida, J.Z.; Moreira, C.N.; Guerrant, R.L.; Figueiredo, J.R. Apolipoprotein E Effects on Mammalian Ovarian Steroidogenesis and Human Fertility. Trends Endocrinol. Metab. 2020, 31, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Jasienska, G.; Ellison, P.T.; Galbarczyk, A.; Jasienski, M.; Kalemba-Drozdz, M.; Kapiszewska, M.; Nenko, I.; Thune, I.; Ziomkiewicz, A. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: A case of antagonistic pleiotropy? Proc. Biol. Sci. 2015, 282, 20142395. [Google Scholar] [CrossRef]
- van Exel, E.; Koopman, J.; Bodegom, D.V.; Meij, J.J.; Knijff, P.; Ziem, J.B.; Finch, C.E.; Westendorp, R.G.J. Effect of APOE ε4 allele on survival and fertility in an adverse environment. PLoS ONE 2017, 12, e0179497. [Google Scholar] [CrossRef]
- Nagy, B.; Bán, Z.; Tóth-Pál, E.; Papp, C.; Fintor, L.; Papp, Z. Apolipoprotein E allele distribution in trisomy 13, 18, and 21 conceptuses in a Hungarian population. Am. J. Clin. Pathol. 2000, 113, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Gambina, G.; Ulizzi, L.; Monini, P.; Broggio, E.; Rosano, A.; Scacchi, R. Combined effect of apolipoprotein e genotype and past fertility on age at onset of Alzheimer’s disease in women. Dement. Geriatr. Cogn. Disord. 2007, 24, 82–85. [Google Scholar] [CrossRef]
- Tavilani, H.; Doosti, M.; Abdi, K.; Vaisi-Raygani, A.; Joshaghani, H.R. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia 2006, 38, 173–178. [Google Scholar] [CrossRef]
- Setarehbadi, R.; Vatannejad, A.; Vaisi-Raygani, A.; Amiri, I.; Esfahani, M.; Fattahi, A.; Tavilani, H. Apolipoprotein E genotypes of fertile and infertile men. Syst. Biol. Reprod. Med. 2012, 58, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Rall, S.C., Jr. Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genom. Hum Genet. 2000, 1, 507–537. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Scacchi, R.; Cresta, M. Differential reproductive efficiency associated with common apolipoprotein e alleles in postreproductive-aged subjects. Fertil. Steril. 2004, 81, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Zedda, S.; Grassetti, D.; Gallo, M.; Corbo, R.M.; Lombardo, F.; Lenzi, A.; Gandini, L. Are apolipoprotein E alleles correlated with semen quality? Int. J. Androl. 2012, 35, 714–719. [Google Scholar] [CrossRef]
- Collazo, M.S.; Porrata-Doria, T.; Flores, I.; Acevedo, S.F. Apolipoprotein E polymorphisms and spontaneous pregnancy loss in patients with endometriosis. Mol. Hum. Reprod. 2012, 18, 372–377. [Google Scholar] [CrossRef][Green Version]
- Conway, G.S.; Agrawal, R.; Betteridge, D.J.; Jacobs, H.S. Risk factors for coronary artery disease in lean and obese women with the polycystic ovary syndrome. Clin. Endocrinol. 1992, 37, 119–125. [Google Scholar] [CrossRef]
- Heinonen, S.; Korhonen, S.; Hippeläinen, M.; Hiltunen, M.; Mannermaa, A.; Saarikoski, S. Apolipoprotein E alleles in women with polycystic ovary syndrome. Fertil. Steril. 2001, 75, 878–880. [Google Scholar] [CrossRef]
- Fan, P.; Liu, H.; Wang, Y.; Zhang, F.; Bai, H. Apolipoprotein E-containing HDL-associated platelet-activating factor acetylhydrolase activities and malondialdehyde concentrations in patients with PCOS. Reprod. Biomed. 2012, 24, 197–205. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhang, F.; Fan, P.; Bai, H.; Zhang, J.X.; Wang, Y. Effects of apolipoprotein E genotypes on metabolic profile and oxidative stress in southwest Chinese women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 146–151. [Google Scholar] [CrossRef]
- Moysich, K.B.; Freudenheim, J.L.; Baker, J.A.; Ambrosone, C.B.; Bowman, E.D.; Schisterman, E.F.; Vena, J.E.; Shields, P.G. Apolipoprotein E genetic polymorphism, serum lipoproteins, and breast cancer risk. Mol. Carcinog. 2000, 27, 2–9. [Google Scholar] [CrossRef]
- Hough, C.D.; Sherman-Baust, C.A.; Pizer, E.S.; Montz, F.J.; Im, D.D.; Rosenshein, N.B.; Cho, K.R.; Riggins, G.J.; Morin, P.J. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000, 60, 6281–6287. [Google Scholar] [PubMed]
- Rindler, M.J.; Traber, M.G.; Esterman, A.L.; Bersinger, N.A.; Dancis, J. Synthesis and secretion of apolipoprotein E by human placenta and choriocarcinoma cell lines. Placenta 1991, 12, 615–624. [Google Scholar] [CrossRef]
- Huvila, J.; Brandt, A.; Rojas, C.R.; Pasanen, S.; Talve, L.; Hirsimäki, P.; Fey, V.; Kytömäki, L.; Saukko, P.; Carpén, O.; et al. Gene expression profiling of endometrial adenocarcinomas reveals increased apolipoprotein E expression in poorly differentiated tumors. Int. J. Gynecol. Cancer 2009, 19, 1226–1231. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Krikunova, L.I.; Ryabchenko, N.I.; Mkrtchyan, L.S.; Khorokhorina, V.A.; Salnikova, L.E. Association of the apolipoprotein E 2 allele with concurrent occurrence of endometrial hyperplasia and endometrial carcinoma. Oxid. Med. Cell. Longev. 2015, 2015, 593658. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Zhao, X.; Qin, Y.; Ding, Y.; Chen, R.; Li, G.; Labrie, M.; Ding, Z.; Zhou, J.; Hu, J. FAK-ERK activation in cell/matrix adhesion induced by the loss of apolipoprotein E stimulates the malignant progression of ovarian cancer. J. Exp. Clin. Cancer Res. 2018, 37, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Pohl, G.; Wang, T.L.; Morin, P.J.; Risberg, B.; Kristensen, G.B.; Yu, A.; Davidson, B.; Shih, I.M. Apolipoprotein E is required for cell proliferation and survival in ovarian cancer. Cancer Res. 2005, 65, 331–337. [Google Scholar]
- Grant, W.B. A multicountry ecological study of risk-modifying factors for prostate cancer: Apolipoprotein E epsilon4 as a risk factor and cereals as a risk reduction factor. Anticancer Res. 2010, 30, 189–199. [Google Scholar]
- Ifere, G.O.; Desmond, R.; Demark-Wahnefried, W.; Nagy, T.R. Apolipoprotein E gene polymorphism influences aggressive behavior in prostate cancer cells by deregulating cholesterol homeostasis. Int. J. Oncol. 2013, 43, 1002–1010. [Google Scholar] [CrossRef]
- Liu, H.; Shui, I.M.; Platz, E.A.; Mucci, L.A.; Giovannucci, E.L. No Association of ApoE Genotype with Risk of Prostate Cancer: A Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1632–1634. [Google Scholar] [CrossRef]
- Yencilek, F.; Yilmaz, S.G.; Yildirim, A.; Gormus, U.; Altinkilic, E.M.; Dalan, A.B.; Bastug, Y.; Turkmen, S.; Turkan, S.; Isbir, T. Apolipoprotein E Genotypes in Patients with Prostate Cancer. Anticancer Res. 2016, 36, 707–711. [Google Scholar] [PubMed]
- Asare, G.A.; Owusu-Boateng, E.; Asiedu, B.; Amoah, B.Y.; Essendoh, E.; Otoo, R.Y. Oxidised low-density lipoprotein, a possible distinguishing lipid profile biomolecule between prostate cancer and benign prostatic hyperplasia. Andrologia 2019, 51, e13321. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; Jukema, J.W.; Katan, M.B.; Blauw, G.J.; Sattar, N.; Buckley, B.; Caslake, M.; Ford, I.; Shepherd, J.; Westendorp, R.G.; et al. Apolipoprotein e genotype, plasma cholesterol, and cancer: A Mendelian randomization study. Am. J. Epidemiol. 2009, 170, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Prakash, S.S.; Veeramanikandan, R.; Kirubakaran, R. Association between apolipoprotein E genotype and cancer susceptibility: A meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Tanaka, F.; Zhang, X.; Mimori, K.; Kamohara, Y.; Inoue, H.; Sawada, T.; Hirakawa, K.; Mori, M. Clinical significance of ApoE expression in human gastric cancer. Oncol. Rep. 2008, 20, 1313–1319. [Google Scholar] [CrossRef]
- Luo, J.; Song, J.; Feng, P.; Wang, Y.; Long, W.; Liu, M.; Li, L. Elevated serum apolipoprotein E is associated with metastasis and poor prognosis of non-small cell lung cancer. Tumour Biol. 2016, 37, 10715–10721. [Google Scholar] [CrossRef]
- Spiro, R.G. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 2002, 12, 43R–56R. [Google Scholar] [CrossRef]
- Kockx, M.; Guo, D.L.; Huby, T.; Lesnik, P.; Kay, J.; Sabaretnam, T.; Jary, E.; Hill, M.; Gaus, K.; Chapman, J.; et al. Secretion of apolipoprotein E from macrophages occurs via a protein kinase A and calcium-dependent pathway along the microtubule network. Circ. Res. 2007, 101, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Rüetschi, U.; Halim, A.; Hesse, C.; Carlsohn, E.; Brinkmalm, G.; Larson, G. Enrichment of glycopeptides for glycan structure and attachment site identification. Nat. Methods 2009, 6, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kockx, M.; Raftery, M.J.; Jessup, W.; Griffith, R.; Kritharides, L. Glycosylation and sialylation of macrophage-derived human apolipoprotein E analysed by SDS-PAGE and mass spectrometry: Evidence for a novel site of glycosylation on Ser290. Mol. Cell. Proteom. 2010, 9, 1968–1981. [Google Scholar] [CrossRef] [PubMed]
- Steentoft, C.; Vakhrushev, S.Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Kong, Y.; Bennett, E.P.; Mandel, U.; Wandall, H.; Levery, S.B.; Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 2011, 8, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Rüetschi, U.; Larson, G.; Nilsson, J. LC-MS/MS characterization of O-glycosylation sites and glycan structures of human cerebrospinal fluid glycoproteins. J. Proteome Res. 2013, 12, 573–584. [Google Scholar] [CrossRef]
- Zannis, V.I.; Breslow, J.L.; SanGiacomo, T.R.; Aden, D.P.; Knowles, B.B. Characterization of the major apolipoproteins secreted by two human hepatoma cell lines. Biochemistry 1981, 20, 7089–7096. [Google Scholar] [CrossRef]
- Rebeck, G.W.; Alonzo, N.C.; Berezovska, O.; Harr, S.D.; Knowles, R.B.; Growdon, J.H.; Hyman, B.T.; Mendez, A.J. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Exp. Neurol. 1998, 149, 175–182. [Google Scholar] [CrossRef]
- Mahley, R.W.; Ji, Z.S. Remnant lipoprotein metabolism: Key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J. Lipid Res. 1999, 40, 1–16. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- MacKay, A.P.; Berg, C.J.; Atrash, H.K. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet. Gynecol. 2001, 97, 533–538. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- Masoura, S.; Kalogiannidis, I.A.; Gitas, G.; Goutsioulis, A.; Koiou, E.; Athanasiadis, A.; Vavatsi, N. Biomarkers in pre-eclampsia: A novel approach to early detection of the disease. J. Obstet. Gynaecol. 2012, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2012, 2, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Shankar, R.; Gude, N.; Cullinane, F.; Brennecke, S.; Purcell, A.W.; Moses, E.K. An emerging role for comprehensive proteome analysis in human pregnancy research. Reproduction 2005, 129, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Ackerman, W.E., 4th; Kniss, D.A.; Takizawa, T.; Vandré, D.D. Proteomics of the human placenta: Promises and realities. Placenta 2008, 29, 135–143. [Google Scholar] [CrossRef]
- Wong, F.; Cox, B. Proteomics Analysis of Preeclampsia, a Systematic Review of Maternal and Fetal Compartments. J. Proteom. Bioinform. 2014, 10, 1. [Google Scholar] [CrossRef]
- Atkinson, K.R.; Blumenstein, M.; Black, M.A.; Wu, S.H.; Kasabov, N.; Taylor, R.S.; Cooper, G.J.; North, R.A. An altered pattern of circulating apolipoprotein E3 isoforms is implicated in preeclampsia. J. Lipid Res. 2009, 50, 71–80. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Dunn, N.A.; Muller, J.M.; Pyke, C.M.; Baade, P.D. The descriptive epidemiology of female breast cancer: An international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012, 36, 237–248. [Google Scholar] [CrossRef]
- Tirona, M.T.; Sehgal, R.; Ballester, O. Prevention of breast cancer (part I): Epidemiology, risk factors, and risk assessment tools. Cancer Investig. 2010, 28, 743–750. [Google Scholar] [CrossRef]
- Karihtala, P.; Winqvist, R.; Syväoja, J.E.; Kinnula, V.L.; Soini, Y. Increasing oxidative damage and loss of mismatch repair enzymes during breast carcinogenesis. Eur. J. Cancer 2006, 42, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.A.; Boffetta, P. Today’s lifestyles, tomorrow’s cancers: Trends in lifestyle risk factors for cancer in low- and middle-income countries. Ann. Oncol. 2011, 22, 2349–2357. [Google Scholar] [CrossRef]
- Ahles, T.A.; Li, Y.; McDonald, B.C.; Schwartz, G.N.; Kaufman, P.A.; Tsongalis, G.J.; Moore, J.H.; Saykin, A.J. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: The impact of APOE and smoking. Psychooncology 2014, 23, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Uen, Y.H.; Liao, C.C.; Lin, J.C.; Pan, Y.H.; Liu, Y.C.; Chen, Y.C.; Chen, W.J.; Tai, C.C.; Lee, K.W.; Liu, Y.R.; et al. Analysis of differentially expressed novel post-translational modifications of plasma apolipoprotein E in Taiwanese females with breast cancer. J. Proteom. 2015, 126, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Gu, D.; Mazzone, T. Oleic acid modulates the post-translational glycosylation of macrophage ApoE to increase its secretion. J Biol Chem. 2004, 279, 29195–29201. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Dhanasekaran, P.; Nickel, M.; Nakatani, R.; Saito, H.; Phillips, M.C.; Lund-Katz, S. Molecular basis for the differences in lipid and lipoprotein binding properties of human apolipoproteins E3 and E4. Biochemistry 2010, 49, 10881–10889. [Google Scholar] [CrossRef] [PubMed]
- Zannis, V.I.; Chroni, A.; Kypreos, K.E.; Kan, H.Y.; Cesar, T.B.; Zanni, E.E.; Kardassis, D. Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer. Curr. Opin. Lipidol. 2004, 15, 151–166. [Google Scholar] [CrossRef]
- Guttman, M.; Prieto, J.H.; Handel, T.M.; Domaille, P.J.; Komives, E.A. Structure of the minimal interface between ApoE and LRP. J. Mol. Biol. 2010, 398, 306–319. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Wang, J. A unified scheme for initiation and conformational adaptation of human apolipoprotein E N-terminal domain upon lipoprotein binding and for receptor binding activity. J. Biol. Chem. 2009, 284, 14657–14666. [Google Scholar] [CrossRef] [PubMed]
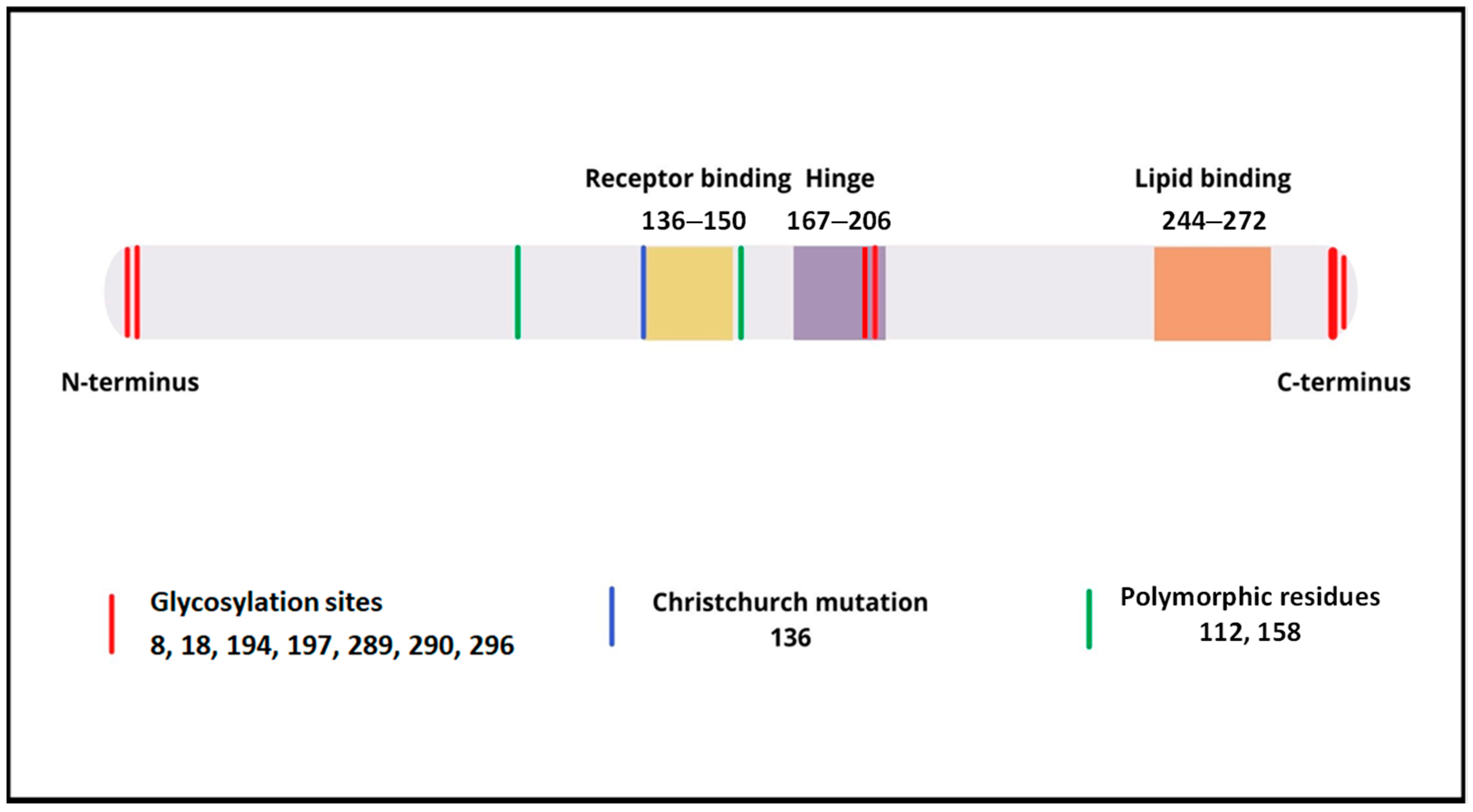

 Neu5Ac—sialic acid (N-acetylneuraminic acid),
Neu5Ac—sialic acid (N-acetylneuraminic acid),  Gal—galactose,
Gal—galactose,  GalNAc—N-acetylgalactosamine, Ser—serine, Thr—threonine. Modification based on Flowers et al. [8].
GalNAc—N-acetylgalactosamine, Ser—serine, Thr—threonine. Modification based on Flowers et al. [8].
 Neu5Ac—sialic acid (N-acetylneuraminic acid),
Neu5Ac—sialic acid (N-acetylneuraminic acid),  Gal—galactose,
Gal—galactose,  GalNAc—N-acetylgalactosamine, Ser—serine, Thr—threonine. Modification based on Flowers et al. [8].
GalNAc—N-acetylgalactosamine, Ser—serine, Thr—threonine. Modification based on Flowers et al. [8].
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacperczyk, M.; Kmieciak, A.; Kratz, E.M. The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information. Int. J. Mol. Sci. 2021, 22, 7197. https://doi.org/10.3390/ijms22137197
Kacperczyk M, Kmieciak A, Kratz EM. The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information. International Journal of Molecular Sciences. 2021; 22(13):7197. https://doi.org/10.3390/ijms22137197
Chicago/Turabian StyleKacperczyk, Monika, Agnieszka Kmieciak, and Ewa Maria Kratz. 2021. "The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information" International Journal of Molecular Sciences 22, no. 13: 7197. https://doi.org/10.3390/ijms22137197
APA StyleKacperczyk, M., Kmieciak, A., & Kratz, E. M. (2021). The Role of ApoE Expression and Variability of Its Glycosylation in Human Reproductive Health in the Light of Current Information. International Journal of Molecular Sciences, 22(13), 7197. https://doi.org/10.3390/ijms22137197






