MSI Analysis in Solid and Liquid Biopsies of Gastroesophageal Adenocarcinoma Patients: A Molecular Approach
Abstract
:1. Introduction
2. Results
2.1. Clinicopathologic Characteristics of Patients
2.2. IHC in FFPE Samples of the Retrospective and Prospective Cohorts
2.3. Validation of the MSI Molecular Assay in FFPE Samples of the Retrospective Cohort
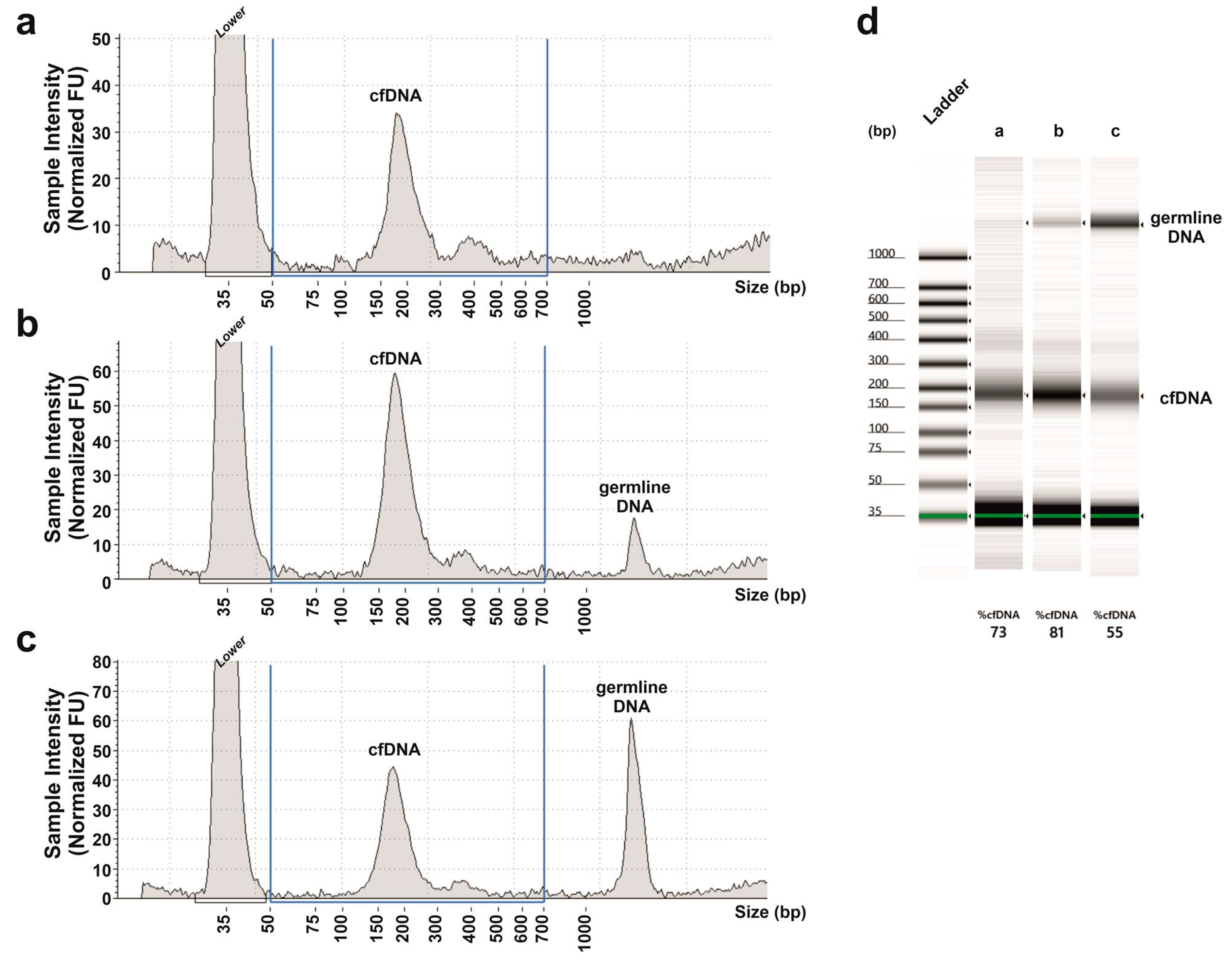
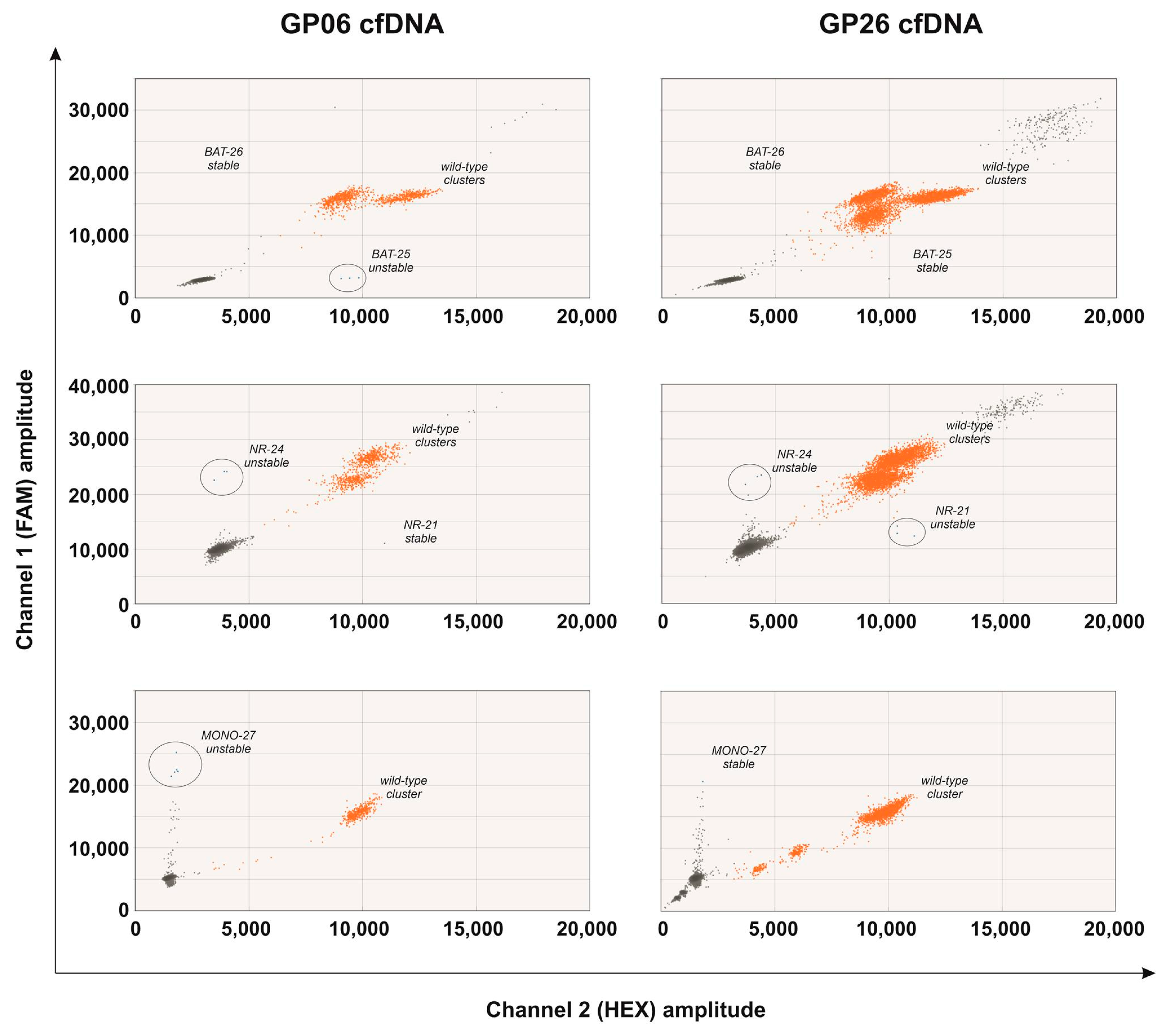
2.4. MSI Molecular Assay in the cfDNA of the Prospective Cohort
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Immunohistochemistry
4.3. DNA Extraction
4.4. MSI Molecular Analysis
4.4.1. Multiplex PCR
4.4.2. Real-Time PCR
4.4.3. ddPCR
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIN | Chromosomal instability |
| CNV | Copy number variation |
| cfDNA | Cell-free DNA |
| ddPCR | Droplet digital PCR |
| EADC | Esophageal adenocarcinoma |
| EBV | Epstein–Barr virus |
| FFPE | Formalin fixed paraffin embedded |
| GAC | Gastric adenocarcinoma |
| GEA | Gastroesophageal adenocarcinoma |
| GS | Genomic stable |
| HER2 | Human epidermal growth factor receptor-2 |
| IHC | Immunohistochemistry |
| MMR | Mismatch Repair |
| MSI | Microsatellite instability |
| MSI-H | Microsatellite instability high |
| MSI-L | Microsatellite instability low |
| MSS | Microsatellite stable |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| SNV | Single nucleotide variation |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domper Arnal, M.J.; Ferrández Arenas, Á.; Lanas Arbeloa, Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J. Gastroenterol. 2015, 21, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Kim, J.; Chao, J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J. Gastroenterol. 2015, 21, 7343–7348. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [CrossRef] [Green Version]
- Dulak, A.M.; Stojanov, P.; Peng, S.; Lawrence, M.S.; Fox, C.; Stewart, C.; Bandla, S.; Imamura, Y.; Schumacher, S.E.; Shefler, E.; et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 2013, 45, 478–486. [Google Scholar] [CrossRef]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Dudley, J.C.; Lin, M.-T.; Le, D.T.; Eshleman, J.R. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Battaglin, F.; Naseem, M.; Puccini, A.; Lenz, H.-J. Molecular biomarkers in gastro-esophageal cancer: Recent developments, current trends and future directions. Cancer Cell Int. 2018, 18, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Zhang, K.; Xi, H.; Cai, A.; Wu, X.; Cui, J.; Li, J.; Qiao, Z.; Wei, B.; Chen, L. Diagnostic and prognostic value of circulating tumor DNA in gastric cancer: A meta-analysis. Oncotarget 2017, 8, 6330–6340. [Google Scholar] [CrossRef] [Green Version]
- Pectasides, E.; Stachler, M.D.; Derks, S.; Liu, Y.; Maron, S.; Islam, M.; Alpert, L.; Kwak, H.; Kindler, H.; Polite, B.; et al. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov. 2018, 8, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Maron, S.B.; Chase, L.M.; Lomnicki, S.; Kochanny, S.; Moore, K.L.; Joshi, S.S.; Landron, S.; Johnson, J.; Kiedrowski, L.A.; Nagy, R.J.; et al. Circulating Tumor DNA Sequencing Analysis of Gastroesophageal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 7098–7112. [Google Scholar] [CrossRef] [Green Version]
- Hamakawa, T.; Kukita, Y.; Kurokawa, Y.; Miyazaki, Y.; Takahashi, T.; Yamasaki, M.; Miyata, H.; Nakajima, K.; Taniguchi, K.; Takiguchi, S.; et al. Monitoring gastric cancer progression with circulating tumour DNA. Br. J. Cancer 2015, 112, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, H.; Nouso, K.; Tanaka, T.; Miyahara, K.; Morimoto, Y.; Dohi, C.; Matsubara, T.; Okada, H.; Yamamoto, K. Droplet digital PCR measurement of HER2 in patients with gastric cancer. Br. J. Cancer 2015, 112, 1652–1655. [Google Scholar] [CrossRef] [Green Version]
- Fang, W.-L.; Lan, Y.-T.; Huang, K.-H.; Liu, C.-A.; Hung, Y.-P.; Lin, C.-H.; Jhang, F.-Y.; Chang, S.-C.; Chen, M.-H.; Chao, Y.; et al. Clinical significance of circulating plasma DNA in gastric cancer. Int. J. Cancer 2016, 138, 2974–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normando, S.R.C.; Delgado, P.D.O.; Rodrigues, A.K.S.B.; David Filho, W.J.; Fonseca, F.L.A.; Cruz, F.J.S.M.; Del Giglio, A. Circulating free plasma tumor DNA in patients with advanced gastric cancer receiving systemic chemotherapy. BMC Clin. Pathol. 2018, 18, 12. [Google Scholar] [CrossRef] [Green Version]
- Shoda, K.; Ichikawa, D.; Fujita, Y.; Masuda, K.; Hiramoto, H.; Hamada, J.; Arita, T.; Konishi, H.; Komatsu, S.; Shiozaki, A.; et al. Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer. Gastric Cancer 2017, 20, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Okamura, R.; Baumgartner, J.M.; Patel, H.; Leichman, L.; Kelly, K.; Sicklick, J.K.; Fanta, P.T.; Lippman, S.M.; Kurzrock, R. Analysis of Circulating Tumor DNA and Clinical Correlates in Patients with Esophageal, Gastroesophageal Junction, and Gastric Adenocarcinoma. Clin. Cancer Res. 2018, 24, 6248–6256. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, C.; Chang, L.; Jia, R.; Liu, R.; Zhang, Y.; Gao, X.; Li, J.; Chen, R.; Xia, X.; et al. Circulating tumor DNA analyses predict progressive disease and indicate trastuzumab-resistant mechanism in advanced gastric cancer. EBioMedicine 2019, 43, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-S.; Liu, Z.-X.; Lu, Y.-X.; Bao, H.; Wu, X.; Zeng, Z.-L.; Liu, Z.; Zhao, Q.; He, C.-Y.; Lu, J.-H.; et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2019, 68, 1152–1161. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Zhang, M.; Li, B.; Niu, Y.; Chen, L.; Yang, J.; Lu, S.; Gao, J.; Shen, L. Chromosomal instability of circulating tumor DNA reflect therapeutic responses in advanced gastric cancer. Cell Death Dis. 2019, 10, 697–699. [Google Scholar] [CrossRef]
- Gonzalez, R.S.; Messing, S.; Tu, X.; McMahon, L.A.; Whitney-Miller, C.L. Immunohistochemistry as a surrogate for molecular subtyping of gastric adenocarcinoma. Hum. Pathol. 2016, 56, 16–21. [Google Scholar] [CrossRef]
- Pös, O.; Biró, O.; Szemes, T.; Nagy, B. Circulating cell-free nucleic acids: Characteristics and applications. Eur. J. Hum. Genet. 2018, 26, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tieng, F.Y.F.; Abu, N.; Lee, L.-H.; Ab Mutalib, N.-S. Microsatellite Instability in Colorectal Cancer Liquid Biopsy—Current Updates on Its Potential in Non-Invasive Detection, Prognosis and as a Predictive Marker. Diagnostics 2021, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Hechtman, J.F.; Rana, S.; Middha, S.; Stadler, Z.K.; Latham, A.; Benayed, R.; Soslow, R.; Ladanyi, M.; Yaeger, R.; Zehir, A.; et al. Retained mismatch repair protein expression occurs in approximately 6% of microsatellite instability-high cancers and is associated with missense mutations in mismatch repair genes. Mod. Pathol. 2020, 33, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Silveira, A.B.; Bidard, F.-C.; Kasperek, A.; Melaabi, S.; Tanguy, M.-L.; Rodrigues, M.; Bataillon, G.; Cabel, L.; Buecher, B.; Pierga, J.-Y.; et al. High-Accuracy Determination of Microsatellite Instability Compatible with Liquid Biopsies. Clin. Chem. 2020, 66, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; De La Chapelle, A.; Rüschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Foy, C.A.; Benes, V.; Emslie, K.; Garson, J.A.; Haynes, R.; Hellemans, J.; Kubista, M.; Mueller, R.D.; Nolan, T.; et al. The Digital MIQE Guidelines: Minimum Information for Publication of Quantitative Digital PCR Experiments. Clin. Chem. 2013, 59, 892–902. [Google Scholar] [CrossRef] [PubMed]


| Retrospective Cohort | Prospective Cohort | |
|---|---|---|
| Patients | Total | Total |
| N (%) 86 (100) | N (%) 35 (100) | |
| Age | ||
| Median (Q1;Q3) | 76 (68;82) | 68 (57;74) |
| (Range) | (44–97) | (40–96) |
| Gender | ||
| Male | 52 (60%) | 21 (60%) |
| Female | 34 (40%) | 14 (40%) |
| Tumor Site | ||
| Cardia | 14 (16%) | 7 (20%) |
| Fundus | 14 (16%) | 7 (20%) |
| Body | 31 (37%) | 10 (29%) |
| Antrum | 27 (31%) | 11 (31%) |
| pTNM Stage | ||
| I/II | 48 (56%) | 11 (31.5%) |
| III/IV | 38 (44%) | 22 (62.8%) |
| unknown | 2 (5.7%) | |
| IHC Typing | ||
| MSI | 15 (17%) | 5 (14%) |
| CIN | 28 (33%) | 11 (32%) |
| EBV+ | 3 (3%) | 0 (0%) |
| GS | 40 (47%) | 19 (54%) |
| MSI Analysis System Version 1.2 | EasyPGX Ready MSI | MSI ddPCR Assay | |
|---|---|---|---|
| Tumor DNA:Normal DNA Dilutions | MSI Status | ||
| 1:0 | ● | ● | ● |
| 1:2 | ● | ● | ● |
| 1:4 | ● | ● | ● |
| 1:8 | ● | ● | ● |
| 1:16 | ○ | ● | ● |
| 1:32 | ○ | ○ | ● |
| 1:64 | ND | ND | ● |
| 1:128 | ND | ND | ● |
| 1:256 | ND | ND | ● |
| 1:512 | ND | ND | ● |
| 1:1024 | ND | ND | ○ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boldrin, E.; Piano, M.A.; Alfieri, R.; Mazza, M.; Vassallo, L.; Scapinello, A.; Pilati, P.; Curtarello, M. MSI Analysis in Solid and Liquid Biopsies of Gastroesophageal Adenocarcinoma Patients: A Molecular Approach. Int. J. Mol. Sci. 2021, 22, 7244. https://doi.org/10.3390/ijms22147244
Boldrin E, Piano MA, Alfieri R, Mazza M, Vassallo L, Scapinello A, Pilati P, Curtarello M. MSI Analysis in Solid and Liquid Biopsies of Gastroesophageal Adenocarcinoma Patients: A Molecular Approach. International Journal of Molecular Sciences. 2021; 22(14):7244. https://doi.org/10.3390/ijms22147244
Chicago/Turabian StyleBoldrin, Elisa, Maria Assunta Piano, Rita Alfieri, Marcodomenico Mazza, Loretta Vassallo, Antonio Scapinello, Pierluigi Pilati, and Matteo Curtarello. 2021. "MSI Analysis in Solid and Liquid Biopsies of Gastroesophageal Adenocarcinoma Patients: A Molecular Approach" International Journal of Molecular Sciences 22, no. 14: 7244. https://doi.org/10.3390/ijms22147244






